Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening of Conocarpus spp.
2.2. Identification of Polyphenols
3. Materials and Methods
3.1. Chemicals
3.2. Collecting of Plant Materials and Extraction Procedure
3.3. Experimental Design
3.4. Phytochemicals Screening Tests
3.5. Determination of Polyphenolic Components Using HPLC
3.6. Determination of Tannins
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Cutter, C. Antimicrobial effect of herb extracts against Escherichia coli O157:H7, Listeria monocytogenes and Salmonella typhimurium associated with beef. J. Food Prot. 2000, 63, 601–607. [Google Scholar] [CrossRef]
- Amal, A.; Ashraf, A.; Hossam, E.S. Chemical compositions and antioxidant/antimicrobial activities of kaff maryam (Anastatica hierochuntica) and doum palm (Hyphaene thebaica) cultivated in Egypt. Biyoloji Bilimleri Arastirma Dergisi 2009, 2, 71–79. [Google Scholar] [CrossRef]
- Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [Google Scholar] [CrossRef]
- Agostini-Costa, T.S.; Vieira, R.F.; Bizzo, H.R.; Silveira, D.; Gimenes, M.A. Secondary metabolites. In Chromatography and Its Applications; IntechOpen: London, UK, 2012; pp. 131–164. [Google Scholar] [CrossRef]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.S.; Campo, S.; Ferlazzo, A.M.; Calatroni, A. Chondroitin sulphate: Antioxidant properties and beneficial effects. Mini Rev. Med. Chem. 2006, 6, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Handique, J.G.; Baruah, J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Nakayama, T.; Yonekura-Sakakibara, K.; Sato, T.; Kikuchi, S.; Fukui-Mizutani, M.; Ueda, T.; Nakao, M.; Tanaka, Y.; Kusumi, T.; Nishino, T. Aureusidin synthase: A polyphenol oxidase homolog responsible for flower coloration. Science 2000, 290, 1163–1166. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar] [CrossRef]
- Austin, D.A. Florida Ethnobotany, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 233, 394, 829. [Google Scholar]
- Abdel-Hameed, E.S.; Bazaid, S.A.; Shohayeb, M.S.; El-Sayed, M.M.; El-Wakil, E.A. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Euro J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Baroon, Z.; Razzaque, M.A. Nutritional evaluation and palatability trial of ensiled Conocarpus greenery residues. Experim. Agri. 2011, 29, 138–147. [Google Scholar] [CrossRef]
- Agra, M.F.; Patrícia, F.F.; Jose, M.B.F. Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Braz. J. Pharmacogn. 2007, 17, 114–140. [Google Scholar] [CrossRef]
- Altschul, S.V.R. Drugs and Foods from Little Known Plants, 2nd ed.; Harvard University Press: Cambridge, MA, USA, 1973; pp. 206–207. [Google Scholar]
- Reis, S.V.; Frank, J.; Lipp, J.R. New Plant Sources for Drugs and Foods from the New York Botanical Garden Herbarium, 2nd ed.; Harvard University Press: Cambridge, MA, USA, 1982; pp. 210–211. [Google Scholar]
- Al-Musayeib, N.M.; Mothana, R.A.; Al-Massarani, S.; Matheeussen, A.; Cos, P.; Maes, L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules 2012, 17, 11379–11390. [Google Scholar] [CrossRef] [PubMed]
- Saadullah, M.; Chaudary, B.A.; Uzair, M.; Afzal, K. Antidiabetic potential of Conocarpus C. lancifolius. Bangladesh J. Pharmacol. 2014, 9, 244–249. [Google Scholar] [CrossRef]
- Touqeer, S.; Saeed, M.A.; Ansari, F.; Zahra, N.; Masood, Z.; Fareed, M.; Javed, A. Antibacterial and antifungal activity of Conocarpus C. lancifolius Engl. (Combretaceae). Appl. Pharmacol. 2014, 6, 153–155. [Google Scholar] [CrossRef]
- Bashir, M.; Uzair, M.; Chaudhry, B.A. A review of phytochemical and biological studies on Conocarpus erectus (Combretaceae). Pak. J. Pharm. Res. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Chung, K.T.; Stevens, S.E., Jr.; Lin, W.F.; Wei, C.I. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett. Appl. Microbiol. 1993, 17, 29–32. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Saadullah, M.; Chaudary, B.A. Muhammad Uzair, M. Antioxidant, phytotoxic and antiurease activities, and total phenolic and flavonoid contents of Conocarpus C. lancifolius (Combretaceae). Trop. J. Pharm. Res. 2016, 15, 555–561. [Google Scholar] [CrossRef]
- Saadullah, M. Studies on Chemical Constituents and Biological Activities of Conocarpus C. lancifolius (CombretaceaeBahauddin). Ph.D. Thesis, Zakariya University, Multan, Pakistan, 2017. [Google Scholar]
- Elizabeth, R.; Iris, S. Fundamentals of Chemistry: Solubility; Department of Chemistry, University of Wisconsin: Madison, WI, USA, 2015. [Google Scholar]
- Sundari, U.T.; Rekha, S.; Parvathi, A. Phytochemical analysis of some therapeutic medicinal flowers. Int. J. Pharm. 2012, 2, 583–585. [Google Scholar]
- Saadullah, M. Medicinally Important Trees: Important Trees with Antidiabetic Activities, 1st ed.; Springer Nature: Cham, Switzerland, 2017; pp. 21–53. [Google Scholar] [CrossRef]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Gómez-Romero, M.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Application and potential of capillary electroseparation methods to determine antioxidant phenolic compounds from plant food material. J. Pharm. Biomed. Anal. 2010, 53, 1130–1160. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Plata-Oviedo, M.S.V.; Mattos, G.; Carpes, S.T.; Branco, I.G. Extraction and quantification of phenolic acids and flavonols from Eugenia pyriformis using different solvents. Food Sci. Technol. 2014, 51, 2862–2866. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, S1700–S1741. [Google Scholar] [CrossRef]
- Harborne, J.B.; Grayer, R.J. The Flavonoids, 1st ed.; Chapman and Hall: London, UK, 1994; pp. 589–618. [Google Scholar]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jianming, W.; Yuan, G.; Ping, L.; Feng, H.; Liying, L. Optimization of ultrasound-assisted extraction procedure to determine total isoflavones in Chinese soybean cheese by box–behnken design. Food Anal. Methods 2013, 6, 221–226. [Google Scholar] [CrossRef]
- Wood, J.R.I. A Handbook of the Yemen Flora; Royal Botanic Gardens, Kew: Richmond, UK, 1997; pp. 1–434. [Google Scholar]
- Myers, R.; Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Myers, R.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 2nd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Jing, Y.; Jing, W.; Chunming, L.; Zhiqiang, L.; Qiang, W. Application of response surface methodology to optimize supercritical carbon dioxide extraction of oil from rapeseed (Brassica napus L.). Int J. Food Sci. Tech. 2012, 47, 1–7. [Google Scholar] [CrossRef]
- Afifi, H.S.; Hashim, I.B.; Altubji, S.I. Optimizing extraction conditions of crude fiber, phenolic compounds, flavonoids and antioxidant activity of date seed powder. J. Food Sci. Tech. 2017, 54, 4149–4161. [Google Scholar] [CrossRef]
- Brain, K.R.; Turner, T.D. The Practical Evaluation of Phytopharmaceuticals, 2nd ed.; Wright-Sciencetechnica: Bristol, UK, 1975; pp. 81–82. [Google Scholar]
- Evans, W.C. Trease and Evans’ Pharmacognosy, 14th ed.; Saunders Company Limited: London, UK, 1996; pp. 545–546. [Google Scholar]
- Fagbemi, T.N.; Oshodi, A.A.; Ipinmoroti, K.O. Processing effects on some antinutritional factors and In vitro multienzyme protein digestibility (IVPD) of three tropical seeds: Breadnut (Artocarpus altilis), cashewnut (Anacardium occidentale) and fluted pumpkin (Telfairia occidentalis). Pak. J. Nutr. 2005, 4, 250–256. [Google Scholar] [CrossRef]


| Standard Order. | Coded and Actual Level of Variables | Leaves | Fruits | Roots | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. lancifolius | C. erectus | C. lancifolius | C. erectus | C. lancifolius | C. erectus | ||||||||||||||||
| Solvent Conc. | Temp. | Time | Phytosteroids | Saponins | Glycosides | Phytosteroids | Saponins | Glycosides | Phytosteroids | Saponins | Glycosides | Phytosteroids | Saponins | Glycosides | Phytosteroids | Saponins | Glycosides | Phytosteroids | Saponins | Glycosides | |
| 1 | 1 (100) | 1 (65) | 0 (2) | -ve | + | -ve | -ve | + | -ve | + | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | + |
| 2 | −1 (50) | 0 (55) | 1 (3) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | -ve | -ve | + | + |
| 3 | 0 (75) | 0 (55) | 0 (2) | -ve | + | + | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | -ve |
| 4 | 0 (75) | 1 (65) | 1 (3) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | -ve |
| 5 | 1 (100) | 0 (55) | −1 (1) | -ve | + | + | + | -ve | -ve | + | -ve | + | -ve | + | + | -ve | -ve | + | -ve | + | -ve |
| 6 | 1 (100) | −1 (45) | 0 (2) | -ve | -ve | + | + | + | -ve | + | -ve | + | -ve | + | + | -ve | -ve | + | -ve | -ve | -ve |
| 7 | −1 (50) | 1 (65) | 0 (2) | -ve | + | -ve | -ve | -ve | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | -ve |
| 8 | 0 (75) | 0 (55) | 0 (2) | -ve | + | + | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | -ve |
| 9 | 0 (75) | 1 (65) | −1 (1) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + |
| 10 | 1 (100) | 0 (55) | 1 (3) | -ve | + | + | + | + | -ve | + | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + |
| 11 | 0 (75) | −1 (45) | 1 (3) | -ve | + | + | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | + |
| 12 | 0 (75) | 0 (55) | 0 (2) | -ve | + | + | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | -ve |
| 13 | −1 (50) | 0 (55) | −1 (1) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | + |
| 14 | 0 (75) | −1 (45) | −1 (1) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | + |
| 15 | −1 (50) | −1 (45) | 0 (2) | -ve | + | -ve | -ve | + | -ve | -ve | -ve | + | -ve | -ve | + | -ve | -ve | + | -ve | + | + |
| Plant | 4-Hydroxy Benzoic Acid | Vanillic Acid | Caffeic Acid | Salicylic Acid | 1,2-Dihydroxy Benzene | Catechin | Benzoic Acid | p-Coumaric Acid | t-Ferulic Acid |
|---|---|---|---|---|---|---|---|---|---|
| CE | 3.33 a | 12.54 a | 4.60 b | 3.26 a | 2.33 a | 1.89 a | 2.21 a | 16.21 a | 15.12 a |
| CL | 1.87 b | 7.32 b | 6.04 a | 0.99 b | 1.02 b | 1.40 b | 1.32 b | 11.48 b | 10.73 b |
| LSD | 0.14 | 0.28 | 0.23 | 0.03 | 0.03 | 0.07 | 0.07 | 0.52 | 0.44 |
| Plant | Sinapic Acid | Chlorogenic Acid | Vanillin | Rutin Hydrate | Cinnamic Acid | Protocatechuic Acid | Quercetin | Flavone | Tannins |
| CE | 32.62 a | 2.33 b | 4.91 a | 135.31 a | 2.13 a | 57.25 a | 79.31 a | 278.64 a | 0.56 a |
| CL | 28.03 b | 5.89 a | 3.18 b | 128.56 b | 1.24 b | 56.24 a | 67.41 b | 123.29 b | 0.43 b |
| LSD | 1.34 | 0.22 | 0.17 | 4.67 | 0.10 | 2.31 | 0.96 | 4.88 | 0.09 |
| Plant Part | 4-Hydroxy Benzoic Acid | Vanillic Acid | Caffeic Acid | Salicylic Acid | 1,2-Dihydroxy Benzene | Catechin | Benzoic Acid | p-Coumaric Acid | t-Ferulic Acid |
| L | 1.80 b | 2.42 c | 1.18 c | 0.15 b | 0.17 b | 0.62 b | 0.67 b | 20.01 a | 29.67 a |
| F | 0.37 c | 4.43 b | 6.60 b | 0.00 c | 0.03 c | 0.12 c | 0.25 c | 8.43 c | 0.94 c |
| R | 5.63 a | 22.93 a | 8.26 a | 6.23 a | 4.83 a | 4.20 a | 4.37 a | 13.09 b | 8.17 b |
| LSD | 0.17 | 0.34 | 0.27 | 0.03 | 0.03 | 0.08 | 0.08 | 0.63 | 0.54 |
| Plant Part | Sinapic Acid | Chlorogenic Acid | Vanillin | Rutin Hydrate | Cinnamic Acid | Protocatechuic Acid | Quercetin | Flavone | Tannins |
| L | 57.03 a | 6.59 a | 5.68 a | 190.21 a | 0.41 b | 30.89 c | 16.75 c | 397.37 a | 0.50 a |
| F | 13.68 c | 0.31 c | 0.83 b | 89.48 c | 0.43 b | 103.31 a | 106.82 a | 96.78 c | 0.51 a |
| R | 20.27 b | 5.44 b | 5.63 a | 116.11 b | 4.22 a | 36.04 b | 96.52 b | 108.73 b | 0.48 a |
| LSD | 1.65 | 0.26 | 0.21 | 5.72 | 0.12 | 2.83 | 1.17 | 5.97 | 0.11 |
| Part | 4-Hydroxy Benzoic Acid | Vanillic Acid | Caffeic Acid | Salicylic Acid | 1,2-Dihydroxy Benzene | Catechin | Benzoic Acid | p-Coumaric Acid | t-Ferulic Acid |
|---|---|---|---|---|---|---|---|---|---|
| CEL | 2.49 ± 2.75 c | 3.47 ± 3.34 d | 1.69 ± 2.21 d | 0.30 ± 1.16 c | 0.18 ± 0.20 c | 0.30 ± 0.29 d | 0.75 ± 0.47 c | 23.72 ± 41.41 a | 32.74 ± 27.58 a |
| CER | 6.83 ± 20.2 1a | 27.45 ± 33.66 a | 11.29 ± 26.63 b | 9.49 ± 35.91 a | 6.76 ± 0.15 a | 5.30 ± 18.87 a | 5.66 ± 20.50 a | 18.55 ± 58.64 b | 11.07 ± 34.18 c |
| CEF | 0.67 ± 1.40 e | 6.70 ± 5.81 c | 0.98 ± 1.20 e | 0.002 ± 0.01 d | 0.05 ± 0.37 d | 0.09 ± 0.23 e | 0.22 ± 0.30 e | 6.37 ± 13.62 f | 1.57 ± 1.89 e |
| CLL | 1.12 ± 2.04 d | 1.38 ± 2.22 f | 0.68 ± 1.76 e | 0.00 ± 0.00 d | 0.16 ± 0.37 c | 0.94 ± 0.92 c | 0.59 ± 0.63 d | 16.30 ± 21.61 c | 26.59 ± 51.00 b |
| CLR | 4.43 ± 11.61 b | 18.42 ± 11.57 b | 5.23 ± 10.19 c | 2.97 ± 11.25 b | 2.90 ± 10.18 b | 3.10 ± 10.63 b | 3.09 ± 11.19 b | 7.64 ± 11.70 e | 5.27 ± 12.37 d |
| CLF | 0.07 ± 0.37 f | 2.15 ± 6.36 e | 12.22 ± 10.29 a | 0.00 ± 0.00 d | 0.01 ± 0.04 d | 0.15 ± 0.17 e | 0.27 ± 0.23 c | 10.49 ± 12.46 d | 0.32 ± 0.63 f |
| Part | Sinapic Acid | Chlorogenic Acid | Vanillin | Rutin Hydrate | Cinnamic Acid | Protocatechuic Acid | Quercetin | Flavone | Tannins |
| CEL | 55.73 ± 47.29 b | 0.44 ± 1.23 d | 4.97 ± 4.27 c | 214.32 ± 328.17 a | 0.72 ± 0.64 c | 33.28 ± 36.65 d | 19.70 ± 35.30 d | 593.14 ± 642.06 a | 0.53 ± 0.10 b |
| CER | 25.40 ± 59.72 c | 5.93 ± 18.01 b | 8.31 ± 14.52 a | 178.21 ± 240.39 b | 5.25 ± 18.85 a | 43.53 ± 26.00 c | 109.68 ± 10.66 a | 135.19 ± 56.44 c | 0.55 ± 0.18 b |
| CEF | 16.73 ± 19.01 d | 0.62 ± 2.23 d | 1.46 ± 2.05 e | 13.39 ± 10.14 e | 0.44 ± 0.10 d | 94.93 ± 33.87 b | 108.57 ± 28.80 a | 107.58 ± 21.06 d | 0.60 ± 0.13 a |
| CLL | 58.33 ± 58.72 a | 12.74 ± 20.26 a | 6.39 ± 3.76 b | 166.10 ± 156.02 c | 0.10 ± 0.30 e | 28.49 ± 26.12 e | 13.80 ± 26.13 e | 201.60 ± 215.75 b | 0.47 ± 0.27 c |
| CLR | 15.14 ± 20.94 d | 4.94 ± 9.15 c | 2.95 ± 9.97 d | 54.00 ± 56.54 d | 3.20 ± 10.83 b | 28.54 ± 24.96 e | 83.36 ± 48.76 c | 82.27 ± 25.57 e | 0.41 ± 0.09 d |
| CLF | 10.64 ± 21.07 e | 00.00 ± 00.00 e | 0.20 ± 0.57 f | 165.57 ± 159.40 c | 0.42 ± 0.21 d | 111.68 ± 22.64 a | 105.07 ± 27.30 b | 85.99 ± 44.40 e | 0.41 ± 0.09 d |
| Component | Solvent Conc. (%) | Temp. (°C) | Time (h) | Plant |
|---|---|---|---|---|
| Vanillic acid | 86.82 | 57.47 | 2.13 | CER |
| p-Coumaric acid | 102.98 | 36.16 | 2.27 | |
| Quercetin | 82.29 | 55.06 | 1.67 | |
| Rutin hydrate | 48.46 | 54.70 | 2.31 | CEL |
| Flavone | 61.46 | 57.66 | 1.56 | |
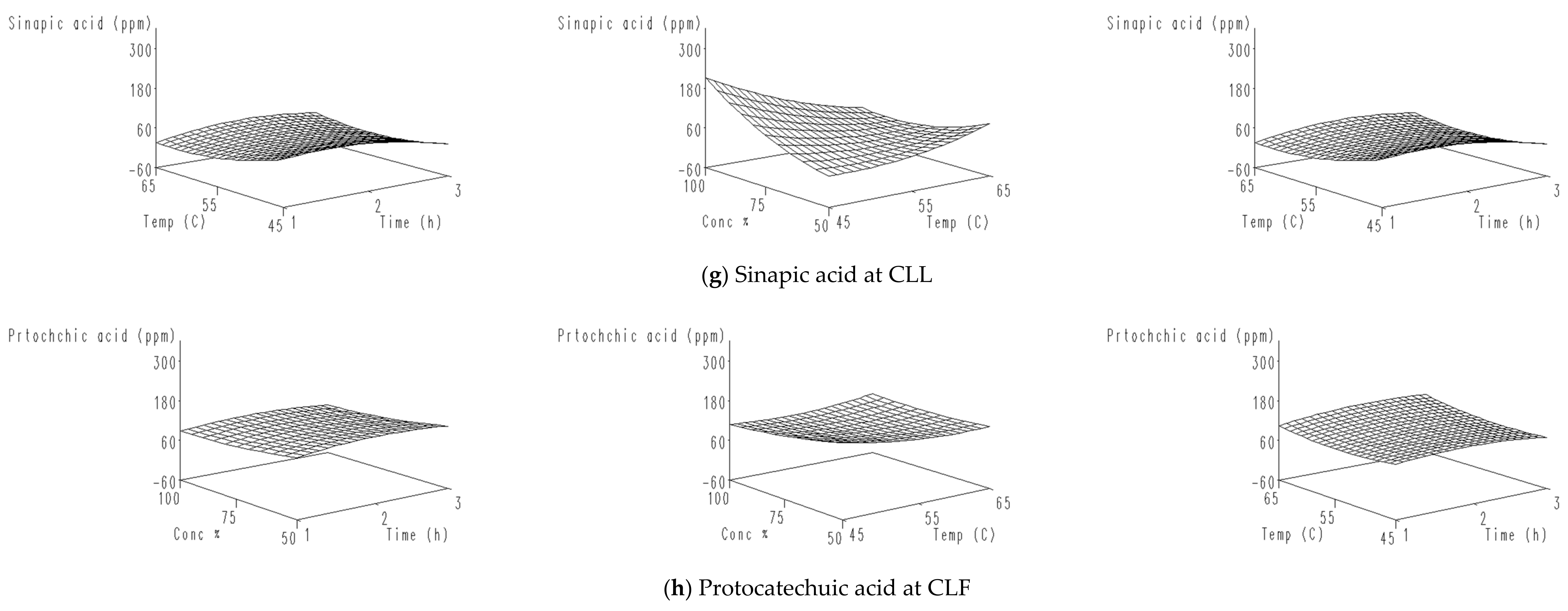
| t-Ferulic acid | 46.04 | 71.05 | 4.05 | CLL |
| Sinapic acid | 71.50 | 59.23 | 1.95 | |
| Protocatechuic acid | 101.37 | 51.40 | 2.05 | CLF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afifi, H.S.; Al Marzooqi, H.M.; Tabbaa, M.J.; Arran, A.A. Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants. Molecules 2021, 26, 1069. https://doi.org/10.3390/molecules26041069
Afifi HS, Al Marzooqi HM, Tabbaa MJ, Arran AA. Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants. Molecules. 2021; 26(4):1069. https://doi.org/10.3390/molecules26041069
Chicago/Turabian StyleAfifi, Hanan S., Hassan M. Al Marzooqi, Mohammad J. Tabbaa, and Ahmed A. Arran. 2021. "Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants" Molecules 26, no. 4: 1069. https://doi.org/10.3390/molecules26041069
APA StyleAfifi, H. S., Al Marzooqi, H. M., Tabbaa, M. J., & Arran, A. A. (2021). Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants. Molecules, 26(4), 1069. https://doi.org/10.3390/molecules26041069





