Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Olea europea L.: Overview on Its Chemical Compounds
3. Olea europea L. By-Products for Human Health
3.1. Secondary Metabolites in Olive Mill Wastewater
3.2. Biological Activity of Olive Mill Wastewater Extracts
3.3. Secondary Metabolites and Biological Activity of Olive Pomace Extracts
3.4. Secondary Metabolites in Olive Leaves
3.5. Biological Activity of Olive Leaf Extracts
3.6. Cosmetic Formulations of Olive Leaf Extracts
3.7. Secondary Metabolites and Biological Activity of Kernel and Seed Extracts
4. Olea europea L. By-Products for Zootechnical Feeding
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.L.; Sumpio, E.B.J. Olive Oil, the Mediterranean Diet, and Cardiovascular Health. Am. Coll. Surg. 2008, 207, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. History and characteristics of the olive tree. In Olive Oil Chemistry and Technology; Boskou, D., Ed.; AOCS Press: Champaign IL, USA, 1996. [Google Scholar]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.C.A. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Tech. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Hanifi, S.; El Hadrami, I. Olive mill wastewaters: Diversity of the fatal product in olive oil industry and its valorisation as agronomical amendment of poor soils: A review. J. Agron. 2009, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jarboui, R.; Sellami, F.; Kharroubi, A.; Gharsallah, N.; Ammar, E. Olive mill wastewater stabilization in open-air ponds: Impact on clay-sandy soil. Bioresour. Technol. 2008, 99, 7699–7708. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Biowastes-to-biofuels. Energy Convers. Manag. 2011, 52, 1815–1828. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Robert, K.W.; Parris, T.M.; Leiserowitz, A.A. What is sustainable development? Goals, indicators, values, and practice. Environ. Sci. Policy 2005, 47, 8–21. [Google Scholar] [CrossRef]
- De Jesus, A.; Antunes, P.; Santos, R.; Mendonça, S. Eco-innovation in the transition to a circular economy: An analytical literature review. J. Cleaner Prod. 2018, 172, 2999–3018. [Google Scholar] [CrossRef]
- Boskou, D. Olive fruit, table olives, and olive oil bioactive constituents. In Olive and olive oil bioactive constituents, 1st ed.; Boskou, D., Ed.; AOCS Press: Champaign IL, USA, 2015; pp. 1–30. [Google Scholar]
- Vermerris, W.; Nicholson, R. Phenolic Compounds Biochemistry, 1st ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–276. [Google Scholar]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, G.; Faccia, M.; Previtali, M.A.; Pati, S.; La Notte, E.; Baiano, A. Effects of olive maturation and stoning on quality indices and antioxidant concentration of extra virgin oils (cv. Coratina) during storage. J. Food Sci. 2010, 75, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Esti, M.; Notte, E. Evaluation of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive mill waste extracts: Polyphenols content, antioxidant, and antimicrobial activities. Adv. Pharmacol. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Uccella, N. Biophenolic components of olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Fanizzi, F.P.; Maffia, M. Composition and statistical analysis of biophenols in Apulian Italian EVOOs. Foods 2017, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitjavila, M.T.; Moreno, J.J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem. Pharmacol. 2012, 84, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Dangles, O.; Rakotomanomana, N.; Baracchinia, S.; Visioli, F. 3-O-Hydroxytyrosol glucuronide and 4-O-hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Varela-López, A.Y.; Forbes-Hernández, T.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [Green Version]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas Aceites 2016, 67, 121–129. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Galleggiante, V.; Scandiffio, L.; Liso, M.; Sommella, E.; Sobolewski, A.; Spilotro, V.; Pinto, A.; Campiglia, P.; Serino, G.; et al. Secretory leukoprotease inhibitor (Slpi) expression is required for educating murine dendritic cells inflammatory response following quercetin exposure. Nutrients 2017, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, C.I.R.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantina, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A.J. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Muller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559–110569. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Martínez-Hovelman, N.; Martínez-Huélamo, M.; Lamuela-Raventos, R.M.; Moreno, J.J. Extra virgin olive oil minor compounds modulate mitogenic action of oleic acid on colon cancer cell line. J. Agric. Food Chem. 2019, 67, 11420–11427. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Sacanella, I.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive compounds of mediterranean cooked tomato sauce (Sofrito) modulate intestinal epithelial cancer cell growth through oxidative stress/arachidonic acid cascade regulation. ACS omega 2020, 5, 17071–17077. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2017, 24, 2829–2841. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248, 185–193. [Google Scholar] [CrossRef]
- Kim, K.; Jung, J.Y.; Kwon, J.H.; Yang, J.W. Dynamic microfiltration with a perforated disk for effective harvesting of microalgae. J. Membr. Sci. 2015, 475, 252–258. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra-and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Cardinali, A.; Cicco, N.; Linsalata, V.; Minervini, F.; Pati, S.; Pieralice, M.; Tursi, N.; Lattanzio, V. Biological activity of high molecular weight phenolics from olive mill wastewater. J. Agric. Food Chem. 2010, 58, 8585–8590. [Google Scholar] [CrossRef]
- Gurr, M.I.; Harwood, J.L.; Frayn, K.N. Lipid Biochemistry, 5th ed.; Blackwells Science: Oxford, UK, 2002. [Google Scholar]
- Nawar, W.W. Chemical changes in lipids produced by thermal processing. J. Chem. 1984, 61, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.) a review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Nadour, M.; Laroche, C.; Pierre, G.; Delattre, C.; Moulti-Mati, F.; Michaud, P. Structural characterization and biological activities of polysaccharides from olive mill wastewater. Appl. Biochem. Biotechnol. 2015, 177, 431–445. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Gardarin, C.; Traikia, M.; Elboutachfaiti, R.; Isogai, A.; Michaud, P. Antioxidant activities of a polyglucuronic acid sodium salt obtained from TEMPO-mediated oxidation of xanthan. Carbohydr. Polym. 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Wu, G.H.; Hu, T.; Li, Z.Y.; Huang, Z.L.; Jiang, J.G. In vitro antioxidant activities of the polysaccharides from Pleurotus tuber-regium (Fr.) Sing. Food Chem. 2014, 148, 351–356. [Google Scholar] [CrossRef]
- Xu, R.; Ye, H.; Sun, Y.; Tu, Y.; Zeng, X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem. Toxicol. 2012, 50, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia Sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; Chen, W.Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective effects induced by two polyphenolic liquid complexes from olive (Olea europaea, mainly Cultivar Coratina) pressing juice in rat isolated tissues challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassani, B.; Rossi, T.; Stefano, D.D.; Pizzichini, D.; Corradino, P.; Macrì, N.; Noonan, D.M.; Albini, A.; Bruno, A.J. Potential chemopreventive activities of a polyphenol rich purified extract from olive mill wastewater on colon cancer cells. Funct. Foods 2016, 27, 236–248. [Google Scholar] [CrossRef]
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Saretto, E.; Vicari, D.; Vacante, M. Olive oil effects on colorectal cancer. Nutrients 2019, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Rossi, T.; Bassani, B.; Gallo, C.; Maramotti, S.; Noonan, D.M.; Bruno, A. Effect of a purified extract of olive mill waste water on endothelial cell proliferation, apoptosis, migration and capillary-like structure in vitro and in vivo. J. Bioanal. Biomed. 2015, 12, 1–8. [Google Scholar]
- Ramos, P.; Santos, S.A.O.; Guerraa, Â.R.; Guerreiro, O.; Felício, L.; Eliana Jerónimo, E.; Silvestrec, A.J.D.; Netoc, C.P.; Duarte, M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-De-La-Lastra, C. An up-date of olive oil phenols in inflammation and cancer: Molecular mechanisms and clinical implications. Curr. Med. Chem. 2013, 20, 4758–4776. [Google Scholar] [CrossRef] [PubMed]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Aβ (1-42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr. Alzheimer Res. 2011, 8, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ 42 by reducing plaque load and motor deficit. PLoS ONE 2013, 8, 58893–58902. [Google Scholar] [CrossRef]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aβ plaque pathology. PLoS ONE 2013, 8, 71702–71715. [Google Scholar] [CrossRef]
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Hass, K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef] [Green Version]
- Moretti, P.; Massari, C.D. Fitocomplesso Polifenolico Standardizzato per la Prevenzione delle Patologie Correlate All’esposizione da Sostanze Reattive Dell’ossigeno e Relativo Metodo di Produzione. BIOENUTRA Srl, N. 102017000118607. Available online: https://www.farmaffari.it/index.php/it/companies-partner/1-2-5-integratori-e-dietetici/bioenutra-srl-1-1 (accessed on 16 January 2020).
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Roselló-Catafau, J.; Pintó, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.T.; Matias, A.A.; Nunes, A.V.M.; Leitao, M.C.; Brito, D.; Bronze, R.; Duarte, C.M. In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. Innov. Food Sci. Emerg. Technol. 2008, 9, 311–319. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Yucetepe, A.; Kasapo, K.N.; Celik, B. High-value compounds from olive oil processing waste. In Edible Oils Extraction, Processing, and Applications, 1st ed.; Chemat, S., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 179–203. [Google Scholar]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Effect of processing conditions, prestorage treatment, and storage conditions on the phenol content and antioxidant activity of olive mill waste. J. Agric. Food Chem. 2008, 56, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Troise, A.D.; Fiore, A.; Colantuono, A.; Kokkinidou, S.; Peterson, D.G.; Fogliano, V. Effect of olive mill wastewater phenol compounds on reactive carbonyl species and maillard reaction end-products in ultrahigh-temperature-treated milk. J. Agric. Food Chem. 2014, 62, 10092–10100. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- Giordano, E.; Dávalos, A.; Visioli, F. Chronic hydroxytyrosol feeding modulates glutation-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef]
- Giordano, E.; Dávalos, A.; Nicod, N.; Visioli, F. Hydroxytyrosol attenuates tunicamycin-induced endoplasmic reticulum stress in human hepatocarcinoma cells. Mol. Nutr. Food Res. 2014, 58, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int. J. Cosmet. Sci. 2008, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Hopia, A.; Huynh-Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.H.; et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Antioxidant activity, lipophilicity and extractability of polyphenols from pig skin–development of analytical methods for skin permeation studies. Biomed. Chromatogr. 2013, 27, 1444–1451. [Google Scholar] [CrossRef]
- Lee, K.K.; Cho, J.J.; Park, E.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Caturla, N.; Castillo, J.; Benavente-Garcia, O.; Alcaraz, M.; Mico, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. 2014, 136, 12–18. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, E.; Kim, S.; Lee, K.E.; Youm, J.K.; Park, D. Antagonist effects of veratric acid against UVB-induced cell damages. Molecules 2013, 18, 5405–5419. [Google Scholar] [CrossRef] [Green Version]
- Potapovich, A.I.; Kostyuk, V.A.; Kostyuk, T.V.; de Luca, C.; Korkina, L.G. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013, 62, 773–780. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid-and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated digestion of olive pomace water-soluble ingredient: Relationship between the compounds bioaccessibility and their potential health benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an extract rich in phenolic compounds from olive pomace by pressurized liquid extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innovative Food Sci. Emerging Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Vergani, L.; Vecchione, G.; Baldini, F.; Voci, A.; Ferrari, P.F.; Aliakbarian, B.; Casazza, A.A.; Perego, P. Antioxidant and hepatoprotective potentials of phenolic compounds from olive pomace. Chem. Eng. Trans. 2016, 49, 475–480. [Google Scholar]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Carreres, J.E.; Micol, V.; García-Pérez, J.V. Influence of olive leaf processing on the bioaccessibility of bioactive polyphenols. J. Agric. Food Chem. 2014, 62, 6190–6198. [Google Scholar] [CrossRef]
- Ghasemi, S.; Koohi, D.E.; Emmamzadehhashemi, M.S.B.; Khamas, S.S.; Moazen, M.; Hashemi, A.K.; Amin, G.; Golfakhrabadi, F.; Yousefi, Z.; Yousefbeyk, F. Investigation of phenolic compounds and antioxidant activity of leaves extracts from seventeen cultivars of Iranian olive (Olea europaea L.). J. Food Sci. Technol. 2018, 55, 4600–4607. [Google Scholar] [CrossRef]
- Guinda, Á.; Pérez Camino, M.C.; Lanzón, A. Supplementation of olis with oleanolic acid from the olive leaf (Olea europaea). Eur. J. Lipid Sci. Technol. 2004, 106, 22–26. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440. [Google Scholar] [CrossRef]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2016, 243, 429–435. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Micucci, M.; Malaguti, M.; Gallina Toschi, T.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and vascular synergic protective effect of Olea europea L. leaves and Hibiscus sabdariffa L. flower extracts. Oxid. Med. Cell. Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micucci, M.; Angeletti, A.; Cont, M.; Corazza, I.; Aldini, R.; Donadio, E.; Chiarini, A.; Budriesi, R. Hibiscus Sabdariffa L. flowers and Olea Europea L. leaves extract-based formulation for hypertension care: In vitro efficacy and toxicological profile. J. Med. Food 2016, 19, 504–512. [Google Scholar] [CrossRef]
- Lockyer, S.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Olive leaf phenolics and cardiovascular risk reduction: Physiological effects and mechanisms of action. Nutr. Aging 2012, 1, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; Yoshimura, H.; Zhong, L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Komaki, E.; Yamaguchi, S.; Maru, I.; Kinoshita, M.; Kakehi, K.; Ohta, Y.; Tsukada, Y. Identification of anti-α-amylase components from olive leaf extracts. Food Sci. Technol. Res. 2003, 9, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Mok, M.; Christensen, A.M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive biophenols reduces alzheimer’s pathology in SH-SY5Y cells and APPswe mice. Int. J. Mol. Sci. 2019, 20, 125. [Google Scholar] [CrossRef] [Green Version]
- Chiaino, E.; Micucci, M.; Cosconati, S.; Novellino, E.; Budriesi, R.; Chiarini, A.; Frosoni, M. Olive leaves and hibicus flowers extracts-based preparation protect brain from oxidative stress-induced injury. Antioxidants 2020, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Moore, R.; Braakhuis, A. The effect of olive leaf extract on upper respiratory illness in high school athletes: A randomised control trial. Nutrients 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β-induced expression of matrix metalloproteinase-1 and-13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef]
- Murakami, A.; Song, M.; Katsumata, S.I.; Uehara, M.; Suzuki, K.; Ohigashi, H. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: Possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 2007, 30, 179–192. [Google Scholar] [CrossRef]
- Perugini, P.; Vettor, M.; Rona, C.; Troisi, L.; Villanova, L.; Genta, I.; Conti, B.; Pavanetto, F. Efficacy of oleuropein against UVB irradiation: Preliminary evaluation. Int. J. Cosmet. Sci. 2008, 30, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R. Ultraviolet radiation and skin cancer: Molecular mechanisms. J. Cutan. Pathol. 2005, 32, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J. The role of UVA rays in skin aging. Eur. J. Dermatol. 2001, 11, 170–171. [Google Scholar] [PubMed]
- Restuccia, D.; Clodoveo, M.L.; Corbo, F.; Loizzo, M.R. De-stoning technology for improving olive oil nutritional and sensory features: The right idea at the wrong time. Food Res. Int. 2018, 106, 636–646. [Google Scholar] [CrossRef]
- Amirante, R.; Clodoveo, M.L.; Distaso, E.; Ruggiero, F.; Tamburrano, P. A tri-generation plant fuelled with olive tree pruning residues in Apulia: An energetic and economic analysis. Renew. Energy 2016, 89, 411–421. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.N.; Alsaad, A.; Rababeh, T. Optimisation and characterisation of various extraction conditions of phenolic compounds and antioxidant activity in olive seeds. Nat. Prod. Res. 2011, 25, 876–889. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Felizón, B.; Brenes, M.; Guillén, R.; Heredia, A. Hydroxytyrosol and tyrosol as the main compounds found in the phenolic fraction of steam-exploded olive stones. J. Am. Oil Chem. Soc. 1998, 75, 1643–1649. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolanos, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- González-Hidalgo, I.; Bañón, S.; Ros, J.M. Evaluation of table olive by-product as a source of natural antioxidants. Int. J. Food Sci. Technol. 2012, 47, 674–681. [Google Scholar] [CrossRef]
- Bolek, S. Olive stone powder: A potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innovative Food Sci. Emerging Technol. 2020, 64, 102423–102429. [Google Scholar] [CrossRef]
- Khadem, S.; Rashidi, L.; Homapour, M. Antioxidant capacity, phenolic composition and physicochemical characteristics of whole olive stone oil extracted from different olive varieties grown in Iran. Eur. J. Lipid Sci. Technol. 2019, 121, 1800365–1800373. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Romero-García, J.M.; Cara, C.; Moya, M.; Castro, E. Low energy-demanding recovery of antioxidants and sugars from olive stones as preliminary steps in the biorefinery context. Ind. Crops Prod. 2014, 60, 30–38. [Google Scholar] [CrossRef]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuels, Bioprod. Biorefin. 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Restuccia, D.; Chiricosta, S.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Picci, N. Olive stones as a source of antioxidants for food industry. J. Food Nutr. Res. 2011, 50, 57–67. [Google Scholar]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and analysis of alternatives for the valorisation of agro-industrial olive oil by-products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel feed including olive oil mill wastewater bioactive compounds enhanced the redox status of lambs. In Vivo 2018, 32, 291–302. [Google Scholar] [PubMed]
- Chiofalo, V.; Liotta, L.; Lo Presti, V.; Gresta, F.; Di Rosa, A.R.; Chiofalo, B. Effect of dietary olive cake supplementation on performance, carcass characteristics, and meat quality of beef cattle. Animals 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Salami, S.A.; Majoka, M.A.; Saha, S.; Garber, A.; Gabarrou, J.F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: Science and market. Avian Biology Research 2015, 8, 65–78. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Barbosa, S.; Pinheiro, V.; Mourão, J.L.; Outor-Monteiro, D. The effect of olive leaves supplementation on the feed digestibility, growth performances of pigs and quality of pork meat. Meat Sci. 2009, 82, 438–443. [Google Scholar] [CrossRef]
- Arco-Pérez, A.; Ramos-Morales, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Martín-García, A.I. Nutritive evaluation and milk quality of including of tomato or olive by-products silages with sunflower oil in the diet of dairy goats. Anim. Feed Sci. Technol. 2017, 232, 57–70. [Google Scholar]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Terzopoulou, Z.; Komini, O.; Kafantaris, I.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Anisimov, N.; Tsatsakis, A.; Kouretas, D. Tissue specific effects of feeds supplemented with grape pomace or olive oil mill wastewater on detoxification enzymes in sheep. Toxicol. Rep. 2017, 4, 364–372. [Google Scholar] [CrossRef]
- Al-Musawi, J.E.; Al-Khalisy, A.F. Effect of grinded olive leave ssuplimentation in milk production and its components and some blood traits in native does. Iraqi J. Agric. Sci. 2016, 47, 1354–1359. [Google Scholar]
- Zangeneh, S.; Torki, M. Effects of b-mannanase supplementing of olive pulp included diet on performance of laying hens, egg quality characteristics, humoral and cellular immune response and blood parameters. Global Vet. 2011, 7, 391–398. [Google Scholar]
- Cayan, H.; Erener, G. Effect of olive leaf (Olea europaea) powder on laying hens performance, egg quality and egg yolk cholesterol levels. Asian-Australas. J. Anim. Sci. 2015, 28, 538–543. [Google Scholar] [CrossRef] [Green Version]



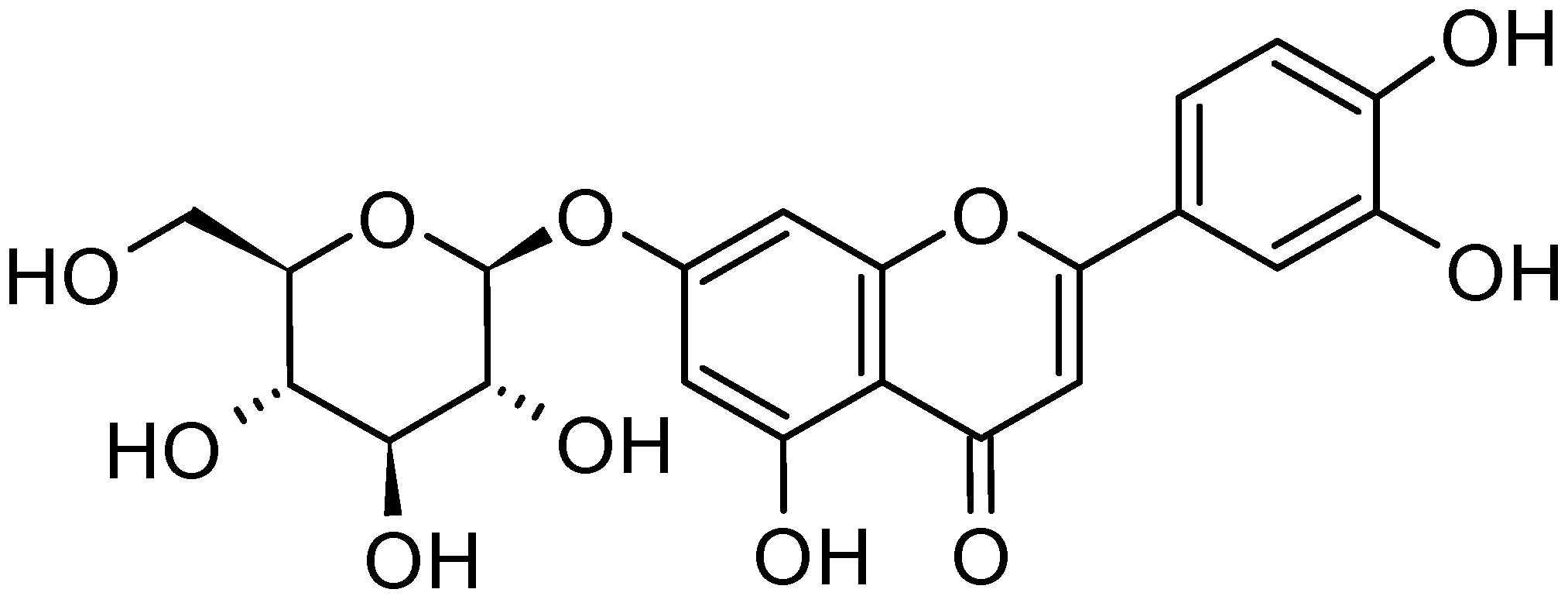
| Seed Oil | Virgin Olive Oil | Skin | Pulp | Wood | Leaves |
|---|---|---|---|---|---|
| Phenolic acid/aldehydes | Phenolic acid/aldehydes | Phenolic acid/aldehydes | Phenolic acid/aldehydes | Phenolic acid/aldehydes | Phenolic acid/aldehydes |
| Tocopherols | Tocopherols | Tocopherols | |||
| Sterols | Sterols | Organic acid and coumarins | Organic acid and coumarins | Organic acid and coumarins | Organic acid and coumarins |
| Flavonoids | Simple phenols and derivatives | Simple phenols and derivatives | Simple phenols and derivatives | ||
| Lignans | Secoiridoids and derivatives | Secoiridoids and derivatives | Secoiridoids and derivatives | ||
| Fatty acids and derivatives | Flavonoids | Flavonoids | |||
| Pentacyclic triterpenes | Tocopherols |
| Composition | OMW a | UF b | AIR c | WSF c | WIF c | UF HSF | UF ETNA O1PP | NF 90 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F a | P a | R a | F a | P a | R a | F a | R a | ||||||
| Total phenols | 1409.0 | 1692.0 | 3.2 | 2.8 | 1.9 | 81.3 | 79.5 | 81.3 | 75.5 | 62.2 | 77.4 | 65.6 | 86.2 |
| Hydroxytyrosol | 3.8 | n.d. | - | - | - | 3.8 | 3.7 | 3.9 | 3.5 | 3.0 | 3.8 | 3.2 | 4.0 |
| Protocatechuic acid | 25.0 | - | - | - | - | 25.0 | 24.0 | 24.5 | 27.0 | 20.6 | 26.0 | 22.0 | 30.0 |
| Catechol | 7.5 | - | - | - | - | 7.5 | 7.1 | 7.2 | 6.0 | 5.0 | 6.2 | 5.5 | 7.5 |
| Tyrosol | 39.0 | n.d. | - | - | - | 39.0 | 38.7 | 39.6 | 34.2 | 30.0 | 36.0 | 31.0 | 40.0 |
| Caffeic acid | 5.0 | - | - | - | - | 5.0 | 4.9 | 5.2 | 4.0 | 3.0 | 4.4 | 3.2 | 3.7 |
| p-Cumaric acid | 1.0 | - | - | - | - | 1.0 | 0.9 | 0.9 | 0.8 | 0.6 | 1.0 | 0.7 | 1.0 |
| Verbascoside | - | n.d. | - | - | - | - | - | - | - | - | - | - | - |
| Isoverbascoside | - | n.d. | - | - | - | - | - | - | - | - | - | - | - |
| Carbohydrates | - | - | 25.0 d | 60.0 d | 5.1 d | - | - | - | - | - | - | - | - |
| Fucose | - | - | 0.5 | 0.6 | 0.4 | - | - | - | - | - | - | - | - |
| Rhamnose | - | - | 14.3 | 13.7 | 16.4 | - | - | - | - | - | - | - | - |
| Arabinose | - | - | 14.1 | 10.7 | 17.6 | - | - | - | - | - | - | - | - |
| Galactose | - | - | 12.6 | 13.1 | 5.9 | - | - | - | - | - | - | - | - |
| Glucose | - | - | 42.2 | 45.1 | 47.7 | - | - | - | - | - | - | - | - |
| Mannose | - | - | 5.5 | 5.4 | 4.4 | - | - | - | - | - | - | - | - |
| Xylose | - | - | 5.0 | 4.9 | 5.3 | - | - | - | - | - | - | - | - |
| Galacturonic acid | - | - | 4.9 | 5.0 | 1.4 | - | - | - | - | - | - | - | - |
| Glucuronic acid | - | - | 1.0 | 1.1 | 0.7 | - | - | - | - | - | - | - | - |
| Proteins | - | - | 3.2 | 11.0 | 0.3 | - | - | - | - | - | - | - | - |
| Cultivars | Total Phenol 1 | FRAP 2 | DPPH 3 |
|---|---|---|---|
| Manzanilla | 134.50 ± 0.01 | 1107.71 ± 0.01 | 33.93 |
| Conservolea | 92.35 ± 0.01 | 1277.33 ± 0.01 | 62.94 |
| Arbequina | 42.35 ± 0.02 | 1760.57 ± 0.01 | 62.56 |
| Mishen | 71.93 ± 0.01 | 1971.37 ± 0.01 | 63.48 |
| Coratina | 155.91 ± 0.06 | 358.66 ± 0.01 | 22.95 |
| Roghani | 121.75 ± 0.02 | 1400.76 ± 0.01 | 29.58 |
| Kalamon | 190.65 ± 0.03 | 532.76 ± 0.01 | 26.74 |
| Amphissis | 50.70 ± 0.01 | 1110.38 ± 0.01 | 95.39 |
| Yellow | 73.85 ± 0.01 | 1400.95 ± 0.01 | 53.80 |
| Amigdalolia | 42.73 ± 0.01 | 1341.05 ± 0.01 | 74.30 |
| Mary | 62.24 ± 0.01 | 1203.81 ± 0.01 | 60.26 |
| Leccino | 59.23 ± 0.01 | 568.28 ± 0.01 | 69.30 |
| Shenge | 61.97 ± 0.01 | 614.19 ± 0.01 | 60.18 |
| Gordal | 184.72 ± 0.01 | 450.86 ± 0.01 | 20.66 |
| Sevillenca | 83.63 ± 0.01 | 432.19 ± 0.01 | 34.92 |
| Fishomi | 109.98 ± 0.06 | 1794.57 ± 0.01 | 32.82 |
| Disease | Type of Experiment | Dose | Effects |
|---|---|---|---|
| Hypertension [100] | Human clinical trial | 1000 mg OLE/die | Lowering systolic and diastolic blood pressures, significant reduction of triglyceride (TG) levels. |
| Atherosclerosis [107] | in vivo | 100 mg OLE/kg body weight | Reduction of the levels of cholesterol, TGs, and LDL cholesterol, and block of the inflammatory response. |
| Thrombosis [109] | in vitro | 1% v/v OLE | Significant dose-dependent reduction in platelet activity. |
| Hypocholesterolemia [111] | Human studies | 1.2 g OLE/die | Reduction of total cholesterol, decreased LDL cholesterol. |
| Diabetes [106,108] | in vitro | IC50 = 4.0–0.02 mg/mL OLE | Inhibition of the activities of α-amylases from human saliva and pancreas. |
| Human clinical trial | 500 mg OLE/die | Significant reduction in HbA1C values. | |
| Alzheimer [110] | in vivo | 50 mg OLE/kg | Reduction of amyloid plaque deposition in cortex and hippocampus. |
| Upper respiratory illness [112] | Randomized controlled trials | 100 mg oleuropein/die | Reduction of the sick days, i.e., acceleration of the recovery. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. https://doi.org/10.3390/molecules26041072
Mallamaci R, Budriesi R, Clodoveo ML, Biotti G, Micucci M, Ragusa A, Curci F, Muraglia M, Corbo F, Franchini C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules. 2021; 26(4):1072. https://doi.org/10.3390/molecules26041072
Chicago/Turabian StyleMallamaci, Rosanna, Roberta Budriesi, Maria Lisa Clodoveo, Giulia Biotti, Matteo Micucci, Andrea Ragusa, Francesca Curci, Marilena Muraglia, Filomena Corbo, and Carlo Franchini. 2021. "Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation" Molecules 26, no. 4: 1072. https://doi.org/10.3390/molecules26041072
APA StyleMallamaci, R., Budriesi, R., Clodoveo, M. L., Biotti, G., Micucci, M., Ragusa, A., Curci, F., Muraglia, M., Corbo, F., & Franchini, C. (2021). Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules, 26(4), 1072. https://doi.org/10.3390/molecules26041072













