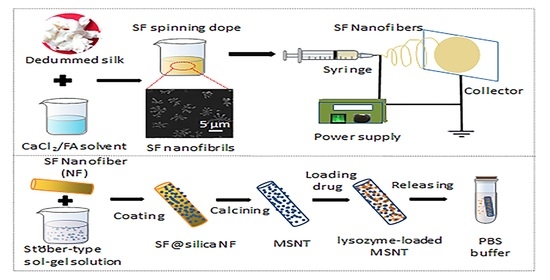
A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Viscosity of SF/CaCl2/FA Solutions
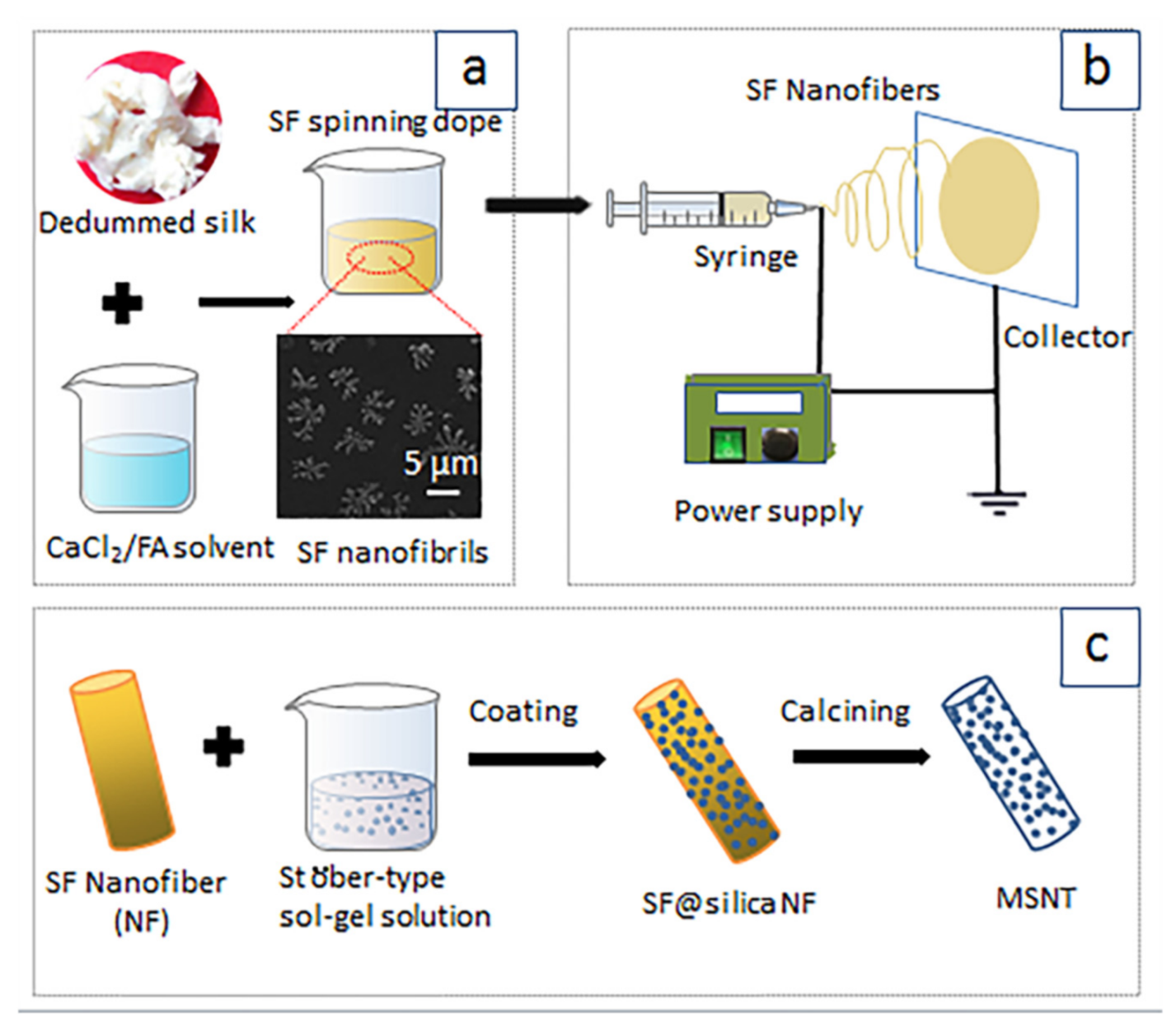
2.2. Morphology of Electrospun SF Nanofiber Templates
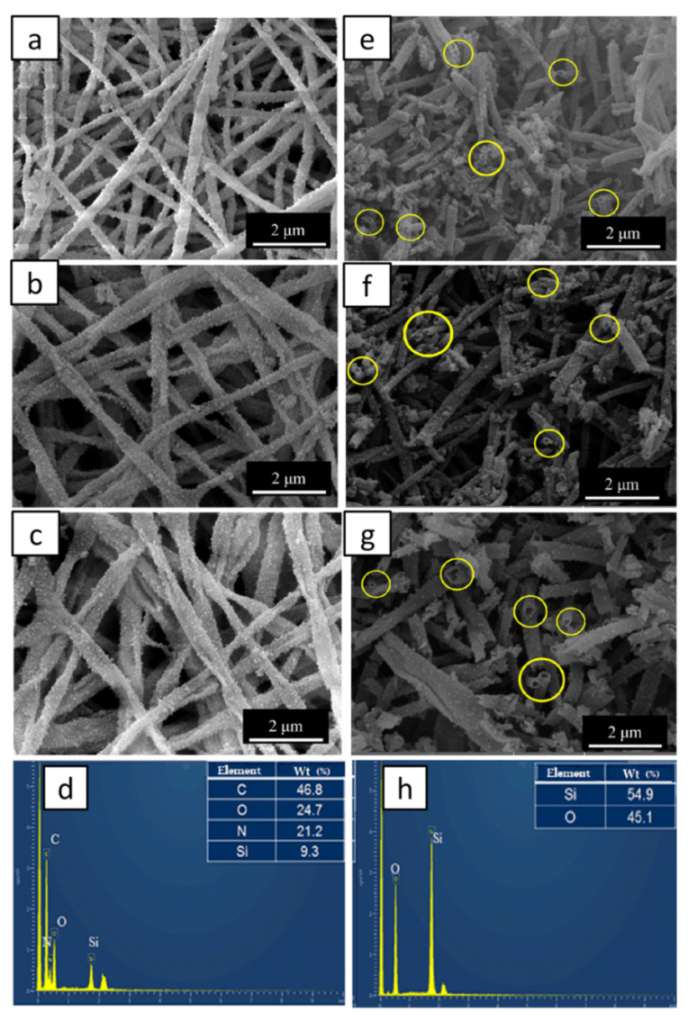
2.3. Morphologies and Compositions of SF@silica NFs and MSNTs
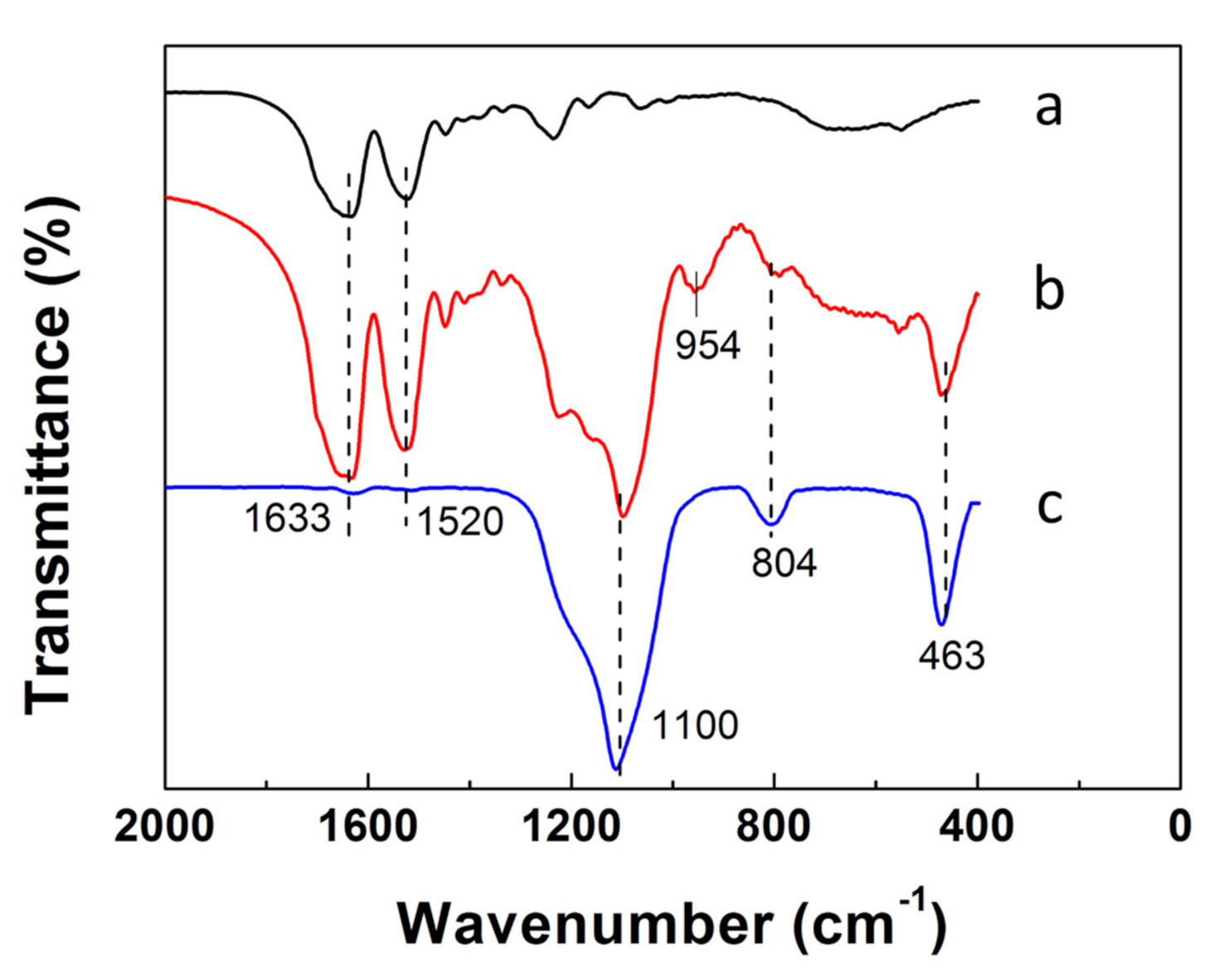
2.4. FTIR Spectra of SF Fiber Templates, SF@silica NFs and MSNTs
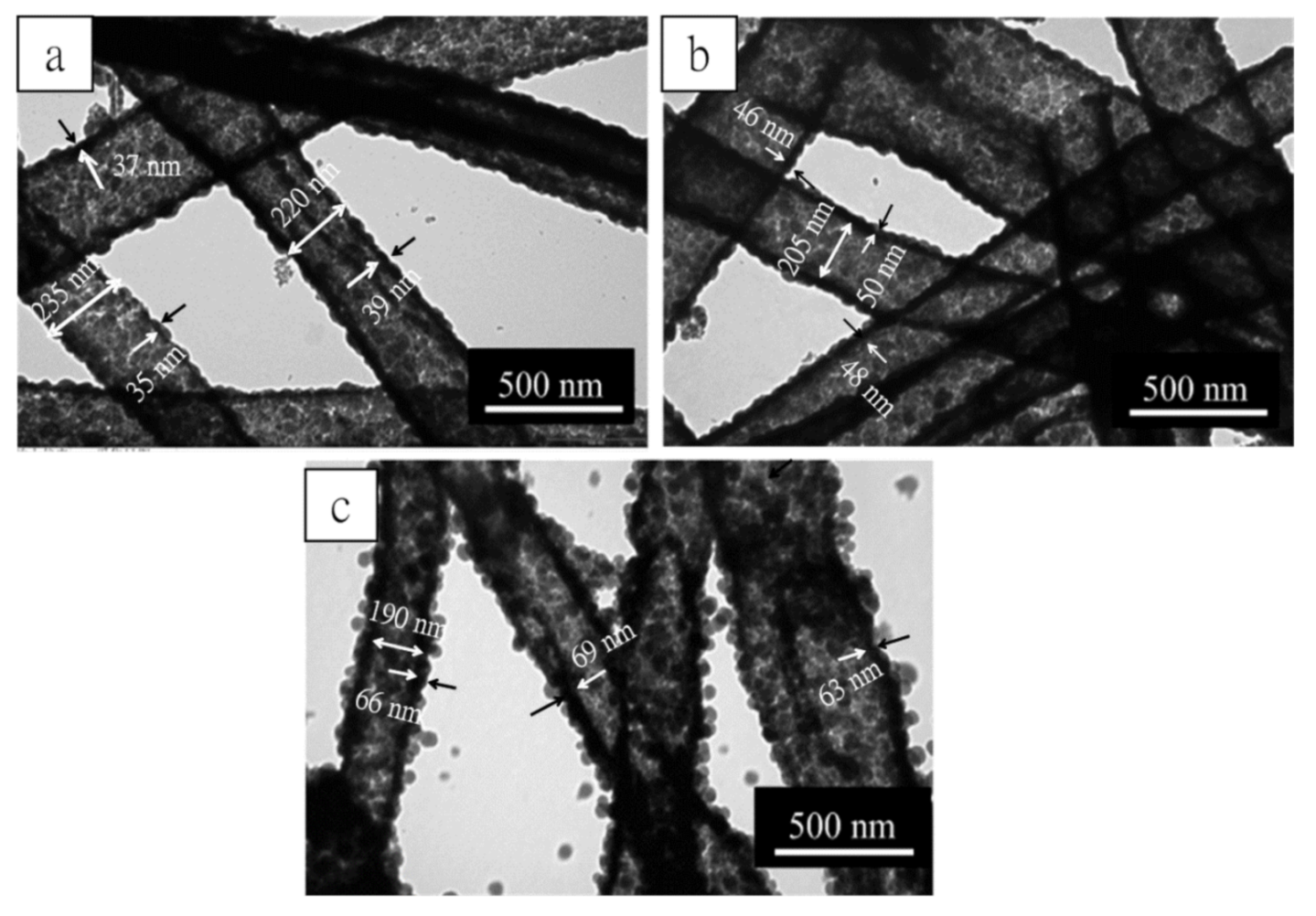
2.5. Effect of TEOS Dosages Changes on the Silica Layer Thickness of MSNTs
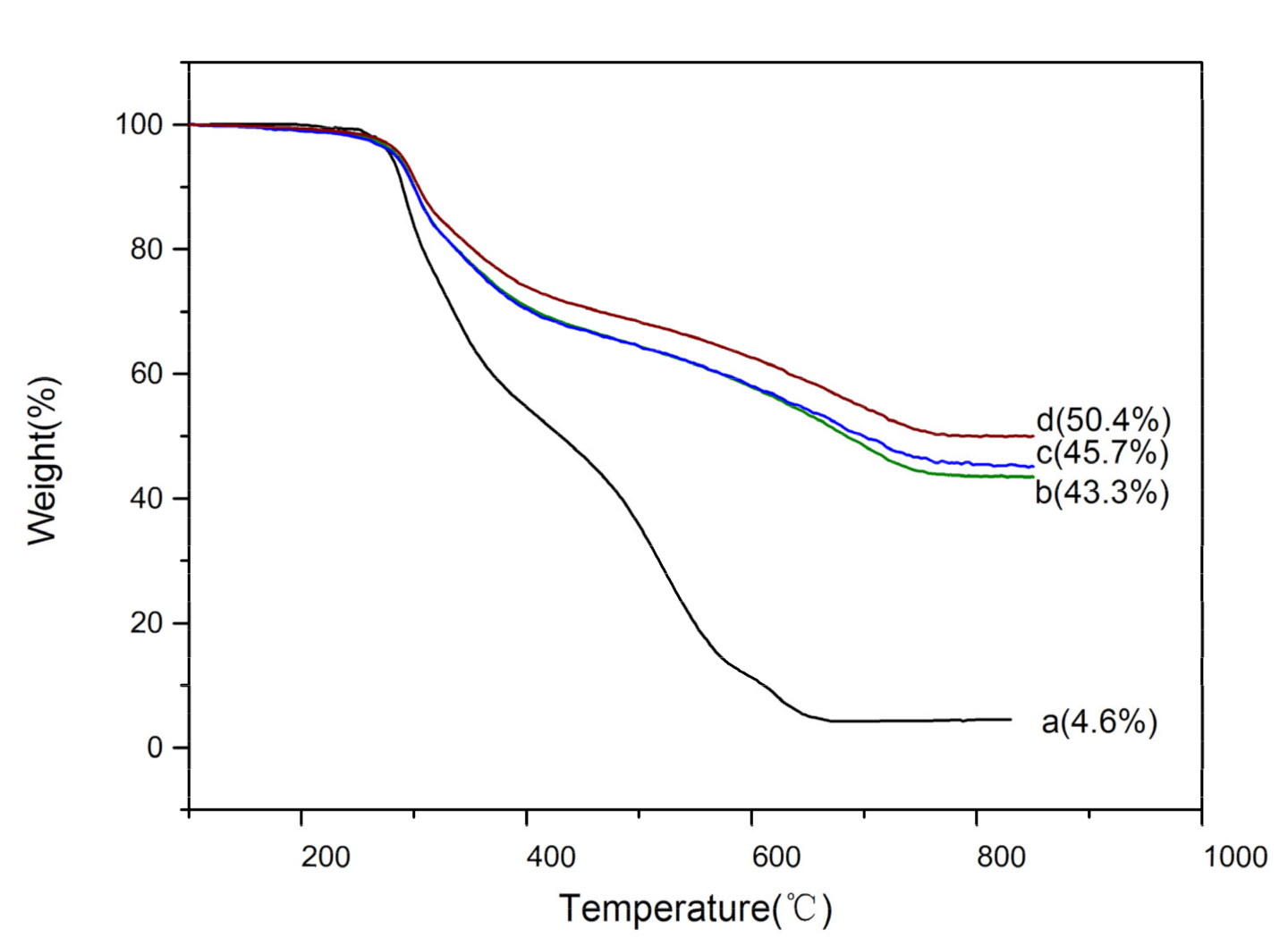
2.6. Thermogravimetric Analysis (TGA) of SF@silica NFs
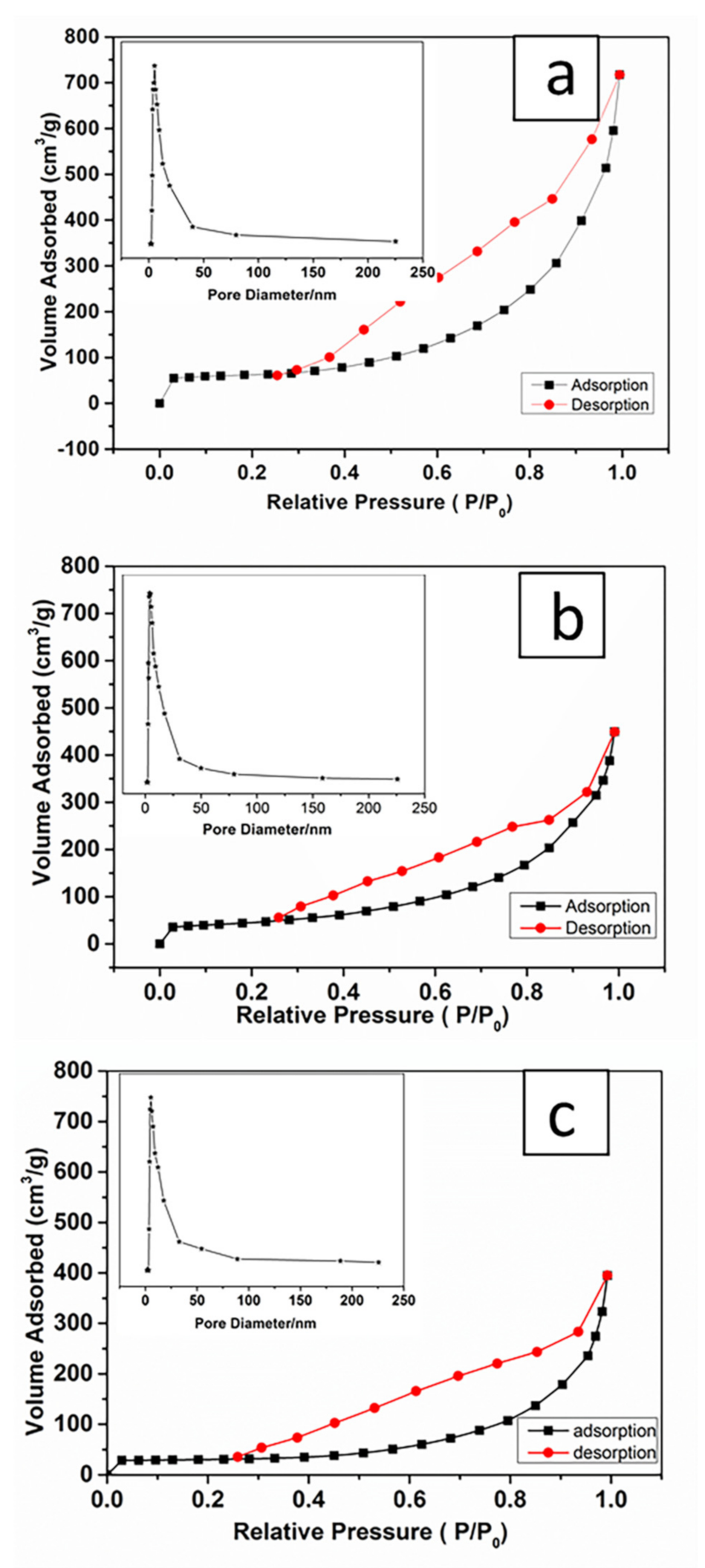
2.7. MSNTs Characterized by Brunauer–Emmett–Teller (BET)
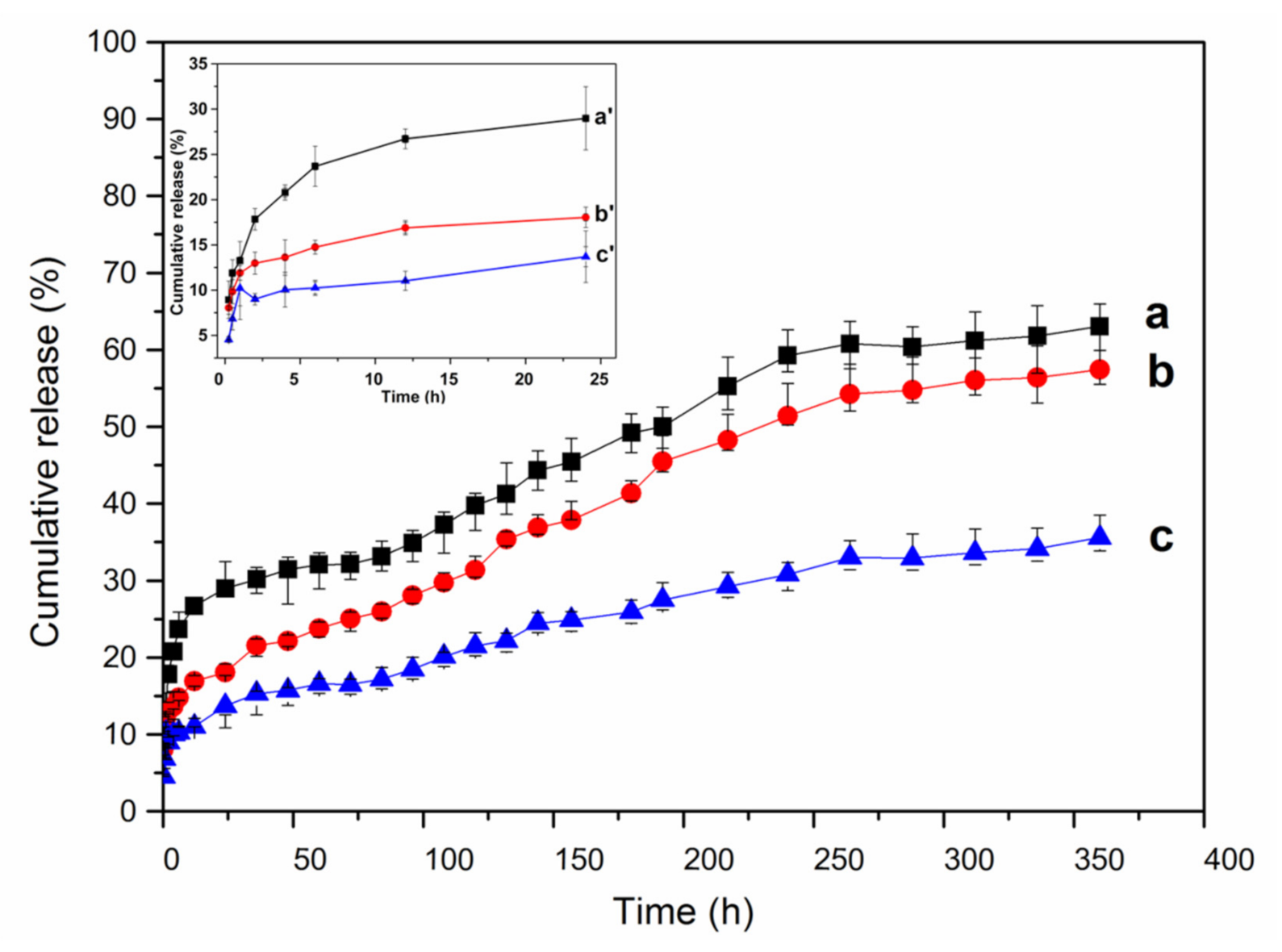
2.8. Drug-Loading and In Vitro Release Behavior of MSNTs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Spinning Dopes
3.3. Electrospinning for SF Nanofiber Templates
3.4. Fabrication of Mesoporous Silica Nanotubes (MSNTs)
3.5. Drug Loading and Release Studies
3.6. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Y.; Shi, C.; Wang, T.; Li, X.; Ma, J. Recent progress in hollow silica: Template synthesis, morphologies and applications. Microporous Mesoporous Mater. 2016, 227, 121–136. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, W.; Al-Deyab, S.S.; Zhang, K.-Q. Tuning porous silica nanofibers by colloid electrospinning for dye adsorption. Appl. Surf. Sci. 2014, 313, 389–395. [Google Scholar] [CrossRef]
- Peng, Y.; Leng, W.; Dong, B.; Ge, R.; Duan, H.; Gao, Y. Bottom-up preparation of gold nanoparticle-mesoporous silica composite nanotubes as a catalyst for the reduction of 4-nitrophenol. Chin. J. Catal. 2015, 36, 1117–1123. [Google Scholar] [CrossRef]
- Yang, X.; He, D.; He, X.; Wang, K.; Tang, J.; Zou, Z.; He, X.; Xiong, J.; Li, L.; Shangguan, J. Synthesis of Hollow Mesoporous Silica Nanorods with Controllable Aspect Ratios for Intracellular Triggered Drug Release in Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 20558–20569. [Google Scholar] [CrossRef]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-Step Synthesis of Amine-Functionalized Hollow Mesoporous Silica Nanoparticles as Efficient Antibacterial and Anticancer Materials. ACS Appl. Mater. Interfaces 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, N.; Pan, W.; Yu, Z.; Yang, L.; Tang, B. Hollow Mesoporous Silica Nanoparticles with Tunable Structures for Controlled Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Tan, L.; Wu, H.-X.; Liu, C.-J.; Zhuo, R.-X. Dual-stimuli-responsive polymer-coated mesoporous silica nanoparticles used for controlled drug delivery. J. Appl. Polym. Sci. 2015, 132, 42395. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Zhang, J.; Huang, L.; Qi, C.; Xu, L.; Liu, X.; Wang, G.; Wang, L.; Wang, Z. Safe and Effective Reversal of Cancer Multidrug Resistance Using Sericin-Coated Mesoporous Silica Nanoparticles for Lysosome-Targeting Delivery in Mice. Small 2017, 13, 1602567. [Google Scholar] [CrossRef]
- Geng, H.; Zhao, Y.; Liu, J.; Cui, Y.; Wang, Y.; Zhao, Q.; Wang, S. Hollow mesoporous silica as a high drug loading carrier for regulation insoluble drug release. Int. J. Pharm. 2016, 510, 184–194. [Google Scholar] [CrossRef]
- Chen, S.; Shi, X.; Chinnathambi, S.; Hanagata, N. Large-Scale Fabrication of Free-Standing, Micropatterned Silica Nanotubes Via a Hybrid Hydrogel-Templated Route. Adv. Health Mater. 2013, 2, 1091–1095. [Google Scholar] [CrossRef]
- Ma, X.; Feng, H.; Liang, C.; Liu, X.; Zeng, F.; Wang, Y. Mesoporous silica as micro/nano-carrier: From passive to active cargo delivery, a mini review. J. Mater. Sci. Technol. 2017, 33, 1067–1074. [Google Scholar] [CrossRef]
- Müllner, M.; Lunkenbein, T.; Breu, J.; Caruso, F.; Müller, A.H.E. Template-Directed Synthesis of Silica Nanowires and Nanotubes from Cylindrical Core–Shell Polymer Brushes. Chem. Mater. 2012, 24, 1802–1810. [Google Scholar] [CrossRef]
- Ma, J.; Lin, H.; Xing, R.; Li, X.; Bian, C.; Xiang, D.; Guo, W.; Qu, F. Synthesis of pH-responsive mesoporous silica nanotubes for controlled release. J. Sol-Gel Sci. Technol. 2013, 69, 364–369. [Google Scholar] [CrossRef]
- Song, J.; Fu, G.; Cheng, Q.; Jin, Y. Bimodal Mesoporous Silica Nanotubes Fabricated by Dual Templates of CTAB and Bare Nanocrystalline Cellulose. Ind. Eng. Chem. Res. 2013, 53, 708–714. [Google Scholar] [CrossRef]
- Wang, P.; Du, M.; Zhang, M.; Zhu, H.; Bao, S. The preparation of tubular heterostructures based on titanium dioxide and silica nanotubes and their photocatalytic activity. Dalton Trans. 2014, 43, 1846–1853. [Google Scholar] [CrossRef]
- Jia, C.; Song, J.; Jin, Y.; Rojas, O.J. Controlled-release drug carriers based hierarchical silica microtubes templated from cellulose acetate nanofibers. J. Appl. Polym. Sci. 2015, 132, 42562. [Google Scholar] [CrossRef]
- Guo, L.; Wang, T.; Jia, L.; Chen, S.; Huang, D.; Chen, W. Synthesis and drug delivery property of silica nanotubes prepared using gelatin nanofibers as novel sacrificed template. Mater. Lett. 2017, 209, 334–337. [Google Scholar] [CrossRef]
- Ha, S.W.; Park, Y.H.; Hudson, S.M.J.B. Dissolution of Bombyx mori silk fibroin in the calcium nitrate tetrahydrate-methanol system and aspects of wet spinning of fibroin solution. Biomacromolecules 2003, 4, 488–496. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, X.; Tao, S.; Xia, J.; Xu, S. Differences in regenerated silk fibroin prepared with different solvent systems: From structures to conformational changes. J. Appl. Polym. Sci. 2015, 132, 41959. [Google Scholar] [CrossRef]
- Reizabal, A.; Costa, C.M.; Saiz, P.G.; Gonzalez, B.; Pérez-Álvarez, L.; Fernández de Luis, R.; Garcia, A.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Processing Strategies to Obtain Highly Porous Silk Fibroin Structures with Tailored Microstructure and Molecular Characteristics and Their Applicability in Water Remediation. J. Hazard. Mater. 2020, 403, 123675. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, J.; Chen, S.; Jia, L.; Ma, Y. Fabrication of aqueous-based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin-loaded RSF nanospheres for drugs controlled release. RSC Adv. 2017, 7, 56550–56558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Lu, Q.; Ming, J.; Dou, H.; Liu, Z.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Silk dissolution and regeneration at the nanofibril scale. J. Mater. Chem. B 2014, 2, 3879–3885. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, F.; Wu, H.; Ming, J.; Fan, Z.; Zuo, B. A novel route to prepare dry-spun silk fibers from CaCl2–formic acid solution. Mater. Lett. 2014, 128, 175–178. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Ming, J.; Bie, S.; Li, J.; Zuo, B. Preparation of electrospun silk fibroin nanofibers from solutions containing native silk fibrils. J. Appl. Polym. Sci. 2015, 132, 41236. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Yue, X.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Regeneration of high-quality silk fibroin fiber by wet spinning from CaCl2–formic acid solvent. Acta Biomater. 2015, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; You, X.; Dou, H.; Liu, Z.; Zuo, B.; Zhang, X. Facile Fabrication of Robust Silk Nanofibril Films via Direct Dissolution of Silk in CaCl2–Formic Acid Solution. ACS Appl. Mater. Interfaces 2015, 7, 3352–3361. [Google Scholar] [CrossRef]
- Ling, S.; Zhang, Q.; Kaplan, D.L.; Omenetto, F.; Buehler, M.J.; Qin, Z. Printing of stretchable silk membranes for strain measurements. Lab. Chip 2016, 16, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Peng, Q.; Lu, L.; Fan, S.; Shao, H.; Zhang, H.; Wu, R.; Hsiao, B.S.; Zhang, Y. Single Molecular Layer of Silk Nanoribbon as Potential Basic Building Block of Silk Materials. ACS Nano 2018, 12, 11860–11870. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, L.; Lv, S.; Shao, H.; Fan, S.; Zhang, Y. Pulse-driven bio-triboelectric nanogenerator based on silk nanoribbons. Nano Energy 2020, 74, 104837. [Google Scholar] [CrossRef]
- Brott, A.S.; Clarke, A.J. Peptidoglycan O-Acetylation as a Virulence Factor: Its Effect on Lysozyme in the Innate Immune System. Antibiotics 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Griswold, K.E.; Bement, J.L.; Teneback, C.C.; Scanlon, T.C.; Wargo, M.J.; Leclair, L.W. Bioengineered lysozyme in combination therapies for Pseudomonas aeruginosa lung infections. Bioengineered 2014, 5, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.H.; Garrido-Pascual, P.; Moreirinha, C.; Almeida, A.; Palomares, T.; Alonso-Varona, A.; Vilela, C.; Freire, C.S. Multifunctional nanofibrous patches composed of nanocellulose and lysozyme nanofibers for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 165, 1198–1210. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [Green Version]
- Putthanarat, S.; Stribeck, N.; Fossey, S.; Eby, R.; Adams, W. Investigation of the nanofibrils of silk fibers. Polymer 2000, 41, 7735–7747. [Google Scholar] [CrossRef]
- Pereira, R.F.P.; Silva, M.M.; Bermudez, V.D.Z. Bombyx moriSilk Fibers: An Outstanding Family of Materials. Macromol. Mater. Eng. 2015, 300, 1171–1198. [Google Scholar] [CrossRef]
- Chen, W.; Takai, C.; Khosroshahi, H.R.; Fuji, M.; Shirai, T. Surfactant-free fabrication of SiO2-coated negatively charged polymer beads and monodisperse hollow SiO2 particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-W.; Kim, H.; Kim, K.; Choi, S.Q.; Lee, H.J. Calcium-Modified Silk as a Biocompatible and Strong Adhesive for Epidermal Electronics. Adv. Funct. Mater. 2018, 28, 1800802. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, A.D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, W.; Zhang, Q.; Ling, S.; Chen, Y.; Kaplan, D.L. Aqueous-Based Coaxial Electrospinning of Genetically Engineered Silk Elastin Core-Shell Nanofibers. Materials 2016, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Du, M.; Zhu, H.; Bao, S.; Yang, T.; Zou, M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. 2015, 286, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, H.-Y.; Gu, Z.-G.; Si, L.; Liu, Q.-C.; Hu, X. Impact of calcium chloride concentration on structure and thermal property of Thai silk fibroin films. J. Therm. Anal. Calorim. 2017, 130, 851–859. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, With Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). IUPAC Stand. Online 2016, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ji, F.; Sun, H.; Qin, Z.; Zhang, E.; Cui, J.; Wang, J.; Li, S.; Yao, F. Engineering Polyzwitterion and Polydopamine Decorated Doxorubicin-Loaded Mesoporous Silica Nanoparticles as a pH-Sensitive Drug Delivery. Polymers 2018, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Tang, X.; Liu, X.; Hou, Y.; Li, H.; Yang, C.; Yi, J.; Song, X.; Zhang, G. Thermo/pH Dual Responsive Mixed-Shell Polymeric Micelles Based on the Complementary Multiple Hydrogen Bonds for Drug Delivery. Chem. Asian J. 2016, 11, 112–119. [Google Scholar] [CrossRef]


| Sample | SBET (m2·g−1) | VPore (cm3·g−1) | DPore (nm) | Drug-Loading Efficiency (wt%) | Encapsulation Efficiency (wt%) |
|---|---|---|---|---|---|
| MSNTs-1 | 200.48 | 1.109 | 5.49 | 9.82 | 31.82 |
| MSNTs-2 | 159.87 | 0.696 | 5.97 | 8.65 | 27.67 |
| MSNTs-3 | 94.87 | 0.611 | 5.75 | 8.04 | 25.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wu, H.; Wang, D.; Ma, Y.; Jia, L. A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates. Molecules 2021, 26, 1073. https://doi.org/10.3390/molecules26041073
Zhu J, Wu H, Wang D, Ma Y, Jia L. A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates. Molecules. 2021; 26(4):1073. https://doi.org/10.3390/molecules26041073
Chicago/Turabian StyleZhu, Jingxin, Haijuan Wu, Ding Wang, Yanlong Ma, and Lan Jia. 2021. "A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates" Molecules 26, no. 4: 1073. https://doi.org/10.3390/molecules26041073
APA StyleZhu, J., Wu, H., Wang, D., Ma, Y., & Jia, L. (2021). A Facile Strategy for Fabrication Lysozyme-Loaded Mesoporous Silica Nanotubes from Electrospun Silk Fibroin Nanofiber Templates. Molecules, 26(4), 1073. https://doi.org/10.3390/molecules26041073