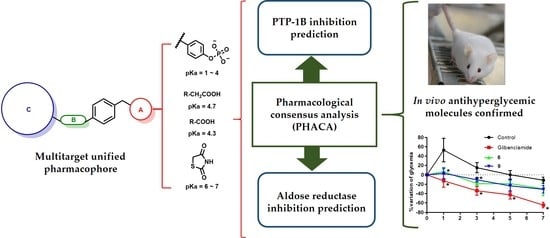
Design, Synthesis, and In Silico Multitarget Pharmacological Simulations of Acid Bioisosteres with a Validated In Vivo Antihyperglycemic Effect
Abstract
Share and Cite
Domínguez-Mendoza, E.A.; Galván-Ciprés, Y.; Martínez-Miranda, J.; Miranda-González, C.; Colín-Lozano, B.; Hernández-Núñez, E.; Hernández-Bolio, G.I.; Palomino-Hernández, O.; Navarrete-Vazquez, G. Design, Synthesis, and In Silico Multitarget Pharmacological Simulations of Acid Bioisosteres with a Validated In Vivo Antihyperglycemic Effect. Molecules 2021, 26, 799. https://doi.org/10.3390/molecules26040799
Domínguez-Mendoza EA, Galván-Ciprés Y, Martínez-Miranda J, Miranda-González C, Colín-Lozano B, Hernández-Núñez E, Hernández-Bolio GI, Palomino-Hernández O, Navarrete-Vazquez G. Design, Synthesis, and In Silico Multitarget Pharmacological Simulations of Acid Bioisosteres with a Validated In Vivo Antihyperglycemic Effect. Molecules. 2021; 26(4):799. https://doi.org/10.3390/molecules26040799
Chicago/Turabian StyleDomínguez-Mendoza, Elix Alberto, Yelzyn Galván-Ciprés, Josué Martínez-Miranda, Cristian Miranda-González, Blanca Colín-Lozano, Emanuel Hernández-Núñez, Gloria I. Hernández-Bolio, Oscar Palomino-Hernández, and Gabriel Navarrete-Vazquez. 2021. "Design, Synthesis, and In Silico Multitarget Pharmacological Simulations of Acid Bioisosteres with a Validated In Vivo Antihyperglycemic Effect" Molecules 26, no. 4: 799. https://doi.org/10.3390/molecules26040799
APA StyleDomínguez-Mendoza, E. A., Galván-Ciprés, Y., Martínez-Miranda, J., Miranda-González, C., Colín-Lozano, B., Hernández-Núñez, E., Hernández-Bolio, G. I., Palomino-Hernández, O., & Navarrete-Vazquez, G. (2021). Design, Synthesis, and In Silico Multitarget Pharmacological Simulations of Acid Bioisosteres with a Validated In Vivo Antihyperglycemic Effect. Molecules, 26(4), 799. https://doi.org/10.3390/molecules26040799