Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS
Abstract
1. Introduction
2. Results
2.1. Influence of Seasonal Changes on the Milk DHI Index
2.2. Effects of Melatonin on Milk DHI Index in Cows with High SCS during Different Seasons
2.3. Influence of Cow’s Age on Milk DHI Index
2.4. Effect of Melatonin on Milk DHI Index in Cows with High SCS of Different Ages
2.5. Influence of Lactation Stages on the Milk DHI Index
2.6. Effects of Melatonin on the DHI Index in Cows with High SCS of Different Lactation Stages
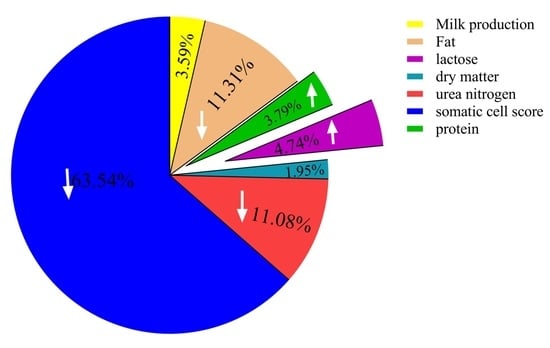
2.7. A Comprehensive Analysis of the Effect of Melatonin on the DHI in Milk
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Study Design
4.4. DHI Measure
4.5. Melatonin Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shock, D.A.; LeBlanc, S.J.; Leslie, K.E.; Hand, K.; Godkin, M.A.; Coe, J.B.; Kelton, D.F. Exploring the Characteristics and Dynamics of Ontario Dairy Herds Experiencing Increases in Bulk Milk Somatic Cell Count During the Summer. J. Dairy Sci. 2015, 98, 3741–3753. [Google Scholar] [CrossRef]
- Haltia, L.; Honkanen-Buzalski, T.; Spiridonova, I.; Olkonen, A.; Myllys, V. A study of bovine mastitis, milking procedures and management practices on 25 Estonian dairy herds. Acta Vet. Scand. 2006, 48, 22. [Google Scholar] [CrossRef][Green Version]
- Volling, O.; Krömker, V. Udder health management practices in dairy enterprises to reduce the incidence of bovine mastitis. Dtsch. Tierarztl. Wochenschr. 2008, 115, 410–420. [Google Scholar] [PubMed]
- Li, N.; Richoux, R.; Boutinaud, M.; Martin, P.; Gagnaire, V. Role of Somatic Cells on Dairy Processes and Products: A Review. Dairy Sci. Technol. 2014, 94, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.E., Jr.; Shuster, D.E. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J. Dairy Sci. 1994, 77, 619–627. [Google Scholar] [CrossRef]
- Cywińska, A.; Baś, M.; Karpiuk, O.; Krzyzowska, M.; Rzewuska, M.; Schollenberger, A.; Niemiałtowski, M. Immunobiology of bovine mammary gland: Apoptosis of somatic cells in milk during naturally occurring mastitis. Pol. J. Vet. Sci. 2006, 9, 63–70. [Google Scholar] [PubMed]
- Berry, D.P.; Lee, J.M.; Macdonald, K.A.; Stafford, K.; Matthews, L.; Roche, J.R. Associations among body condition score, body weight, somatic cell count, and clinical mastitis in seasonally calving dairy cattle. J. Dairy Sci. 2007, 90, 637–648. [Google Scholar] [CrossRef]
- Green, M.J.; Bradley, A.J.; Newton, H.; Browne, W.J. Seasonal variation of bulk milk somatic cell counts in UK dairy herds: Investigations of the summer rise. Prev. Vet. Med. 2006, 74, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Zambelis, A.; Robles, I.; DeVries, T.J. Comparison of physical and behavioral traits between dairy cows with low and high somatic cell count. Prev. Vet. Med. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Delos Campos, G.; Gianola, D.; Heringstad, B. A structural equation model for describing relationships between somatic cell score and milk yield in first-lactation dairy cows. J. Dairy Sci. 2006, 89, 4445–4455. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Zhang, C.; Jones, A.D.; Sordillo, L.M. Differences in the Oxylipid Profiles of Bovine Milk and Plasma at Different Stages of Lactation. J. Agric. Food Chem. 2017, 65, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Barkema, H.W.; DeVries, T.J.; Rushen, J.; Pajor, E.A. Impact of automatic milking systems on dairy cattle producers’ reports of milking labour management, milk production and milk quality. Animal 2018, 12, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Rhoda, D.A.; Pantoja, J.C. Using Mastitis Records and Somatic Cell Count Data. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Romero-Velarde, E.; Delgado-Franco, D.; García-Gutiérrez, M.; Gurrola-Díaz, C.; Larrosa-Haro, A.; Montijo-Barrios, E.; Muskiet, F.A.J.; Vargas-Guerrero, B.; Geurts, J. The Importance of Lactose in the Human Diet: Outcomes of a Mexican Consensus Meeting. Nutrients 2019, 11, 2737. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; D’Oria, V.; De Cosmi, V.; Bettocchi, S.; Milani, G.P.; Silano, M.; Agostoni, C. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Kim, J.W.; Cheon, S.; Nam, M.S.; Kim, K.K. Alpha-Casein and Beta-Lactoglobulin from Cow Milk Exhibit Antioxidant Activity: A Plausible Link to Antiaging Effects. J. Food Sci. 2019, 84, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- De Seram, E.L.; Penner, G.B.; Mutsvangwa, T. Nitrogen utilization whole-body urea-nitrogen kinetics, omasal nutrient flow, and production performance in dairy cows fed lactose as a partial replacement for barley starch. J. Dairy Sci. 2019, 102, 6088–6108. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.G.; Chapman, C.E.; Aragona, K.M.; Clark, E.; Lunak, M.; Erickson, P.S. Predicting colostrum quality from performance in the previous lactation and environmental changes. J. Dairy Sci. 2016, 99, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, A.; Havenaar, R.; He, T.; Bellmann, S. Protein Digestion and Quality of Goat and Cow Milk Infant Formula and Human Milk Under Simulated Infant Conditions. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, J.; Tian, J.; Tao, J.; Chai, M.; Wang, J.; Xu, Z.; Song, Y.; Zhu, K.; Ji, P.; et al. Exogenous melatonin reduces somatic cell count of milk in Holstein cows. Sci. Rep. 2017, 7, 43280. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pérez, J.L.; López-Patiño, M.A.; Álvarez-Otero, R.; Gesto, M.; Soengas, J.L.; Míguez, J.M. Characterization of melatonin synthesis in the gastrointestinal tract of rainbow trout (Oncorhynchus mykiss): Distribution, relation with serotonin, daily rhythms and photoperiod regulation. J. Comp. Physiol. B 2016, 186, 471–484. [Google Scholar] [CrossRef]
- Zawilska, J.B. The role of dopamine in the regulation of melatonin biosynthesis in vertebrate retina. Acta Neurobiol. Exp. 1994, 54, 47–56. [Google Scholar]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 24, 509. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Valtonen, M.; Laitinen, J.T.; Paananen, M.; Kaikkonen, M. Diurnal rhythm of melatonin in bovine milk: Pharmacokinetics of exogenous melatonin in lactating cows and goats. Acta Vet. Scand. 1998, 39, 301–310. [Google Scholar] [CrossRef]
- Avilés, R.; Delgadillo, J.A.; Flores, J.A.; Duarte, G.; Vielma, J.; Flores, M.J.; Petrovski, K.; Zarazaga, L.A.; Hernández, H. Melatonin administration during the dry period stimulates subsequent milk yield and weight gain of offspring in subtropical does kidding in summer. J. Dairy Sci. 2019, 102, 11536–11543. [Google Scholar] [CrossRef]
- Kurjogi, M.; Issa Mohammad, Y.H.; Alghamdi, S.; Abdelrahman, M.; Satapute, P.; Jogaiah, S. Detection and determination of stability of the antibiotic residues in cow’s milk. PLoS ONE 2019, 14, e0223475. [Google Scholar] [CrossRef]
- Young, A.J.; Walters, J.L. Relationship Between DHI Production Values and Myers-Briggs Type Indicator as a Measure of Management Ability. J. Dairy Sci. 2002, 85, 2046–2052. [Google Scholar] [CrossRef]
- Vasicek, C.A.; Malpaux, B.; Fleming, P.A. Melatonin secretion in the Mashona mole-rat, Cryptomys darlingi-influence of light on rhythmicity. Physiol. Behav. 2005, 83, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Stelwagen, K.; Phyn, C.V.; Davis, S.R.; Guinard-Flament, J.; Pomiès, D.; Roche, J.R.; Kay, J.K. Invited review: Reduced milking frequency: Milk production and management implications. J. Dairy Sci. 2013, 96, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Mercan, E.; Aydemir, S.; Civelek, M. Effects of milk somatic cell counts on some physicochemical and functional characteristics of skim and whole milk powders. J. Dairy Sci. 2016, 99, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Condas, L.A.Z.; De Buck, J.; Nobrega, D.B.; Carson, D.A.; Roy, J.P.; Keefe, G.P.; DeVries, T.J.; Middleton, J.R.; Dufour, S.; Barkema, H.W. Distribution of non-aureus staphylococci species in udder quarters with low and high somatic cell count, and clinical mastitis. J. Dairy Sci. 2017, 100, 5613–5627. [Google Scholar] [CrossRef]
- Ivemeyer, S.; Maeschli, A.; Walkenhorst, M.; Klocke, P.; Heil, F.; Oser, S.; Notz, C. Effects of a two-year dairy herd health management programme on udder health, use of antibiotics and longevity. Schweiz Arch. Tierheilkd. 2008, 150, 499–505. [Google Scholar] [CrossRef]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; Long, Y.; Wan, H.; Che, L.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; Wu, D.; et al. Mammary inflammatory gene expression was associated with reproductive stage and regulated by docosahexenoic acid: In vitro and in vivo studies. Lipids Health Dis. 2016, 15, 215. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Geraily, G.; Norouzi, F.; Heidari, M.; Rezapoor, S. The melatonin immunomodulatory actions in radiotherapy. Biophys. Rev. 2017, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, L.; Nunez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and its Therapeutic Potential. Curr. Pharm. Des. 2018, 24, 1563–1588. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.H.; Tsao, J.H.; Lu, Y.P.; Lee, J.W.; Zhao, X.; Chien, F.L.; Mao, S.J.T. Neutrophils as one of the major haptoglobin sources in mastitis affected milk. Vet. Res. 2009, 40, 17. [Google Scholar] [CrossRef]
- Shin, I.S.; Shin, N.R.; Park, J.W.; Jeon, C.M.; Hong, J.M.; Kwon, O.K.; Kim, J.S.; Lee, I.C.; Kim, J.C.; Oh, S.R.; et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J. Pineal Res. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef]
- Nooshinfar, E.; Safaroghli-Azar, A.; Bashash, D.; Akbari, M.E. Melatonin an inhibitory agent in breast cancer. Breast Cancer 2017, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Michalski, M.C. Structure-function relationship of the milk fat globule. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Palhière, I.; Maroteau, C.; Bardou, P.; Canale-Tabet, K.; Sarry, J.; Woloszyn, F.; Bertrand-Michel, J.; Racke, I.; Besir, H.; et al. A genome scan for milk production traits in dairy goats reveals two new mutations in Dgat1 reducing milk fat content. Sci. Rep. 2017, 7, 1872. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, B.; Lacasse, P.; Ollier, S.; Zhao, X. Effects of photoperiod modulation and melatonin feeding around drying-off on bovine mammary gland involution. J. Dairy Sci. 2017, 100, 8496–8506. [Google Scholar] [CrossRef]
- Auldist, M.J.; Turner, S.A.; McMahon, C.D.; Prosser, C.G. Effects of melatonin on the yield and composition of milk from grazing dairy cows in New Zealand. J. Dairy Res. 2007, 74, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.; Xu, H.; Tang, K.; Zan, L.; Yang, W. Melatonin suppresses milk fat synthesis by inhibiting the mTOR signaling pathway via the MT1 receptor in bovine mammary epithelial cells. J. Pineal Res. 2019, 67, e12593. [Google Scholar] [CrossRef] [PubMed]
- Valentina, C.; Besian, I.S.; Haydee, M.G.; Haleh, A.; Sergei, Y.N.; Siewert, J.M.; Tieleman, D.P. Emerging Diversity in Lipid-Protein Interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar]
- Diego, H.; Pedro, F.M.; Joana, R. Nutritional Regulation of Gene Expression: Carbohydrate-Fat- and Amino Acid-Dependent Modulation of Transcriptional Activity. Int. J. Mol. Sci. 2019, 20, 1386. [Google Scholar]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]

| Items | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| somatic cell score | 4.5 ± 0.04 ab | 5.1 ± 0.46 a | 4.3 ± 0.20 ab | 3.8 ± 0.28 b |
| milk yield (kg/d) | 34.9 ± 0.14 a | 33.6 ± 0.19 b | 33.6 ± 0.33 b | 34.2 ± 0.22 ab |
| Protein (%) | 3.5 ± 0.02 a | 3.3 ± 0.03 b | 3.5 ± 0.02 a | 3.5 ± 0.05 a |
| Lactose (%) | 5.0 ± 0.02 b | 5.1 ± 0.02 a | 5.1 ± 0.01 a | 5.1 ± 0.07 a |
| fat (%) | 4.5 ± 0.17 | 4.4 ± 0.35 | 4.5 ± 0.01 | 4.4 ± 0.32 |
| dry matter (%) | 13.8 ± 0.13 a | 13.3 ± 0.17 b | 14.0 ± 0.02 a | 13.7 ± 0.23 ab |
| urea nitrogen (mg/dL) | 14.6 ± 1.98 | 14.3 ± 2.26 | 13.5 ± 1.11 | 16.6 ± 2.79 |
| Day | 0 d | 1 d | 2 d | 3 d | 4 d | 15 d |
|---|---|---|---|---|---|---|
| MT | 9.3 ± 1.15 d | 17.7 ± 1.31 αc | 21.6 ± 1.37 αb | 23.8 ± 2.24 αb | 37.4 ± 2.68 a | 10.3 ± 2.34 d |
| control | 8.4 ± 1.32 | 6.5 ± 2.22 β | 8.8 ± 1.45 β | 7.5 ± 1.53 β | 9.2 ± 1.43 β | 8.9 ± 1.56 |
| Items | Treatment | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|---|
| somatic cell score | Before MT | 6.0 ± 0.08 α | 6.1 ± 0.12 α | 6.0 ± 0.11 α | 6.1 ± 0.11 α |
| After MT | 4.9 ± 0.15 aβ | 4.1 ± 0.20 cβ | 4.6 ± 0.16 bβ | 4.3 ± 0.17 bcβ | |
| milk yield (kg/d) | Before MT | 32.1 ± 1.04 ab | 34.1 ± 1.06 a | 32.9 ± 1.02 ab | 31.7 ± 1.11 b |
| After MT | 32.1 ± 1.15 | 32.7 ±1.00 | 32.1 ± 1.05 | 31.8 ±1.17 | |
| Protein (%) | Before MT | 3.5 ± 0.04 a | 3.5 ± 0.06 a | 3.5 ± 0.05 aα | 3.39 ± 0.05 b |
| After MT | 3.5 ± 0.04 ab | 3.5 ± 0.04 ab | 3.6 ± 0.05 aβ | 3.43 ± 0.05 b | |
| Lactose (%) | Before MT | 4.9 ± 0.02 bα | 5.0 ± 0.03 aα | 5.0 ± 0.03 aα | 4.7 ± 0.07 c |
| After MT | 4.9 ± 0.02 bβ | 5.1 ± 0.02 aβ | 5.1 ± 0.04 aβ | 4.8 ± 0.04 c | |
| fat (%) | Before MT | 5.2 ± 0.12 aα | 4.5 ± 0.16 c | 4.8 ± 0.11 b | 4.8 ± 0.10 b |
| After MT | 4.8 ± 0.14 aβ | 4.2 ± 0.12 b | 4.7 ± 0.13 a | 4.8 ± 0.11 a | |
| dry matter (%) | Before MT | 14.2 ± 0.14 a | 13.6 ± 0.17 b | 14.0 ± 0.13 a | 13.7 ± 0.10 b |
| After MT | 13.9 ± 0.15 b | 13.4 ± 0.13 c | 14.1 ± 0.14 a | 13.7 ± 0.12 b | |
| urea nitrogen (mg/dL) | Before MT | 13.1 ± 0.39 b | 16.6 ± 0.64 a | 12.1 ± 0.43 b | 17.4 ± 0.47 a |
| After MT | 14.4 ± 0.45 b | 14.2 ± 0.50 b | 11.7 ± 0.36 c | 15.9 ± 0.51 a |
| Items | Two | Three | Four | Five | Six | Seven |
|---|---|---|---|---|---|---|
| somatic cell score | 4.0 ± 0.24 d | 4.3 ± 0.21 cd | 4.9 ± 0.15 ab | 4.8 ± 0.20 abc | 4.7 ± 0.19 bc | 5.3 ± 0.17 a |
| milk yield (kg/d) | 32.9 ± 0.58 bc | 35. 7 ± 0.66 a | 31.9 ± 0.34 cd | 34.7 ± 0.56 ab | 34.3 ± 0.75 ab | 30.2 ± 1.57 d |
| Protein (%) | 3.3 ± 0.03 c | 3.4 ± 0.02 bc | 3.5 ± 0.50 a | 3.5 ± 0.07 ab | 3.4 ± 0.05 abc | 3.5 ± 0.75 abc |
| Lactose (%) | 5.2 ± 0.02 a | 5.1 ± 0.02 a | 5.0 ± 0.05 bc | 4.8 ± 0.06 c | 5.0 ± 0.03 b | 4.9 ± 0.04 bc |
| fat (%) | 4.5 ± 0.14 b | 4.5 ± 0.10 b | 4.7 ± 0.12 ab | 4.8 ± 0.08 ab | 4.9 ± 0.14 a | 4.5 ± 0.24 ab |
| dry matter (%) | 13.6 ± 0.14 a | 13.6 ± 0.10 a | 14.0 ± 0.15 a | 13.9 ± 0.10 a | 14.0 ± 0.13 a | 13.6 ± 0.21 a |
| urea nitrogen (mg/dL) | 14.4 ± 0.86 a | 14.0 ± 0.15 a | 14.6 ± 1.01 a | 14.7 ± 1.04 a | 15.2 ± 1.09 a | 14.9 ± 1.37 a |
| Items | Treatment | Two | Three | Four | Five | Six | Seven |
|---|---|---|---|---|---|---|---|
| Somatic cell score | Before MT | 5.3 ± 0.27 cα | 5.7 ± 0.15 abcα | 5.9 ± 0.17 aα | 5.5 ± 0.18 bcα | 5.6 ± 0.26 abc | 5.9 ± 0.20 aα |
| After MT | 3.9 ± 0.33 dβ | 4.3 ± 0.18 cdβ | 4.7 ± 0.18 bβ | 4.0 ± 0.19 cdβ | 5.2 ± 0.27 a | 5.0 ± 0.29 abβ | |
| milk yield (kg/d) | Before MT | 32.1 ± 0.95 b | 31.7 ± 1.04 b | 30.9 ± 1.19 b | 35.0 ± 1.54 a | 33.7 ± 2.45 ab | 33.5 ± 1.73 ab |
| After MT | 32.1 ± 1.63 ab | 32.1 ± 0.96 ab | 30.0 ± 1.22 b | 33.6 ± 1.52 ab | 35.2 ± 2.67 a | 30.3 ± 2.08 b | |
| Protein (%) | Before MT | 3.4 ± 0.07 b | 3.4 ± 0.04 b | 3.6 ± 0.06 a | 3.4 ± 0.06 b | 3.4 ± 0.07 b | 3.2 ± 0.06 c |
| After MT | 3.4 ± 0.09 b | 3.4 ± 0.04 b | 3.7 ± 0.06 a | 3.6 ± 0.07 a | 3.4 ± 0.07 b | 3.3 ± 0.07 b | |
| Lactose (%) | Before MT | 5.2 ± 0.04 a | 5.0 ± 0.03 bα | 4.8 ± 0.04 cα | 4.8 ± 0.04 c | 4.9 ± 0.05 bc | 4.9 ± 0.06 bc |
| After MT | 5.2 ± 0.03 a | 5.0 ± 0.02 bcβ | 4.9 ± 0.03 cβ | 4.9 ± 0.05 c | 4.9 ± 0.06 b | 4.9 ± 0.06 b | |
| Fat (%) | Before MT | 4.7 ± 0.24 ab | 4.7 ± 0.12 bα | 5.0 ± 0.16 a | 4.8 ± 0.14 ab | 5.1 ± 0.18 a | 5.2 ± 0.37 a |
| After MT | 4.5 ± 0.26 bc | 4.4 ± 0.11 cβ | 4.7 ± 0.13 b | 4.6 ± 0.16 bc | 5.3 ± 0.21 a | 4.7 ± 0.37 abc | |
| dry matter(%) | Before MT | 13.9 ± 0.22 ab | 13.8 ± 0.12 b | 14.2 ± 0.17 a | 13.8 ± 0.17 b | 14.1 ± 0.20 ab | 13.8 ± 0.36 b |
| After MT | 13.9 ± 0.27 ab | 13.6 ± 0.13 b | 14.2 ± 0.15 a | 13.9 ± 0.18 ab | 14.1 ± 0.24 a | 13.5 ± 0.32 b | |
| urea nitrogen (mg/dL) | Before MT | 12.8 ± 0.90 c | 14.1 ± 0.53 b | 14.3 ± 0.61 ab | 15.0 ± 0.52 ab | 15.4 ± 0.90 ab | 16.5 ± 1.64 a |
| After MT | 13.9 ± 0.80 bc | 13.6 ± 0.46 bc | 13.3 ± 0.49 c | 14.1 ± 0.59 bc | 15.8 ± 0.94 a | 15.1 ± 1.34 ab |
| Items | Peak Lactation Stage | Middle Lactation Stage | End Lactation Stage |
|---|---|---|---|
| somatic cell score | 2.3 ± 0.31 b | 3.1 ± 0.25 ab | 3.1 ± 0.19 a |
| milk yield (kg/d) | 33.2 ± 2.09 a | 38.9 ± 1.19 a | 32.1 ± 0.93 b |
| Protein (%) | 3.4 ± 0.05 a | 3.4 ± 0.03 a | 3.4 ± 0.03 a |
| Lactose (%) | 5.2 ± 0.04 a | 5.2 ± 0.03 a | 5.2 ± 0.02 a |
| fat (%) | 4.4 ± 0.15 a | 4.1 ± 0.11 a | 4.0 ± 0.08 a |
| dry matter (%) | 13.7 ± 0.14 a | 13.4 ± 0.12 a | 13.3 ± 0.08 a |
| urea nitrogen (mg/dL) | 12.1 ± 0.51 a | 12.5 ± 0.50 a | 11.8 ± 0.33 a |
| Items | Treatment | Peak Lactation | Middle Lactation | End Lactation |
|---|---|---|---|---|
| somatic cell score | Before MT | 2.9 ± 0.32 b | 3.9 ± 0.25 aα | 4.3 ± 0.18 aα |
| After MT | 2.0 ± 0.24 | 2.0 ± 0.17β | 2.0 ± 0.15β | |
| milk yield (kg/d) | Before MT | 32.9 ± 2.34 b | 40.0 ± 1.35 a | 31.6 ± 1.22 b |
| After MT | 38.8 ± 2.06 a | 39.1 ± 1.79 a | 31.3 ± 1.29 b | |
| Protein (%) | Before MT | 3.5 ± 0.06 | 3.5 ± 0.04 | 3.5 ± 0.04 |
| After MT | 3.4 ± 0.06 ab | 3.5 ± 0.05 a | 3.4 ± 0.04 b | |
| Lactose (%) | Before MT | 5.2 ± 0.05 aα | 5.1 ± 0.03 b | 5.1 ± 0.03 bα |
| After MT | 5.3 ± 0.04 aβ | 5.2 ± 0.02 b | 5.2 ± 0.02 bβ | |
| Fat (%) | Before MT | 4.5 ± 0.19 a | 4.2 ± 0.12 b | 4.1 ± 0.08 b |
| After MT | 3.9 ± 0.26 | 4.2 ± 0.11 | 4.2 ± 0.12 | |
| dry matter (%) | Before MT | 13.8 ± 0.16 a | 13.5 ± 0.14 ab | 13.4 ± 0.09 b |
| After MT | 13.2 ± 0.29 b | 13.6 ± 0.14 a | 13.5 ± 0.13 a | |
| urea nitrogen (mg/dL) | Before MT | 11.9 ± 0.65 a | 12.7 ± 0.65 a | 11.5 ± 0.38 a |
| After MT | 11.1 ± 0.64 a | 12.1 ± 0.54 a | 12.0 ± 0.38 a |
| Farm 1 | Lactation Period | Peak | Middle | Late | |||
| (summer, age 4) | Number | 21 | 54 | 98 | |||
| Farm 2 | Seasons | Spring | Summer | Autumn | Winter | ||
| (middle, ages 3 to 4) | Number | 728 | 702 | 662 | 686 | ||
| Farm 3 | Age | Two | Three | Four | Five | Six | Seven |
| (summer, middle) | Number | 507 | 681 | 601 | 502 | 159 | 62 |
| Farm 1 | Lactation Period | Peak | Middle | Late | |||
| (summer, age 4) | Number | 15 | 38 | 59 | |||
| Farm 2 | Seasons | Spring | Summer | Autumn | Winter | ||
| (middle, ages 3 to 4) | Number | 90 | 74 | 83 | 81 | ||
| Farm 3 | Age | Two | Three | Four | Five | Six | Seven |
| (summer, middle) | Number | 23 | 80 | 66 | 56 | 27 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Yao, S.; Wang, T.; Wang, J.; Ren, K.; Yang, H.; Ma, W.; Ji, P.; Lu, Y.; Ma, H.; et al. Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS. Molecules 2021, 26, 834. https://doi.org/10.3390/molecules26040834
Wu H, Yao S, Wang T, Wang J, Ren K, Yang H, Ma W, Ji P, Lu Y, Ma H, et al. Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS. Molecules. 2021; 26(4):834. https://doi.org/10.3390/molecules26040834
Chicago/Turabian StyleWu, Hao, Songyang Yao, Tiankun Wang, Jun Wang, Kang Ren, Hai Yang, Wenkui Ma, Pengyun Ji, Yongqiang Lu, Hui Ma, and et al. 2021. "Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS" Molecules 26, no. 4: 834. https://doi.org/10.3390/molecules26040834
APA StyleWu, H., Yao, S., Wang, T., Wang, J., Ren, K., Yang, H., Ma, W., Ji, P., Lu, Y., Ma, H., He, C., Wei, W., Zhang, L., & Liu, G. (2021). Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS. Molecules, 26(4), 834. https://doi.org/10.3390/molecules26040834