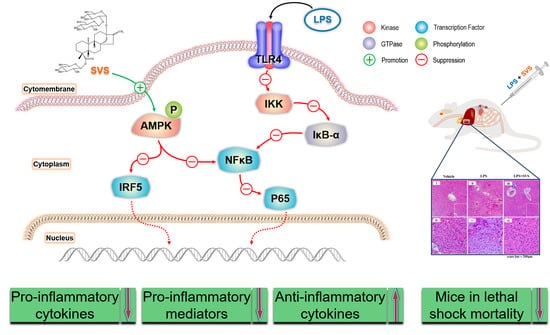
Stevioside Activates AMPK to Suppress Inflammation in Macrophages and Protects Mice from LPS-Induced Lethal Shock
Abstract
:1. Introduction
2. Results
2.1. Stevioside Inhibited IL-6 Expression in LPS-Stimulated Macrophages
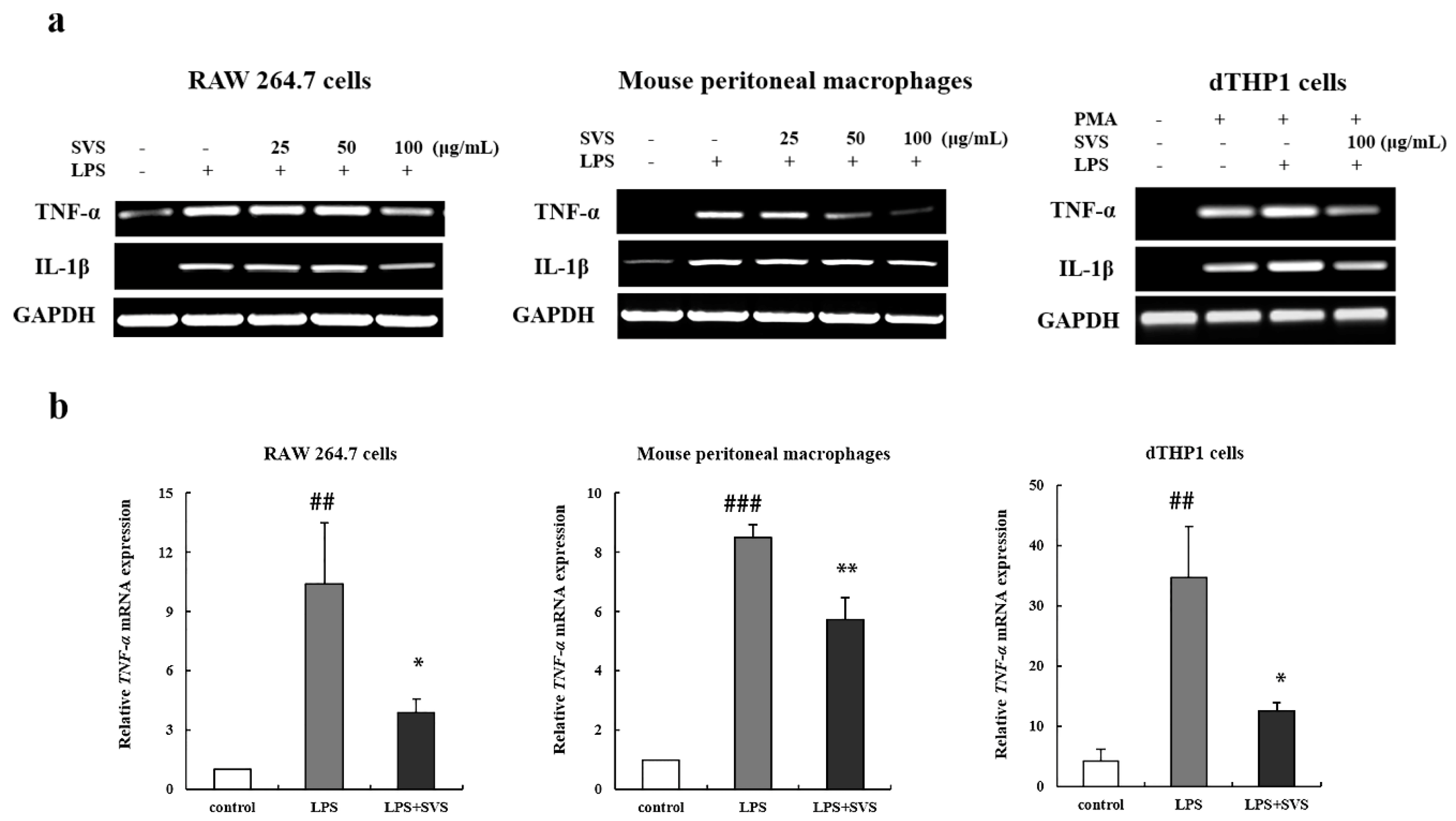
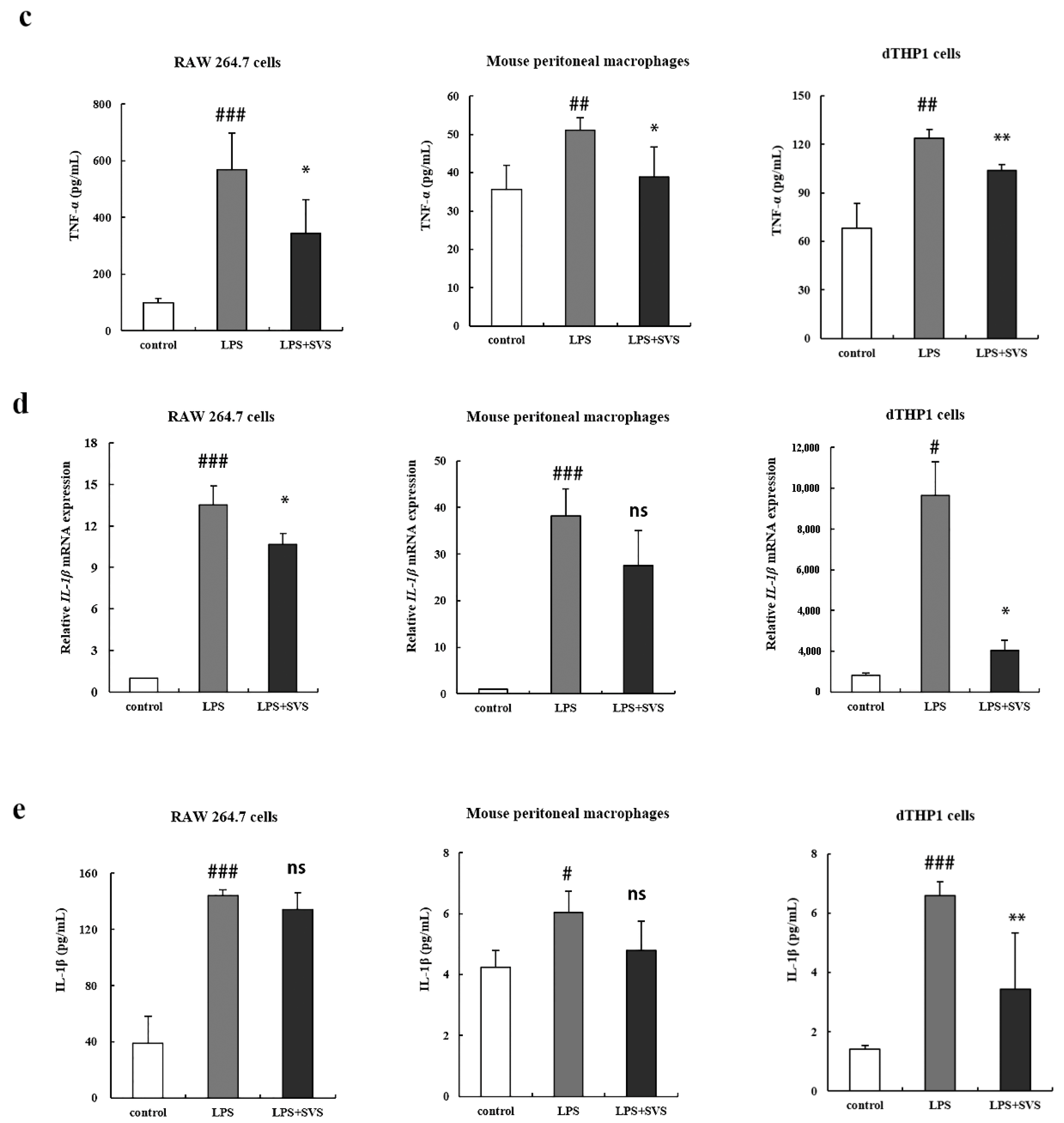
2.2. Stevioside Repressed LPS-Induced Expression of Pro-Inflammatory Cytokine TNF-α and IL-1β in Macrophages
2.3. Stevioside Enhanced Expression of Anti-Inflammatory Cytokines in LPS-Stimulated Macrophages
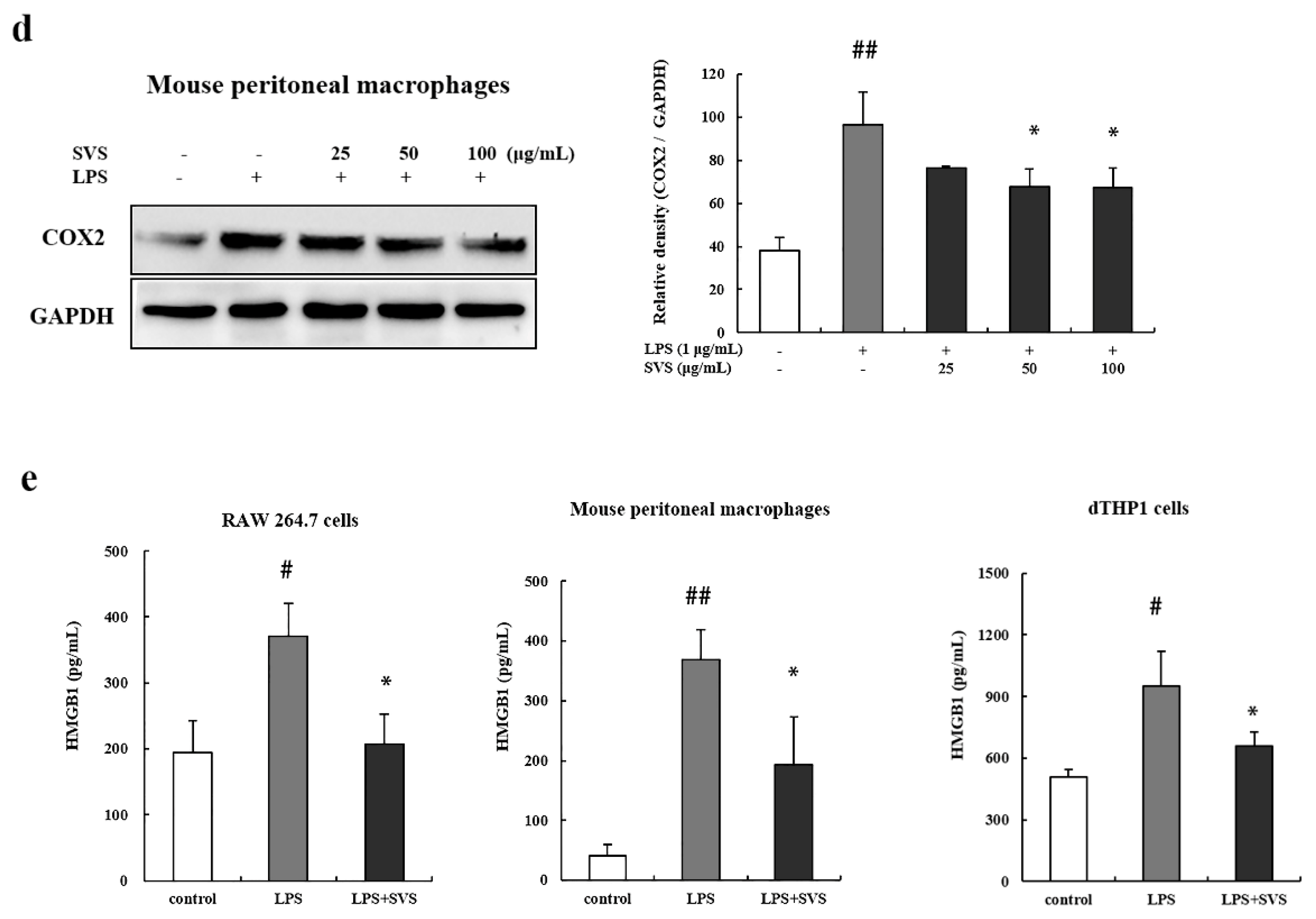
2.4. Stevioside Suppressed LPS-Induced iNOS, COX-2, and HMGB1 Expression in Macrophages
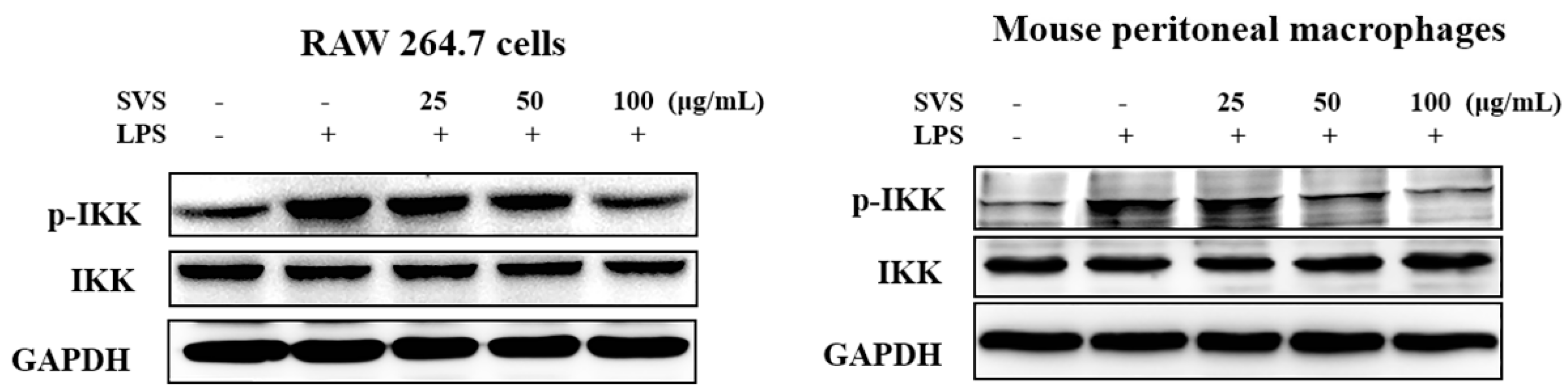
2.5. Stevioside Repressed LPS-Induced NF-κB and IRF5 Activation in Macrophages
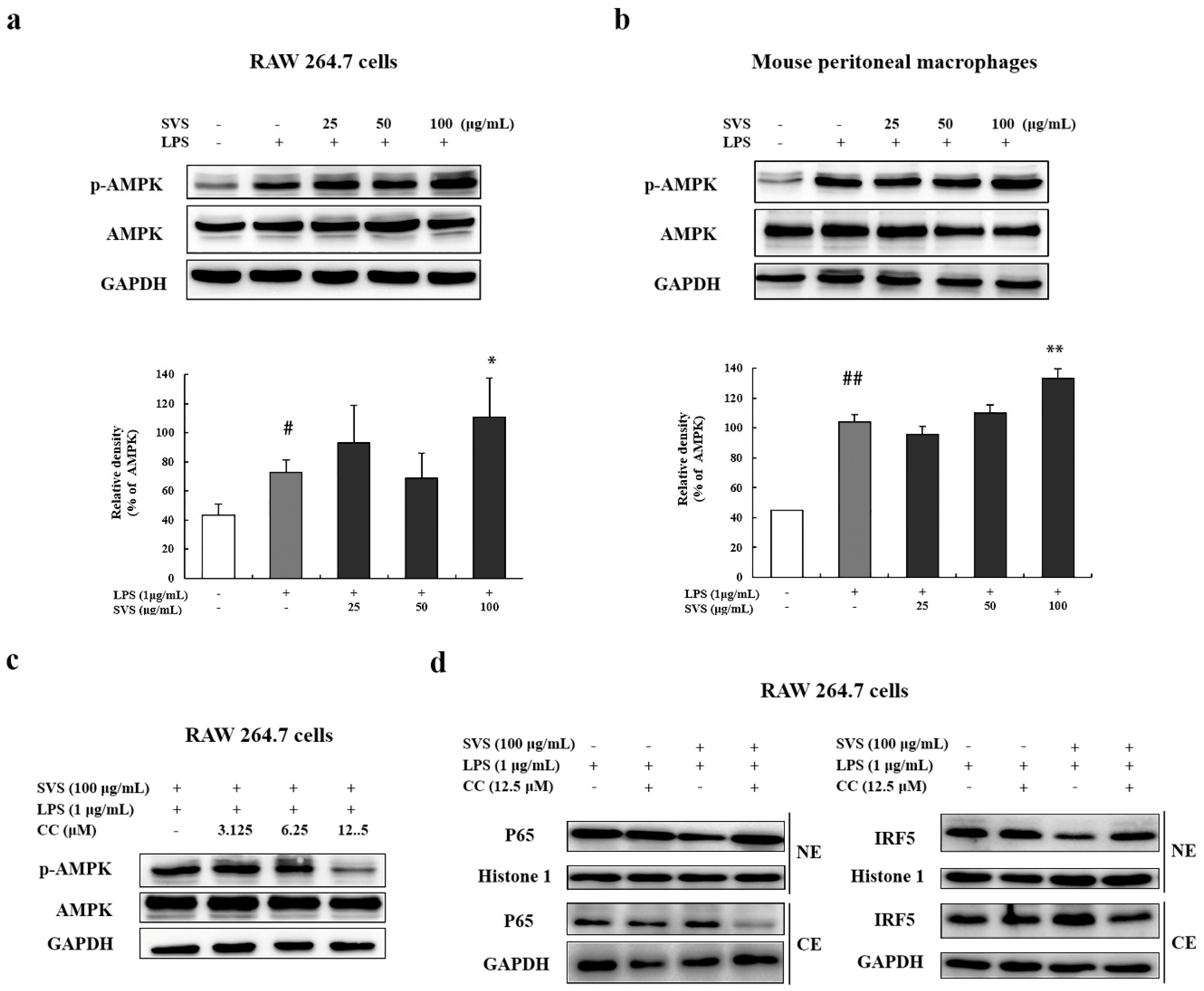
2.6. Stevioside Inhibited IRF5/NF-κB Pathway through AMPK Activation
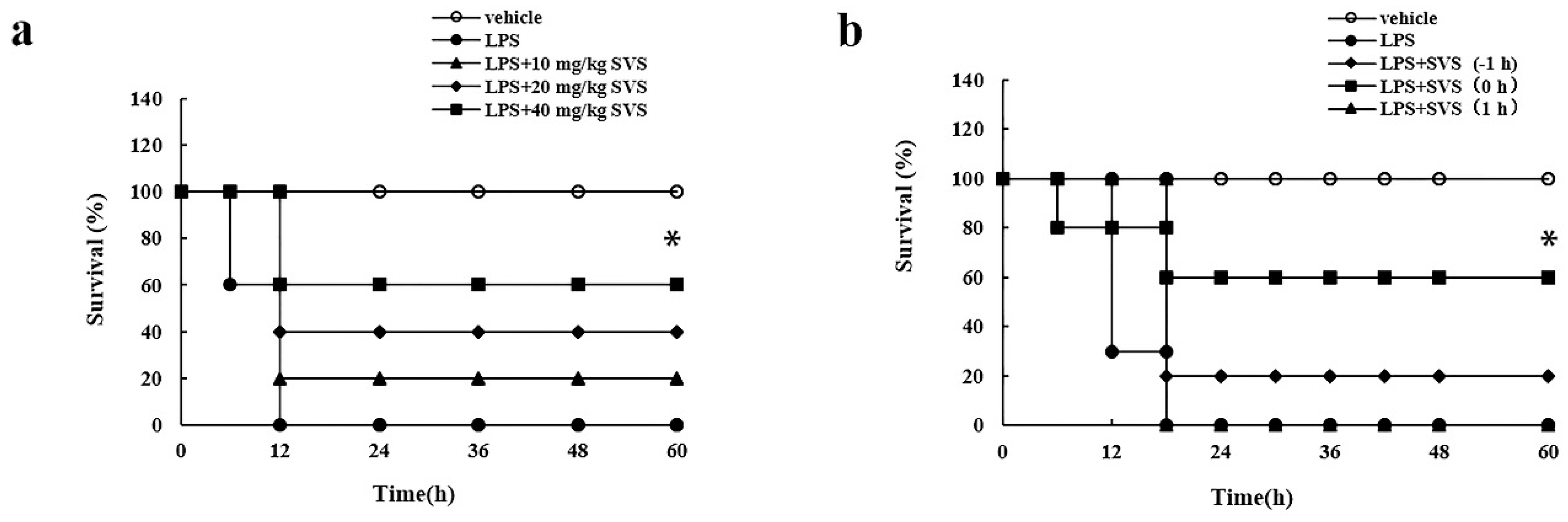
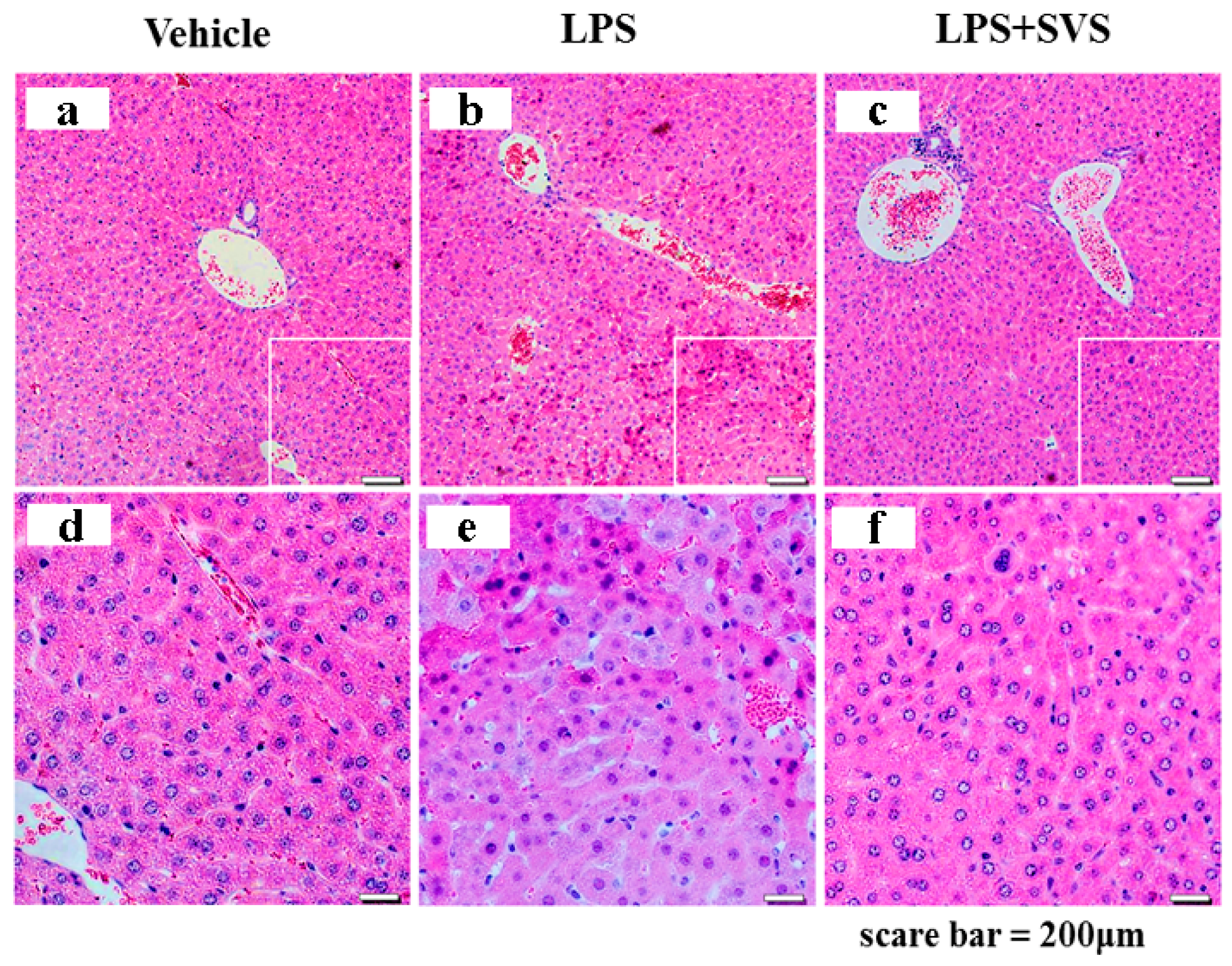
2.7. Stevioside Attenuated the Inflammatory Response and Protected Mice from Excessive Cytokine-Mediated Lethal Shock
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Animals
4.4. RNA Extraction, RT-PCR, and Real-Time RT-PCR (RT-qPCR)
4.5. ELISA
4.6. NO Determination
4.7. Protein Extraction and Western Blotting Analysis
4.8. Lethal Shock Model
4.9. Histological Analysis of Mouse Livers
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives/Prepared by the Sixty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA); International Programme on Chemical Safety; WHO: Geneva, Switzerland, 2006; pp. 117–141. [Google Scholar]
- Ullah, A.; Munir, S.; Mabkhot, Y.; Badshah, S.L. Bioactivity Profile of the Diterpene Isosteviol and its Derivatives. Molecules 2019, 24, 678. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yang, P.; Sifa, D.; Wen, Z. Effect of dietary stevioside supplementation on growth performance, nutrient digestibility, serum parameters, and intestinal microflora in broilers. Food Funct. 2019, 10, 2340–2346. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Qin, L.; Zhang, X.; Mei, Y. Stevioside improved hyperglycemia-induced cardiac dysfunction by attenuating the development of fibrosis and promoting the degradation of established fibrosis. J. Funct. Foods 2020, 68, 103895. [Google Scholar] [CrossRef]
- Jia, C.-H.; Zhang, J.-Y.; Shen, W.; Zhao, X.; Xie, M.-L. Attenuation of high-fat diet-induced fatty liver through PPARα activation by stevioside. J. Funct. Foods 2019, 57, 392–398. [Google Scholar] [CrossRef]
- Wang, J.; Shen, W.; Zhang, J.Y.; Jia, C.H.; Xie, M.L. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-kappaB/TGF-beta1/Smad signaling pathway. Food Funct 2019, 10, 1179–1190. [Google Scholar] [CrossRef]
- Rotimi, S.O.; Rotimi, O.A.; Adelani, I.B.; Onuzulu, C.; Obi, P.; Okungbaye, R. Stevioside modulates oxidative damage in the liver and kidney of high fat/low streptozocin diabetic rats. Heliyon 2018, 4, e00640. [Google Scholar] [CrossRef] [Green Version]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Ilić, V.; Vukmirović, S.; Stilinović, N.; Čapo, I.; Arsenović, M.; Milijašević, B. Insight into anti-diabetic effect of low dose of stevioside. Biomed. Pharmacother. 2017, 90, 216–221. [Google Scholar] [CrossRef]
- Alavala, S.; Nalban, N.; Sangaraju, R.; Kuncha, M.; Jerald, M.K.; Kilari, E.K.; Sistla, R. Anti-inflammatory effect of stevioside abates Freund’s complete adjuvant (FCA)-induced adjuvant arthritis in rats. Inflammopharmacology 2020, 28, 1579–1597. [Google Scholar] [CrossRef] [PubMed]
- Fengyang, L.; Yunhe, F.; Bo, L.; Zhicheng, L.; Depeng, L.; Dejie, L.; Wen, Z.; Yongguo, C.; Naisheng, Z.; Xichen, Z.; et al. Stevioside Suppressed Inflammatory Cytokine Secretion by Downregulation of NF-κB and MAPK Signaling Pathways in LPS-Stimulated RAW264.7 Cells. Inflammation 2012, 35, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, M.; Song, X.; Zhang, Z.; Jiang, H.; Wang, W.; Fu, Y.; Cao, Y.; Zhu, L.; Zhang, N. Stevioside Plays an Anti-inflammatory Role by Regulating the NF-κB and MAPK Pathways in S. aureus-infected Mouse Mammary Glands. Inflammation 2014, 37, 1837–1846. [Google Scholar] [CrossRef]
- Boonkaewwan, C.; Burodom, A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J. Sci. Food Agric. 2013, 93, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Boonkaewwan, C.; Toskulkao, C.; Vongsakul, M. Anti-Inflammatory and Immunomodulatory Activities of Stevioside and Its Metabolite Steviol on THP-1 Cells. J. Agric. Food Chem. 2006, 54, 785–789. [Google Scholar] [CrossRef]
- Mollaei, M.; Abbasi, A.; Hassan, Z.M.; Pakravan, N. The intrinsic and extrinsic elements regulating inflammation. Life Sci. 2020, 260, 118258. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Doseff, A.I. The Targeted Impact of Flavones on Obesity-Induced Inflammation and the Potential Synergistic Role in Cancer and the Gut Microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martínez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef] [Green Version]
- Tsung, A.; Tohme, S.; Billiar, T.R. High-mobility group box-1 in sterile inflammation. J Intern Med 2014, 276, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Dickensheets, H.; Finbloom, D.S. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 1999, 19, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.H.; Zhang, J.; Zhang, X.; Hlavaty, K.A.; Ricci, C.F.; Leonard, J.N.; Shea, L.D.; Gower, R.M. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 2016, 80, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Li, L.C.; Pan, Z.H.; Ning, D.S.; Fu, Y.X. Anti-Inflammatory Effect of Simonsinol on Lipopolysaccharide Stimulated RAW264.7 Cells through Inactivation of NF-κB Signaling Pathway. Molecules 2020, 25, 3573. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.E.; Huang, D.-B.; Chen, Y.-Q.; Ghosh, G. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 1998, 391, 410–413. [Google Scholar] [CrossRef]
- Lazzari, E.; Jefferies, C.A. IRF5-mediated signaling and implications for SLE. Clin. Immunol. 2014, 153, 343–352. [Google Scholar] [CrossRef]
- Khoyratty, T.E.; Udalova, I.A. Diverse mechanisms of IRF5 action in inflammatory responses. Int. J. Biochem. Cell Biol. 2018, 99, 38–42. [Google Scholar] [CrossRef]
- Stempel, M.; Chan, B.; Juranić Lisnić, V.; Krmpotić, A.; Hartung, J.; Paludan, S.R.; Füllbrunn, N.; Lemmermann, N.A.; Brinkmann, M.M. The herpesviral antagonist m152 reveals differential activation of STING-dependent IRF and NF-κB signaling and STING’s dual role during MCMV infection. EMBO J. 2019, 38. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5 ‘-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-H.; Zhang, S.-D.; Wang, L.-T.; Yu, L.; Zhao, X.-L.; Ni, H.-Y.; Wang, Y.-Q.; Wang, J.-D.; Shan, C.-H.; Fu, Y.-J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Wu, X.; Li, Y. Experimental Methodology of Pharmacology. Pharmacological Experiment Design and Statistical Analysis; Peoples’ Medical Publishing House: Beijing, China, 2010; pp. 70–72. [Google Scholar]
- Chamberlain, L.M.; Holt-Casper, D.; Gonzalez-Juarrero, M.; Grainger, D.W. Extended culture of macrophages from different sources and maturation results in a common M2 phenotype. J. Biomed. Mater. Res. A 2015, 103, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.S. Anticatabolic and Anti-Inflammatory Effects of Myricetin 3-O-β-d-Galactopyranoside in UVA-Irradiated Dermal Cells via Repression of MAPK/AP-1 and Activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef] [Green Version]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, D.M.; Song, Z.B.; Hou, Y.Q.; Bao, Y.L.; Sun, L.G.; Yu, C.L.; Li, Y.X. Protumoral TSP50 Regulates Macrophage Activities and Polarization via Production of TNF-α and IL-1β, and Activation of the NF-κB Signaling Pathway. PLoS ONE 2015, 10, e0145095. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Cao, X.; Liu, B.; Huang, H.; Wang, X.; Sui, L.; Yin, W.; Ma, K. CaMK IV phosphorylates prohibitin 2 and regulates prohibitin 2-mediated repression of MEF2 transcription. Cell Signal. 2011, 23, 1686–1690. [Google Scholar] [CrossRef]
- Sun, R.; Sun, L.; Bao, M.; Zhang, Y.; Wang, L.; Wu, X.; Hu, D.; Liu, Y.; Yu, Y.; Wang, L. A human microsatellite DNA-mimicking oligodeoxynucleotide with CCT repeats negatively regulates TLR7/9-mediated innate immune responses via selected TLR pathways. Clin. Immunol. 2010, 134, 262–276. [Google Scholar] [CrossRef]






| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IL-6 Mouse | 5′-TCCAGTTGCCTTCTTGGGAC-3′ | 5′-GTGTAATTAAGCCTCCGACTTG-3′ |
| Human | 5′-GTGAAAGCAGCAAAGAGGCACT-3′ | 5′-ACCAGGCAAGTCTCCTCATTGA-3′ |
| TNF-α Mouse | 5′-GACCCTCACACTCAGATCATCTTCT-3′ | 5′-CCTCCACTTGGTGGTTTGCT-3′ |
| Human | 5′-TCAATCGGCCCGACTATCTC-3′ | 5′-CAGGGCAATGATCCCAAAGT-3′ |
| IL-1β Mouse | 5′-TCGTGCTGTCGGACCCATAT-3′ | 5′-GTCGTTGCTTGGTTCTCCTTGT-3′ |
| Human | 5′-GCACGATGCACCTGTACGAT-3′ | 5′-AGACATCACCAAGCTTTTTTGCT-3′ |
| TGF-β1 Mouse | 5′-CAAGGGCTACCATGCCAACT-3′ | 5′-AGGGCCAGGACCTTGCTG-3′ |
| Human | 5′-CAAGGGCTACCATGCCAACT-3′ | 5′-AGGGCCAGGACCTTGCTG-3′ |
| IL-10 Mouse | 5′-CTGTCATCGATTTCTCCCCTGTG-3′ | 5′-TGGTCTTGGAGCTTATTAAAATCAC-3′ |
| Human | 5′-CTGTCATCGATTTCTCCCCTGTG-3′ | 5′-TGGTCTTGGAGCTTATTAAAATCAC-3′ |
| GAPDH Mouse | 5′-AAATGGTGAAGGTCGGTGTG-3′ | 5′-TGAAGGGGTCGTTGATGG-3′ |
| Human | 5′-AAATGGTGAAGGTCGGTGTG-3′ | 5′-TGAAGGGGTCGTTGATGG-3′ |
| iNOS Mouse | 5′-GGGTCGTAATGTCCAGGAAGT-3′ | 5′-TCTTGGAGCGAGTTGTGGAT-3′ |
| Human | 5′-GGGTCGTAATGTCCAGGAAGT-3′ | 5′-TCTTGGAGCGAGTTGTGGAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Zhu, H.; Li, N.; Yu, C.; Song, Z.; Wang, S.; Sun, Y.; Zheng, L.; Wang, G.; Huang, Y.; et al. Stevioside Activates AMPK to Suppress Inflammation in Macrophages and Protects Mice from LPS-Induced Lethal Shock. Molecules 2021, 26, 858. https://doi.org/10.3390/molecules26040858
Wei F, Zhu H, Li N, Yu C, Song Z, Wang S, Sun Y, Zheng L, Wang G, Huang Y, et al. Stevioside Activates AMPK to Suppress Inflammation in Macrophages and Protects Mice from LPS-Induced Lethal Shock. Molecules. 2021; 26(4):858. https://doi.org/10.3390/molecules26040858
Chicago/Turabian StyleWei, Fuyao, Hong Zhu, Na Li, Chunlei Yu, Zhenbo Song, Shuyue Wang, Ying Sun, Lihua Zheng, Guannan Wang, Yanxin Huang, and et al. 2021. "Stevioside Activates AMPK to Suppress Inflammation in Macrophages and Protects Mice from LPS-Induced Lethal Shock" Molecules 26, no. 4: 858. https://doi.org/10.3390/molecules26040858