Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Surface Tension Measurements
2.3. Contact Angle Measurements
3. Results and Discussion
3.1. Surface Activity Parameters of the Extended Surfactants
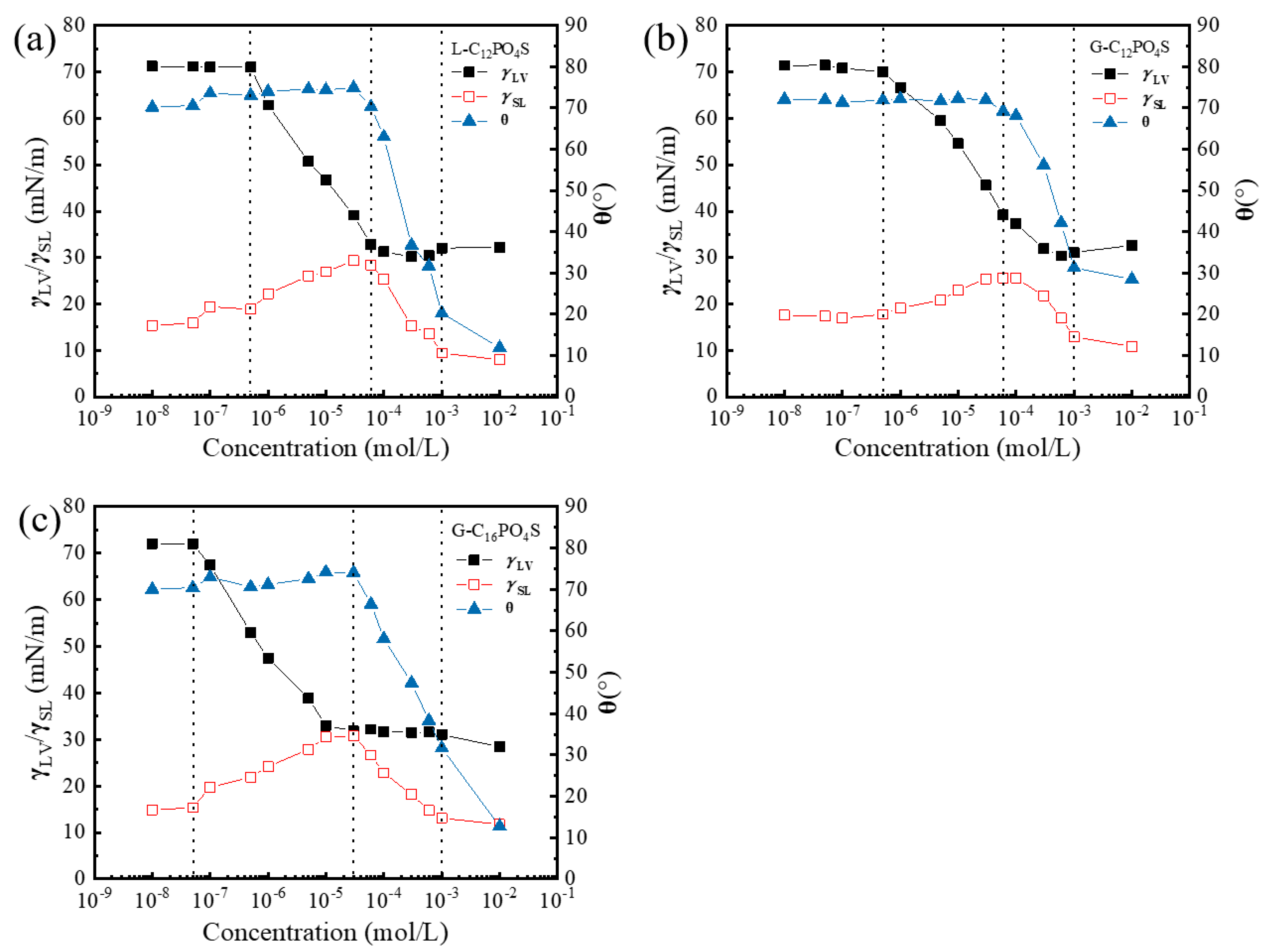
3.2. Contact Angles of the Extended Surfactants at PMMA Surface
3.3. Adhesional Tension of the Extended Surfactants at PMMA–Liquid Interface
3.4. Interfacial Tension of the PMMA–Liquid Interface
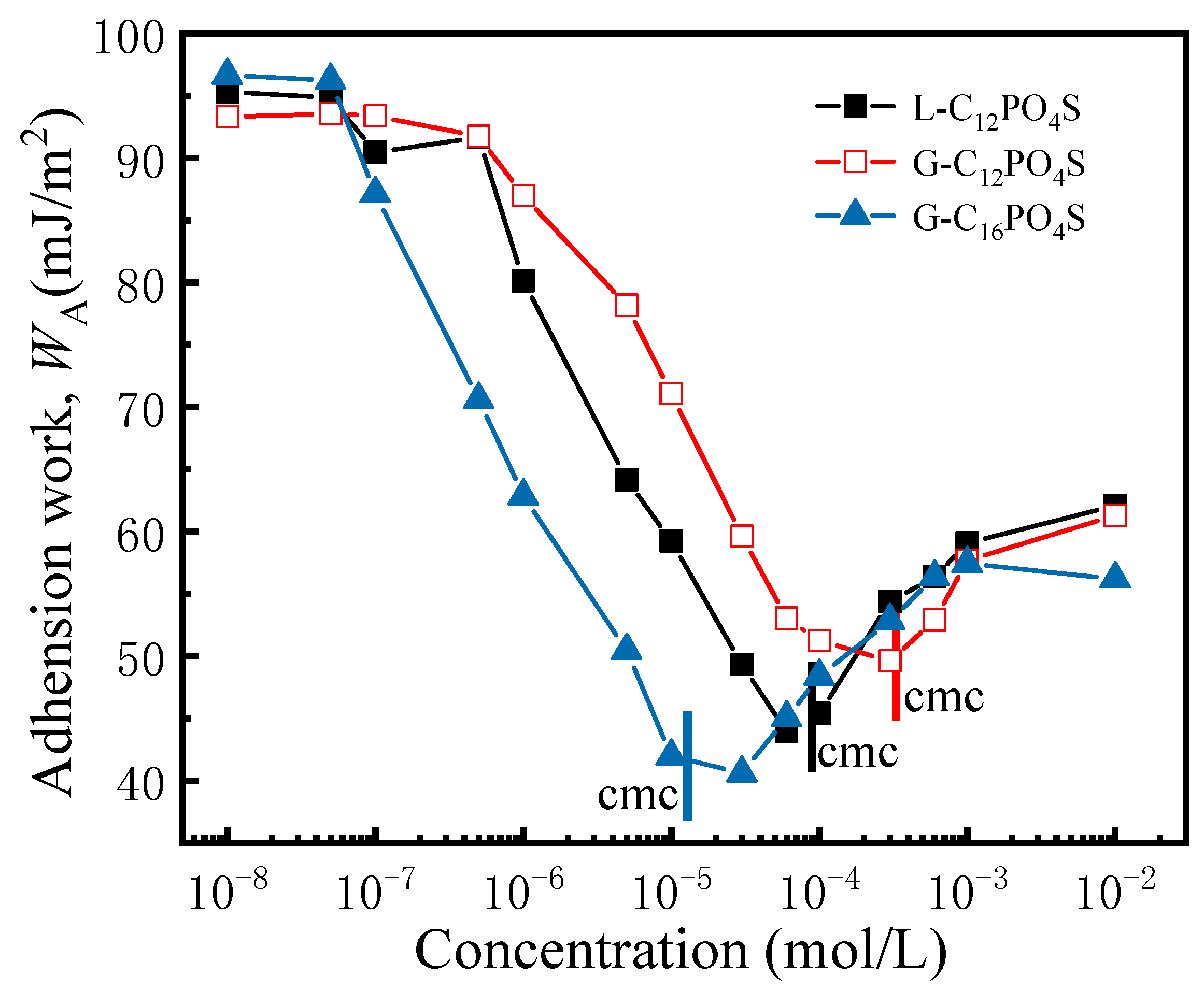
3.5. Adhesion Work of the Extended Surfactants on PMMA Surface
3.6. Adsorption Mechanism of the Extended Surfactants on PMMA Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Jiang, Y.; Choi, C.H. Droplet Retention on Superhydrophobic Surfaces: A Critical Review. Adv. Mater. Interfaces 2021, 8, 2001205. [Google Scholar] [CrossRef]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Gu, Z.; Wan, X.; Jiang, L.; Wang, S. Bioinspired Multiscale Wet Adhesive Surfaces: Structures and Controlled Adhesion. Adv. Funct. Mater. 2020, 30, 1905287. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Kunugi, T. Wettability model for various-sized droplets on solid surfaces. Phys. Fluids 2014, 26, 082110. [Google Scholar] [CrossRef]
- Yuan, T.; Liu, Z.; Gao, R.; Hu, G.; Zhang, G.; Zhao, J. Enhanced oil recovery from high-salinity reservoirs by cationic gemini surfactants. J. Appl. Polym. Sci. 2018, 135, 46086. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Aghazadeh, S. A review of the role of wetting and spreading phenomena on the flotation practice. Curr. Opin. Colloid Interface Sci. 2014, 19, 266–282. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, J.H.V.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Barma, S.D.; Banerjee, B.; Chatterjee, K.; Paria, S. Natural Surfactants-Based Ag Nanofluids for Enhanced Wettability on Hair Surface. ACS Sustain.Chem. Eng. 2018, 6, 3615–3623. [Google Scholar] [CrossRef]
- Singh, H.; Bhowmick, H. Lubrication characteristics and wear mechanism mapping for hybrid aluminium metal matrix composite sliding under surfactant functionalized MWCNT-oil. Tribol. Int. 2020, 145, 106152. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, X.; Liu, Y.; Song, S. AFM study on the wettability of mica and graphite modified with surfactant DTAB. J. Dispers. Sci. Technol. 2018, 39, 1060–1064. [Google Scholar] [CrossRef]
- Alexandrova, L.; Rao, K.H.; Forsberg, K.S.E.; Grigorov, L.; Pugh, R.J. The influence of mixed cationic-anionic surfactants on the three-phase contact parameters in silica-solution systems. Colloids Surf. A 2011, 373, 145–151. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, Q.; Wang, H.Z.; Xu, Z.C.; Zhang, L.; Liu, D.D.; Zhang, L. Wettability of a PTFE Surface by Aqueous Solutions of Zwitterionic Surfactants: Effect of molecular structure. Colloids Surf. A 2016, 489, 370–377. [Google Scholar] [CrossRef]
- Chang, H.; Cui, Y.; Wei, W.; Li, X.; Gao, W.; Zhao, X.; Yin, S. Adsorption behavior and wettability by Gemini surfactants with ester bond at polymer-solution-air systems. J. Mol. Liq. 2017, 230, 429–436. [Google Scholar] [CrossRef]
- Leon, J.M.; Bernardo, V.; Rodriguez-Perez, M.A. Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale. Materials 2019, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Jaeblon, T. Polymethylmethacrylate: Properties and Contemporary Uses in Orthopaedics. J. Am. Acad. Orthop. Surg. 2010, 18, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.M.; Kaczinski, M.B.; Dwight, D.W. Characterization of polymer surface sites with contact angles of test solutions. 1. Phenol and iodine adsorption from methylene iodide onto PMMA films. Langmuir 1991, 7, 2464–2470. [Google Scholar] [CrossRef]
- Harkot, J.; Janczuk, B. The role of adsorption of sodium bis(2-ethylhexyl) sulfosuccinate in wetting of glass and poly(methyl methacrylate) surface. Appl. Surf. Sci. 2008, 254, 2825–2830. [Google Scholar] [CrossRef]
- Harkot, J.; Janczuk, B. The role of adsorption of dodecylethyldimethylammonium bromide and benzyldimethyldodecylammonium bromide surfactants in wetting of polytetrafluoroethylene and poly(methyl methacrylate) surfaces. Appl. Surf. Sci. 2009, 255, 3623–3628. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Janczuk, B. Behavior of cationic surfactants and short-chain alcohols in mixed surface layers at water-air and polymer-water interfaces with regard to polymer wettability II. Wettability of polymers. J. Colloid Interface Sci. 2010, 350, 568–576. [Google Scholar] [CrossRef]
- Zdziennicka, A. The wettability of polytetrafluoroethylene and polymethylmethacrylate by aqueous solutions of Triton X-100 and propanol mixtures. Appl. Surf. Sci. 2009, 255, 3801–3810. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Krawczyk, J.; Janczuk, B. Correlation between wetting, adhesion and adsorption in the polymer-aqueous solutions of ternary surfactant mixtures-air systems. Appl. Surf. Sci. 2014, 288, 488–496. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Janczuk, B. The relationship between the adhesion work, the wettability and composition of the surface layer in the systems polymer/aqueous solution of anionic surfactants and alcohol mixtures. Appl. Surf. Sci. 2010, 257, 1034–1042. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.H.; Hu, S.S.; Li, S.M.; Ma, D.S.; Zhou, X.Y.; Han, L.; Zhang, L.; Zhang, L. Wettability of a Polymethylmethacrylate Surface in the Presence of Benzyl-Substituted Alkyl Betaines. J. Mol. Liq. 2019, 277, 571–576. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.G.; Lv, W.F.; Zhou, Z.H.; Zhang, Q.; Ma, D.S.; Wang, H.Z.; Yan, F.; Zhang, L.; Zhang, L. Adsorption behaviors of branched cationic Gemini surfactants and wettability in quartz-solution-air systems. Soft Matter 2020, 16, 5450–5457. [Google Scholar] [CrossRef]
- Lv, W.F.; Zhou, Z.H.; Zhang, Q.; Luo, W.L.; Wang, H.Z.; Ma, D.S.; Zhang, L.; Wang, R.; Zhang, L. Wetting of Polymer Surfaces by Aqueous Solutions of Branched Cationic Gemini Surfactants. Soft Matter 2019, 15, 6725–6731. [Google Scholar] [CrossRef]
- Liu, D.D.; Xu, Z.C.; Zhao, Q.; Zhang, L.; Zhang, L.; Zhao, S. Adsorption behavior of cationic and zwitterionic surfactants on poly(methyl methacrylate). Acta Phys. Chim. Sin. 2013, 29, 569–575. [Google Scholar]
- Wu, W.; Liu, D.D.; Xu, Z.C.; Gong, Q.T.; Huang, J.B.; Zhang, L.; Zhang, L. Adsorption and wettability of branched betaine and cationicsurfactants on a poly(methyl methacrylate) surface. Acta Phys. Chim. Sin. 2016, 32, 1214–1220. [Google Scholar] [CrossRef]
- He, Z.Q.; Zhang, M.J.; Fang, Y.; Jin, G.Y.; Chen, J. Extended surfactants: A well-designed spacer to improve interfacial performance through a gradual polarity transition. Colloids Surf. A 2014, 450, 83–92. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.Y.; Fang, Y.; Liu, H.H.; Xia, Y.M. Comparative Study of Conventional/Ethoxylated/Extended n-Alkylsulfate Surfactants. Langmuir 2019, 35, 3116–3125. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.Y.; Fang, Y.; Jin, G.Y.; Xia, Y.M. What dominates the interfacial properties of extended surfactants: Amphipathicity or surfactant shape? J. Colloid Interface Sci. 2019, 547, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Zhou, Z.H.; Zhang, L.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhao, S. Adsorption Behaviors of Novel Betaines on the Wettability of Quartz Surface. Soft Matter 2015, 11, 7960–7968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Li, Q.; Niu, J. Surface tension, interfacial tension and emulsification of sodium dodecyl sulfate extended surfactant. Colloids Surf. A 2016, 494, 201–208. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B.; Wójcik, W. The wettability of polytetrafluoroethylene and polymethyl methacrylate by aqueous solution of two cationic surfactants mixture. J. Colloid Interface Sci. 2006, 293, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sritapunya, T.; Kitiyanan, B.; Scamehorn, J.F.; Grady, B.P.; Chavadej, S. Wetting of polymer surfaces by aqueous surfactant solutions. Colloids Surf. A 2012, 409, 30–41. [Google Scholar] [CrossRef]
- Szymczyk, K. Wettability of polymeric solids by ternary mixtures composed of hydrocarbon and fluorocarbon nonionic surfactants. J. Colloid Interface Sci. 2011, 363, 223–231. [Google Scholar] [CrossRef]
- Wang, C.; Cao, X.L.; Guo, L.L.; Xu, Z.C.; Zhang, L.; Gong, Q.T.; Zhang, L.; Zhao, S. Effect of Adsorption of Catanionic Surfactant Mixtures on Wettability of Quartz Surface. Colloids Surf. A 2016, 509, 564–573. [Google Scholar] [CrossRef]
- Yuan, F.Q.; Liu, D.D.; Guo, L.L.; Zhu, Y.W.; Xu, Z.C.; Huang, J.B.; Zhang, L.; Zhang, L. Effect of branched cationic and betaine surfactants on the wettability of a poly(tetrafluoroethylene) surface. Acta Phys. Chim. Sin. 2015, 31, 715–721. [Google Scholar]
- Hu, S.S.; Zhang, L.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhao, S. Effect of Benzyl-Substituted Alkyl Betaine on the Wettability of a Poly(tetrafluoroethylene) Surface. Acta Phys. Chim. Sin. 2015, 31, 1924–1931. [Google Scholar] [CrossRef]
- Hu, S.S.; Zhang, L.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhao, S. Wettability alteration by novel betaines at polymer-aqueous solution interfaces. Appl. Surf. Sci. 2015, 355, 868–877. [Google Scholar] [CrossRef]

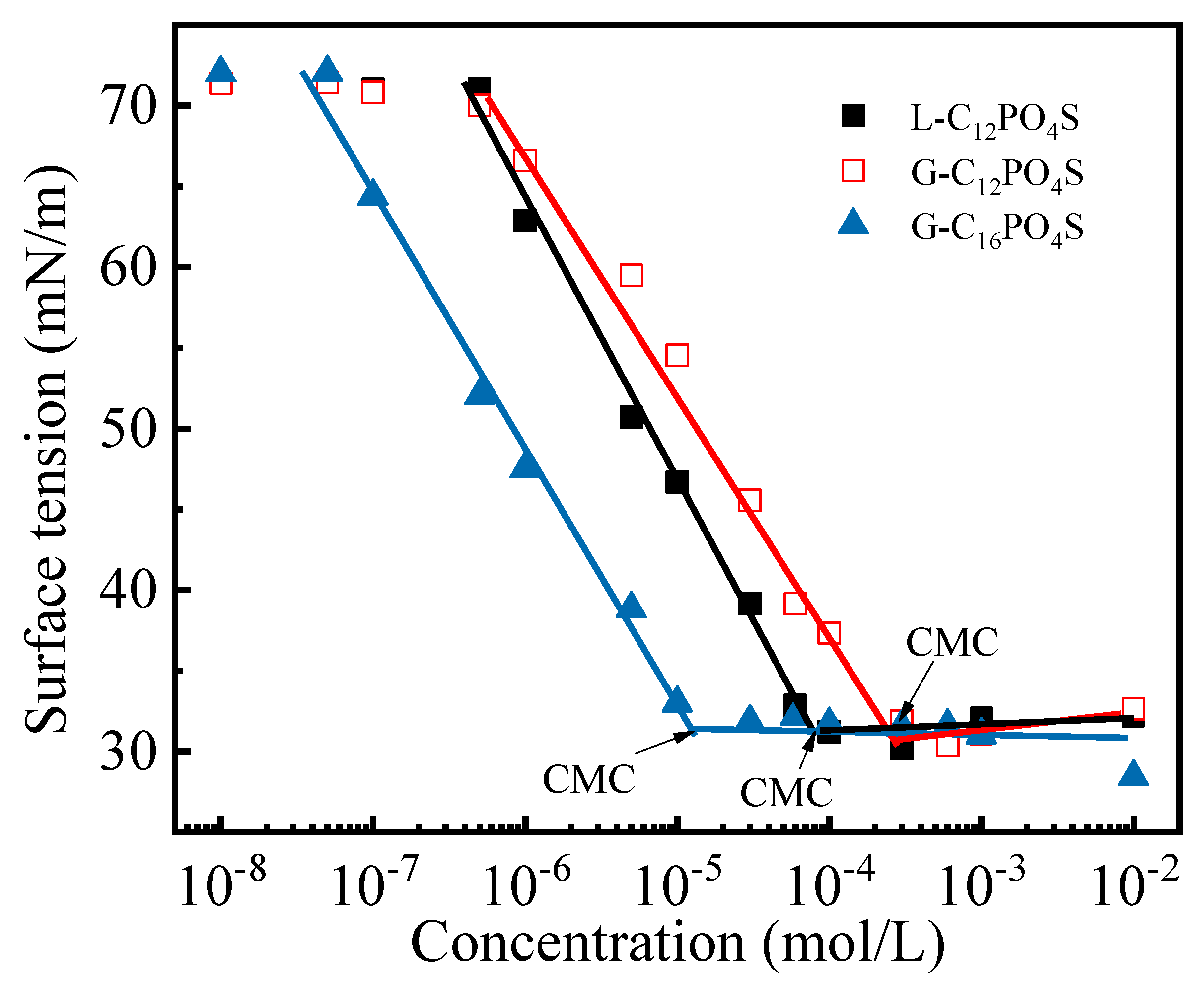
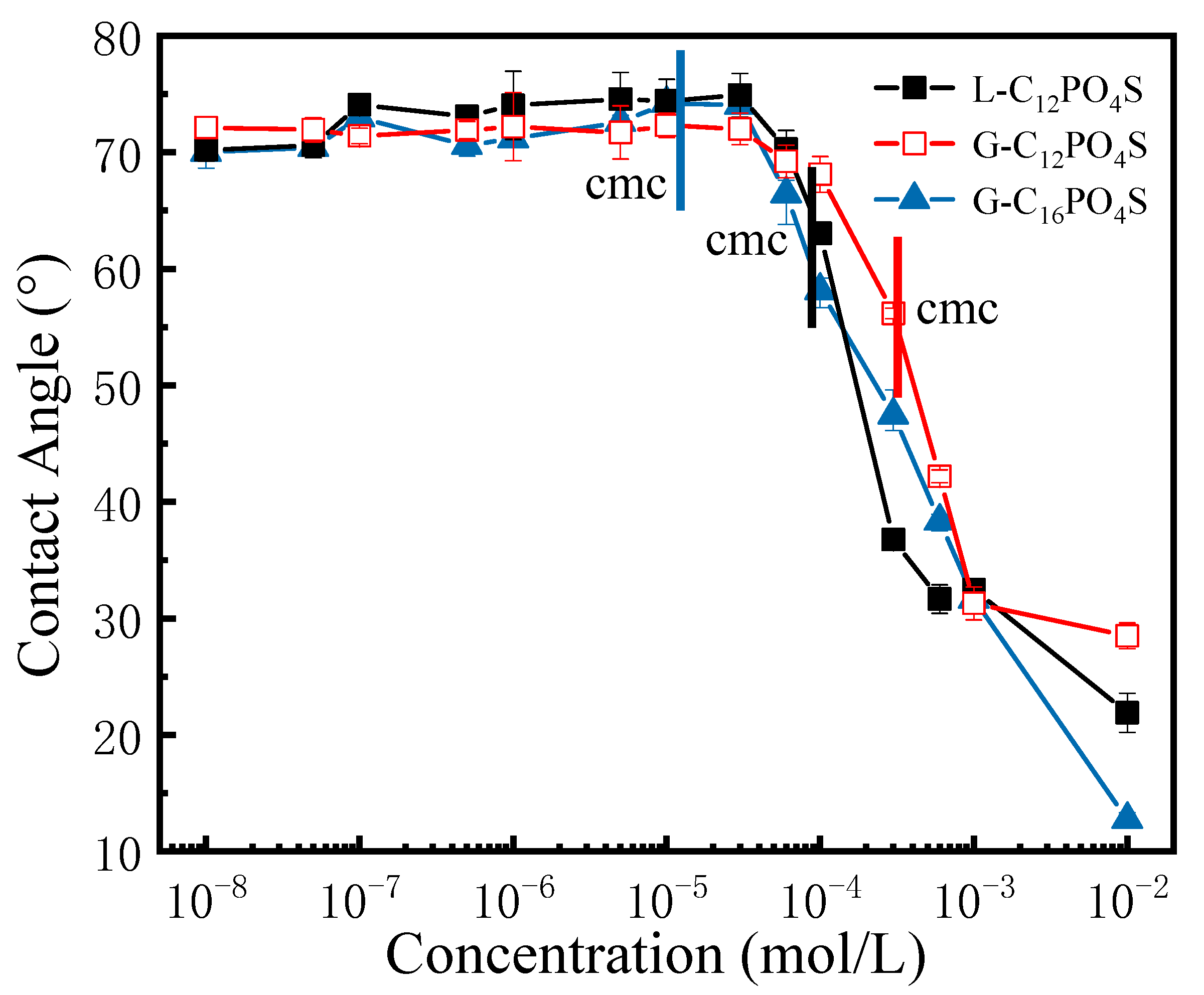
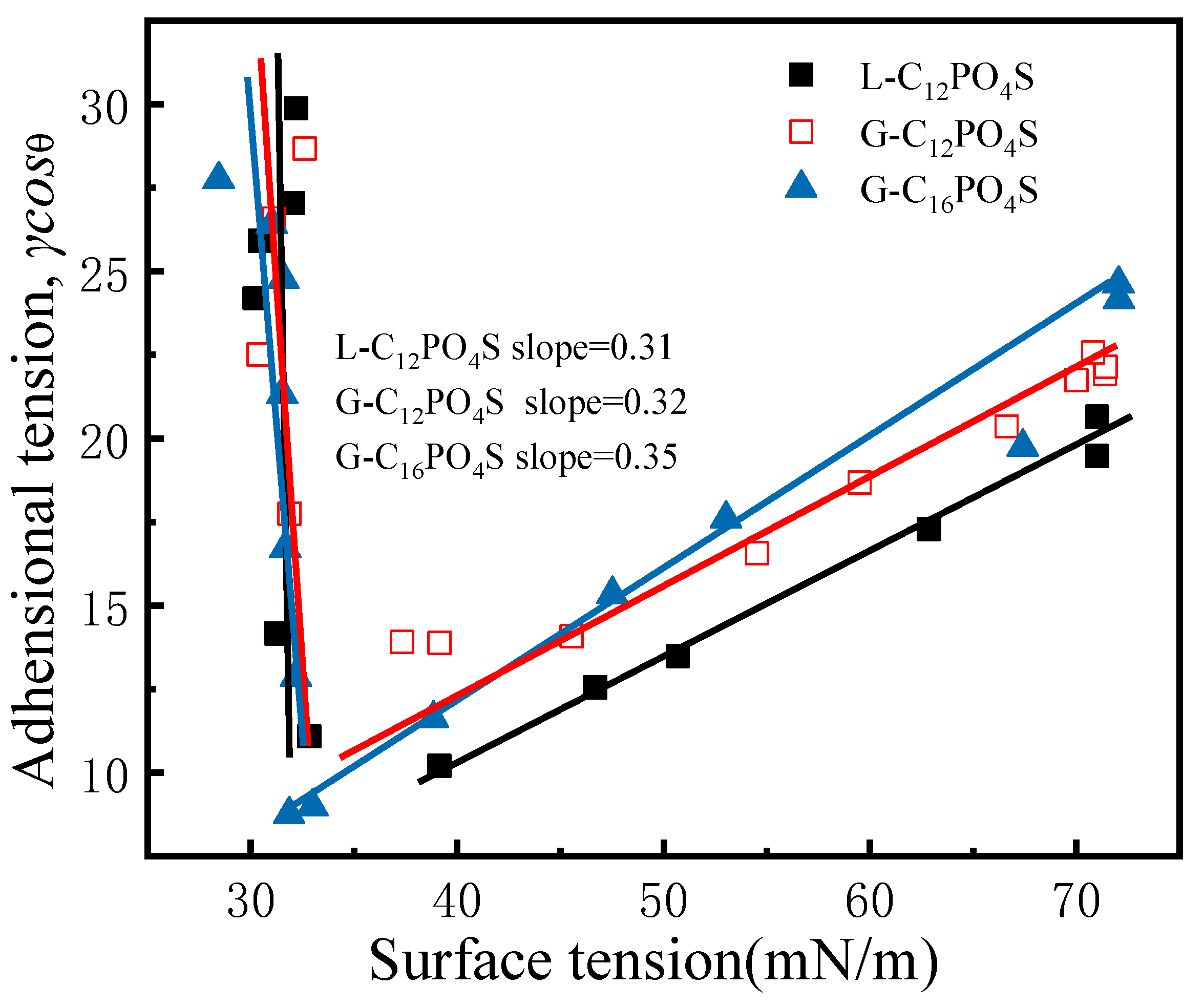
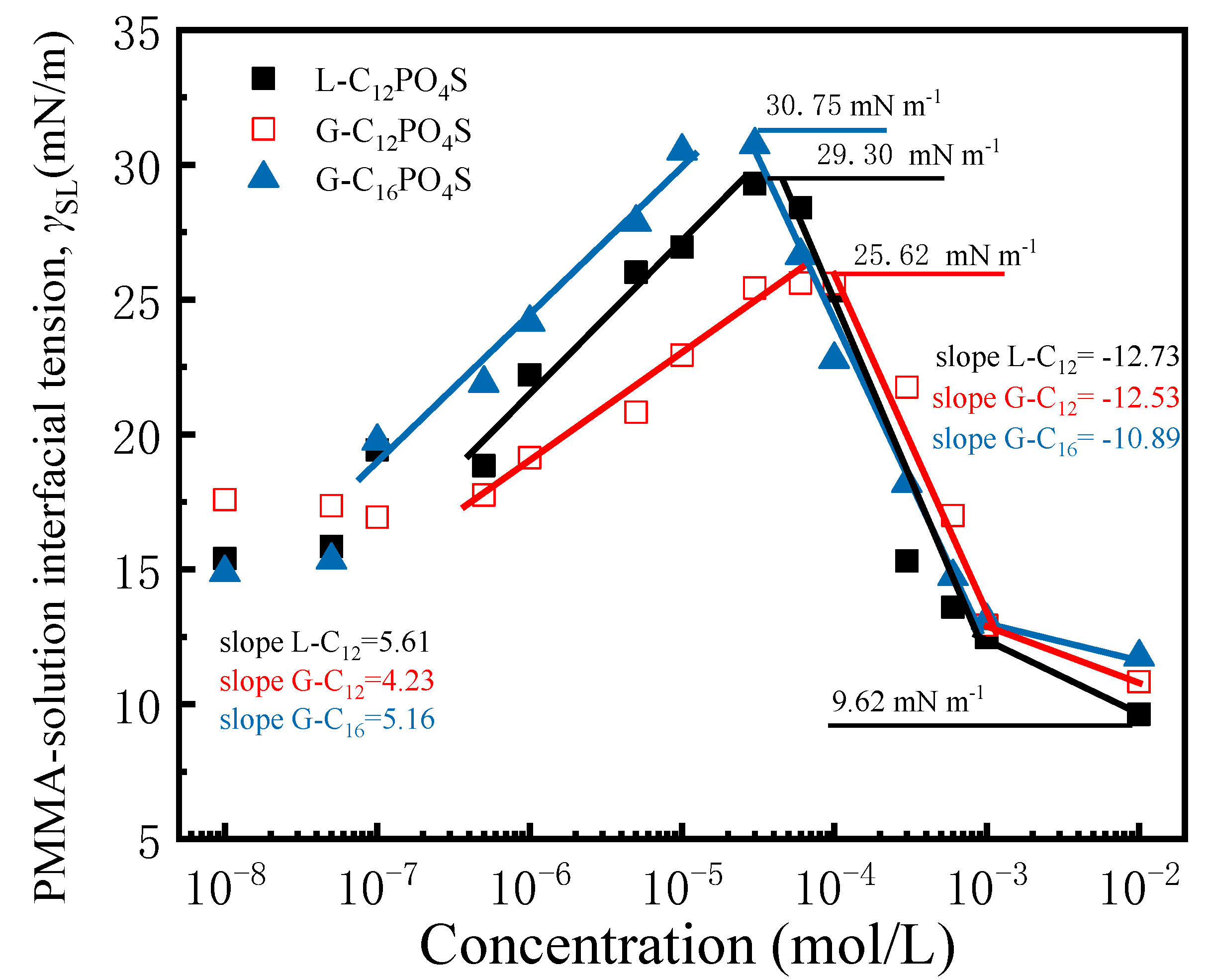

| Abrr. | CMC/(10−5 mol L−1) | γCMC/(mN m−1) | 1010Γmax1/(mol cm−2) | Amin1/(nm2) |
|---|---|---|---|---|
| L-C12PO4S | 9.9 | 30.1 | 1.47 | 1.13 |
| G-C12PO4S | 30.0 | 31.4 | 1.25 | 1.33 |
| G-C16PO4S | 1.5 | 30.7 | 1.24 | 1.34 |
| C12P4S33 | 42.0 | 40.0 | 1.00 | 1.66 |
| C12P8S33 | 7.8 | 35.5 | 1.08 | 1.54 |
| C12P12S33 | 4.3 | 33.7 | 1.01 | 1.64 |
| PP3S29 | 5.9 | 34.4 | 1.66 | 1.00 |
| PP6S29 | 3.0 | 34.1 | 1.29 | 1.29 |
| PP9S29 | 1.6 | 33.7 | 1.17 | 1.41 |
| PP12S29 | 1.2 | 36.9 | 0.97 | 1.71 |
| Surfactants | Slopes | Amin2/(nm2) |
|---|---|---|
| L-C12PO4S | 0.31 | 3.65 |
| G-C12PO4S | 0.32 | 4.16 |
| G-C16PO4S | 0.35 | 3.83 |
| CTAB34 | −0.34 | - |
| CPyB34 | −0.34 | - |
| C12(EDMAB)19 | −0.30 | - |
| BDDAB19 | −0.31 | - |
| C16PC27 | 0.045 | - |
| C16(EO)3PC27 | 0.033 | - |
| C16PB27 | 0.136 | - |
| C16(EO)3PB27 | 0.063 | - |
| C16GPC28 | 0.11 | 7.36 |
| C16G(EO)3PC28 | 0.11 | 6.36 |
| C16GPB28 | 0.11 | 6.00 |
| C16G(EO)3PB28 | 0.11 | 6.18 |
| C326 | 0.13 | 8.69 |
| C626 | 0.13 | 9.62 |
| 18C40 | 0.26 | 2.84 |
| 18S40 | 0.28 | 3.01 |
| BCB24 | 0.30 | 2.00 |
| BSB24 | 0.34 | 2.05 |
| Abbr. | 1010Γmax3/(mol/cm2) (<CMC) | Amin3/(nm2) (<CMC) | 1010Γmax4/(mol/cm2) (>CMC) | Amin4/(nm2) (>CMC) |
|---|---|---|---|---|
| L-C12PO4S | 0.48 | 3.44 | 1.10 | 1.51 |
| G-C12PO4S | 0.36 | 4.56 | 1.08 | 1.54 |
| G-C16PO4S | 0.44 | 3.74 | 0.94 | 1.77 |
| Surfactants | ΔγSL | The Minimum γSL |
|---|---|---|
| L-C12PO4S | 13.91 | 9.62 |
| G-C12PO4S | 8.69 | 10.83 |
| G-C16PO4S | 15.87 | 11.75 |
| C16PC27 | 1.1 | 21.75 |
| C16(EO)3PC27 | 4.25 | 26.8 |
| C16PB27 | 5.4 | 27.2 |
| C16(EO)3PB27 | 4.1 | 9.75 |
| C16GPC28 | 2.7 | 18.8 |
| C16G(EO)3PC28 | 4.4 | 18 |
| C16GPB28 | 5 | 18 |
| C16G(EO)3PB28 | 4.9 | 17.5 |
| C326 | 2.8 | 29.8 |
| C626 | 3 | 28.1 |
| 18C40 | 9 | 21 |
| 18S40 | 9.2 | 24 |
| BCB24 | 11.8 | 22.5 |
| BSB24 | 13 | 27.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Du, Y.; Zhang, L.; Ma, W.; Yan, F.; Zhang, L.; Zhao, S. Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains. Molecules 2021, 26, 863. https://doi.org/10.3390/molecules26040863
Jiang Q, Du Y, Zhang L, Ma W, Yan F, Zhang L, Zhao S. Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains. Molecules. 2021; 26(4):863. https://doi.org/10.3390/molecules26040863
Chicago/Turabian StyleJiang, Qin, Yuechun Du, Lei Zhang, Wangjing Ma, Feng Yan, Lu Zhang, and Sui Zhao. 2021. "Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains" Molecules 26, no. 4: 863. https://doi.org/10.3390/molecules26040863
APA StyleJiang, Q., Du, Y., Zhang, L., Ma, W., Yan, F., Zhang, L., & Zhao, S. (2021). Wettability of a Polymethylmethacrylate Surface by Extended Anionic Surfactants: Effect of Branched Chains. Molecules, 26(4), 863. https://doi.org/10.3390/molecules26040863






