Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil
Abstract
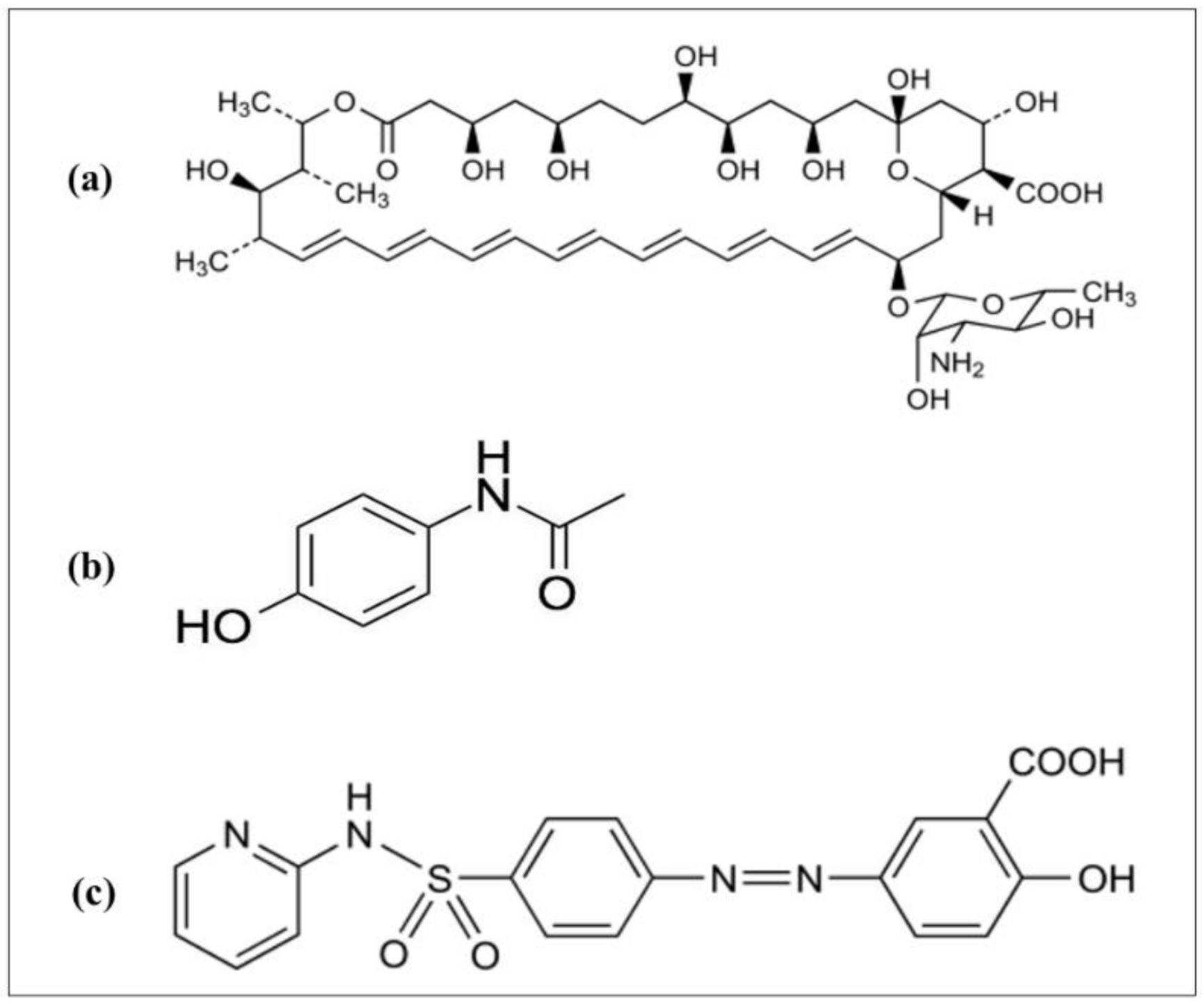
:1. Introduction
2. Results and Discussion
2.1. Physical Appearance of the Formulations
2.2. Nanoparticle Tracking Analysis (NTA)
2.3. Dynamic Light Scattering (DLS) Analysis
2.4. In Vitro Drug Release Studies
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of SLNs
3.3. Particle Size Analysis
3.4. FTIR Analysis
3.5. Drug Release Studies
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mühlen, A.Z.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Mã¼LlerR. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mehnert, W. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Porter, C.J.; Charman, W.N. In vitro assessment of oral lipid based formulations. Adv. Drug Deliv. Rev. 2001, 50, S127–S147. [Google Scholar] [CrossRef]
- Sek, L.; Porter, C.J.; Charman, W.N. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situ densitometric analysis. J. Pharm. Biomed. Anal. 2001, 25, 651–661. [Google Scholar] [CrossRef]
- Charman, W.N. Lipids, Lipophilic Drugs, and Oral Drug Delivery—Some Emerging Concepts. J. Pharm. Sci. 2000, 89, 967–978. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Current State-of-Art and New Trends on Lipid Nanoparticles (SLN and NLC) for Oral Drug Delivery. J. Drug Deliv. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Ribeiro, L.N.; Breitkreitz, M.C.; Guilherme, V.A.; Da Silva, G.H.; Couto, V.M.; Castro, S.R.; De Paula, B.O.; Machado, D.; De Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef]
- Stefanov, S.; Andonova, V. Natural Lipids as Structural Components of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Topical Delivery. Curr. Pharm. Des. 2020, 26, 4524–4535. [Google Scholar] [CrossRef]
- Mustiga, G.M.; Morrissey, J.; Stack, J.C.; Duval, A.; Royaert, S.; Jansen, J.; Bizzotto, C.; Villela-Dias, C.; Mei, L.; Cahoon, E.B.; et al. Identification of Climate and Genetic Factors That Control Fat Content and Fatty Acid Composition of Theobroma cacao L. Beans. Front. Plant Sci. 2019, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.; Nunes, F.M. Authentication of beeswax (Apis mellifera) by high-temperature gas chromatography and chemometric analysis. Food Chem. 2013, 136, 961–968. [Google Scholar] [CrossRef]
- Amekyeh, H.; Billa, N.; Yuen, K.-H.; Chin, S.L.S. A Gastrointestinal Transit Study on Amphotericin B-Loaded Solid Lipid Nanoparticles in Rats. AAPS PharmSciTech 2015, 16, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amekyeh, H.; Billa, N.; Roberts, C.J. Correlating gastric emptying of amphotericin B and paracetamol solid lipid nanoparticles with changes in particle surface chemistry. Int. J. Pharm. 2017, 517, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A. Preparation of finasteride capsules-loaded drug nanoparticles: Formulation, optimization, in vitro, and pharmacokinetic evaluation. Int. J. Nanomed. 2016, 11, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, S.; Hu, J.; Kearney, C.J.; Skaat, H.; Gu, L.; Gentili, M.; VanDenburgh, H.H.; Mooney, D.J. Sequential release of nanoparticle payloads from ultrasonically burstable capsules. Biomaterials 2016, 75, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, M.-G.; Tian, Y.-Q.; Zhang, J.; Li, S.-J.; Yang, D.-S.; Chen, Y.-D.; Liang, Z.-Y.; Cen, Y.-Y. Development of oral dispersible tablets containing prednisolone nanoparticles for the management of pediatric asthma. Drug Des. Dev. Ther. 2015, 9, 5815–5825. [Google Scholar] [CrossRef] [Green Version]
- Murgia, D.; Angellotti, G.; D’Agostino, F.; De Caro, V. Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization. Polymer 2019, 11, 1801. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.A.; Sarmento, B.; Das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control. Release 2016, 243, 43–53. [Google Scholar] [CrossRef]
- Mobley, W.; Schreier, H. Phase transition temperature reduction and glass formation in dehydroprotected lyophilized liposomes. J. Control. Release 1994, 31, 73–87. [Google Scholar] [CrossRef]
- Heiati, H.; Tawashi, R.; Phillips, N.C. Drug retention and stability of solid lipid nanoparticles containing azidothymidine palmitate after autoclaving, storage and lyophilization. J. Microencapsul. 1998, 15, 173–184. [Google Scholar] [CrossRef]
- Kim, J.-H.; Baek, J.-S.; Park, J.-K.; Lee, B.-J.; Kim, M.-S.; Hwang, S.-J.; Lee, J.-Y.; Cho, C.-W. Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release. Molecules 2017, 22, 2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazief, A.M.; Hassaan, P.S.; Khalifa, H.M.; Sokar, M.S.; El-Kamel, A.H. Lipid-Based Gliclazide Nanoparticles for Treatment of Diabetes: Formulation, Pharmacokinetics, Pharmacodynamics and Subacute Toxicity Study. Int. J. Nanomed. 2020, 15, 1129–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, L.N.D.M.; Couto, V.M.; Fraceto, L.F.; De Paula, E. Use of nanoparticle concentration as a tool to understand the structural properties of colloids. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonte, P.; Soares, S.; Costa, A.; Andrade, J.C.; Seabra, V.; Reis, S.; Sarmento, B. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2012, 2, 329–339. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef]
- Woitiski, C.B.; Carvalho, R.A.; Ribeiro, A.J.; Neufeld, R.J.; Veiga, F. Strategies toward the Improved Oral Delivery of Insulin Nanoparticles via Gastrointestinal Uptake and Translocation. BioDrugs 2008, 22, 223–237. [Google Scholar] [CrossRef]
- Reddy, L.H.; Vivek, K.; Bakshi, N.; Murthy, R. Tamoxifen Citrate Loaded Solid Lipid Nanoparticles (SLN™): Preparation, Characterization, In Vitro Drug Release, and Pharmacokinetic Evaluation. Pharm. Dev. Technol. 2006, 11, 167–177. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2010, 55, 495–503. [Google Scholar] [CrossRef]
- Riddick, T.M. Control of Colloid Stability through Zeta Potential; Livingston Publishing Company: Wynnewood, PA, USA, 1968. [Google Scholar]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Somani, A.A.; Thelen, K.; Zheng, S.; Trame, M.N.; Coboeken, K.; Meyer, M.; Schnizler, K.; Ince, I.; Willmann, S.; Schmidt, S. Evaluation of changes in oral drug absorption in preterm and term neonates for Biopharmaceutics Classification System (BCS) class I and II compounds. Br. J. Clin. Pharmacol. 2015, 81, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özyazıcı, M.; Gökçe, E.H.; Ertan, G. Release and diffusional modeling of metronidazole lipid matrices. Eur. J. Pharm. Biopharm. 2006, 63, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.-H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef]
- Nahar, M.; Jain, N.K. Preparation, Characterization and Evaluation of Targeting Potential of Amphotericin B-Loaded Engineered PLGA Nanoparticles. Pharm. Res. 2009, 26, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Al-Quadeib, B.T.; Radwan, M.A.; Siller, L.; Horrocks, B.R.; Wright, M.C. Stealth Amphotericin B nanoparticles for oral drug delivery: In vitro optimization. Saudi Pharm. J. 2015, 23, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehenni, L.; Lahiani-Skiba, M.; Ladam, G.; Hallouard, F.; Skiba, M. Preparation and Characterization of Spherical Amorphous Solid Dispersion with Amphotericin B. Pharmaceutics 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-L.; Lin, S.-Y.; Wei, Y.-S. Transformation of metastable forms of acetaminophen studied by thermal Fourier transform infrared (FT-IR) microspectroscopy. Chem. Pharm. Bull. 2002, 50, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.A.; Khames, A.; Alrobaian, M.M.; Hamaidi, M.; Abourehab, M.A. Glucosamine-paracetamol spray-dried solid dispersions with maximized intrinsic dissolution rate, bioavailability and decreased levels of in vivo toxic metabolites. Drug Des. Dev. Ther. 2018, 12, 3071–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, A.A.; Mohamed, G.G.; Hosny, W.M.; El-Mawgood, M.A. Synthesis, Spectroscopic and Thermal Characterization of New Sulfasalazine Metal Complexes. Synth. React. Inorg. Met. Chem. 2005, 35, 483–490. [Google Scholar] [CrossRef]






| Formulation | R2 | n Value | |||
|---|---|---|---|---|---|
| First Order | Higuchi | Hixson-Crowell | Korsmeyer–Peppas | ||
| Fresh AMB-SLNs | 0.8997 | 0.9889 | 0.8964 | 0.9704 | 0.5960 1 |
| Lyophilized AMB-SLNs | 0.9206 | 0.9826 | 0.9174 | 0.9606 | 0.6740 1 |
| Fresh PAR-SLNs | 0.9360 | 0.9858 | 0.9077 | 0.9728 | 0.4963 |
| Lyophilized PAR-SLNs | 0.9820 | 0.9825 | 0.9592 | 0.957 | 0.6392 |
| Fresh SSZ-SLNs | 0.9631 | 0.9743 | 0.9559 | 0.9563 | 0.7565 1 |
| Lyophilized SSZ-SLNs | 0.8932 | 0.9765 | 0.8821 | 0.9297 | 0.6035 1 |
| Model | Expression |
|---|---|
| Zero order | Q = Kt |
| First order log | QR = Kt/2.303 |
| Higuchi | |
| Hixson–Crowell | |
| Korsmeyer–Peppas | log Q = log K + nlog t |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amekyeh, H.; Billa, N. Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil. Molecules 2021, 26, 908. https://doi.org/10.3390/molecules26040908
Amekyeh H, Billa N. Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil. Molecules. 2021; 26(4):908. https://doi.org/10.3390/molecules26040908
Chicago/Turabian StyleAmekyeh, Hilda, and Nashiru Billa. 2021. "Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil" Molecules 26, no. 4: 908. https://doi.org/10.3390/molecules26040908
APA StyleAmekyeh, H., & Billa, N. (2021). Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil. Molecules, 26(4), 908. https://doi.org/10.3390/molecules26040908







