Construction of Supramolecular Polymers with Different Topologies by Orthogonal Self-Assembly of Cryptand–Paraquat Recognition and Metal Coordination
Abstract
1. Introduction
2. Results and Discussion
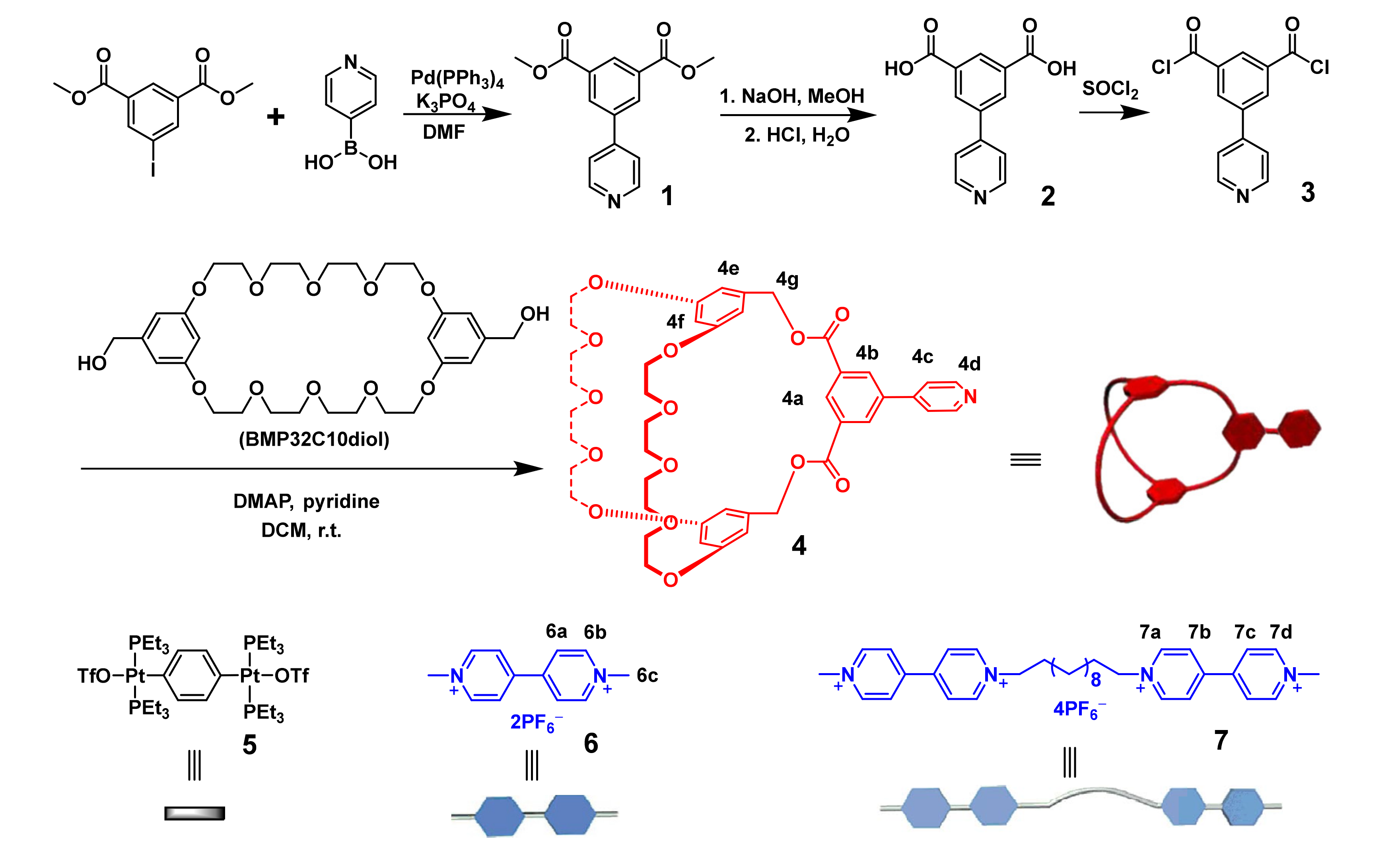
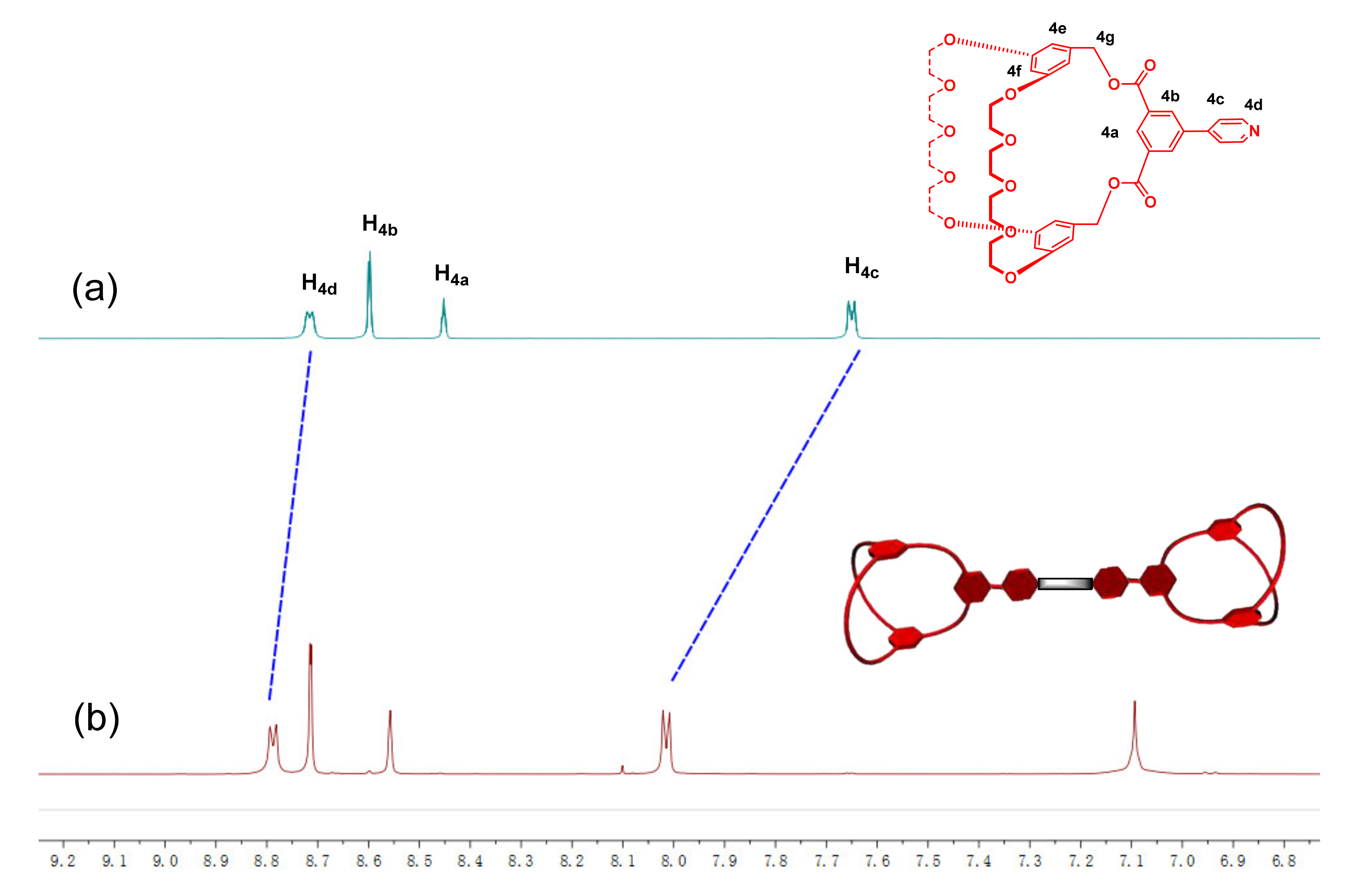
2.1. Design, Synthesis, and Characterization of Cryptand 4
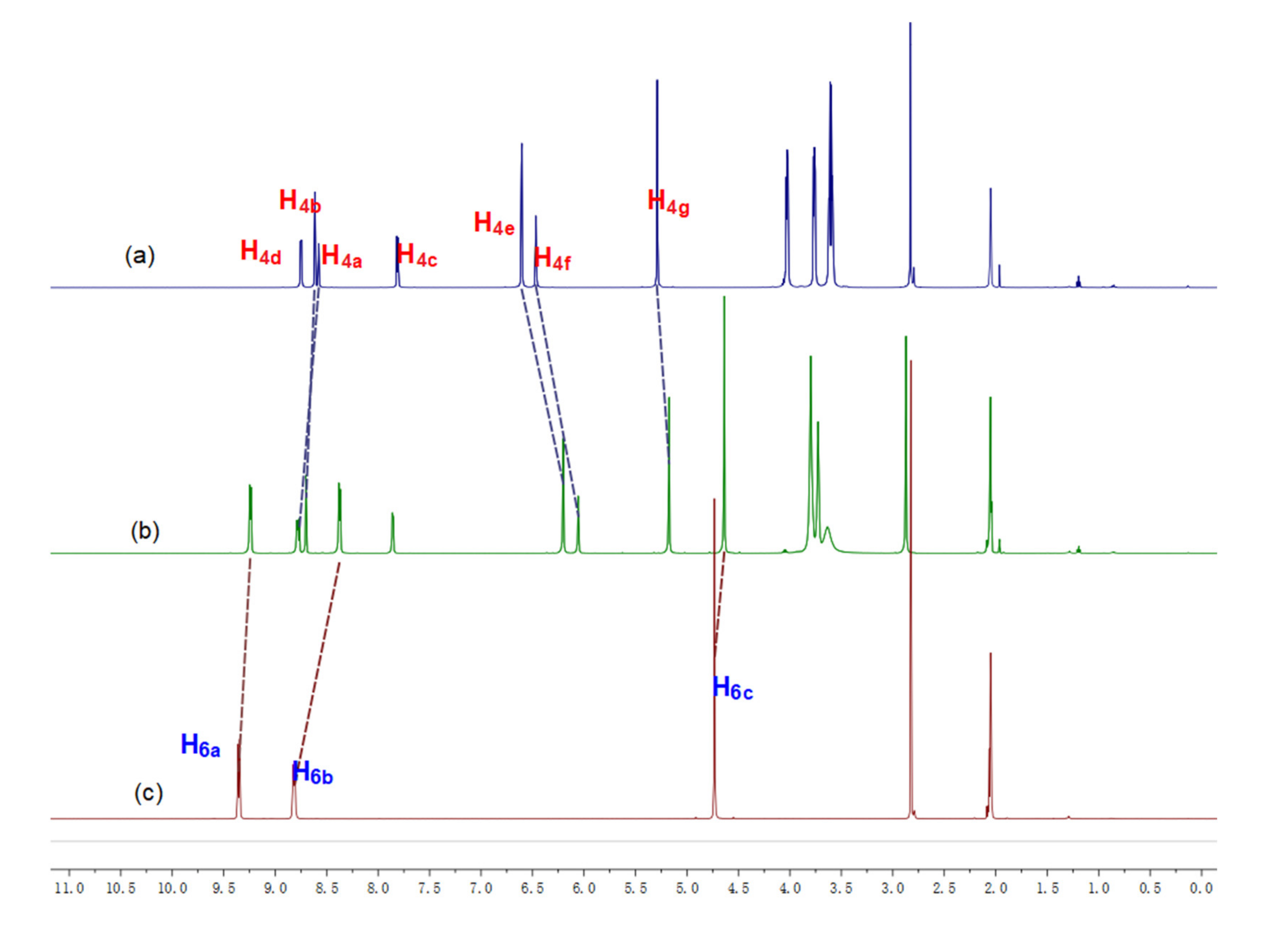
2.2. Investigation of the Host–Guest Complexation between Cryptand 4 and Paraquat
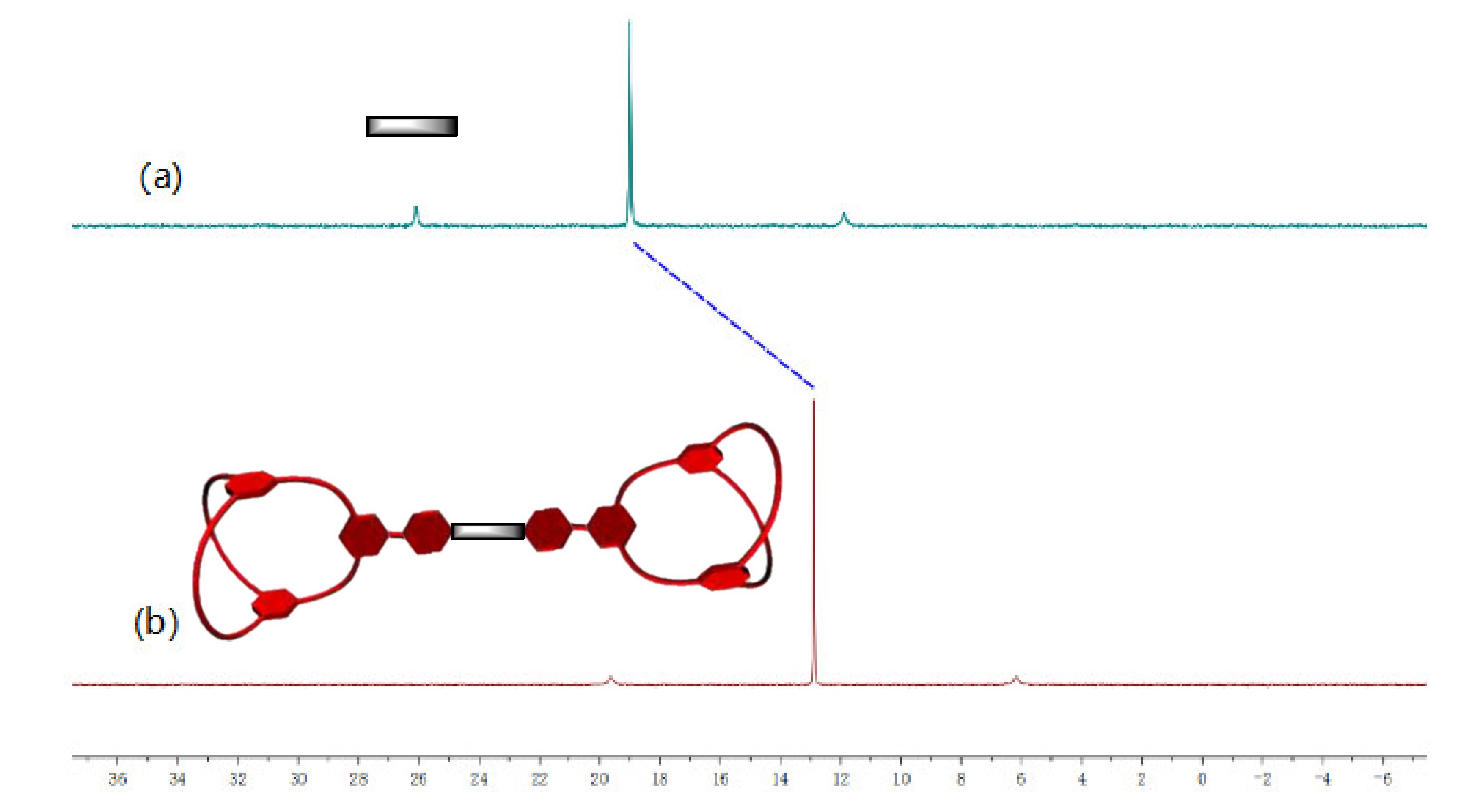
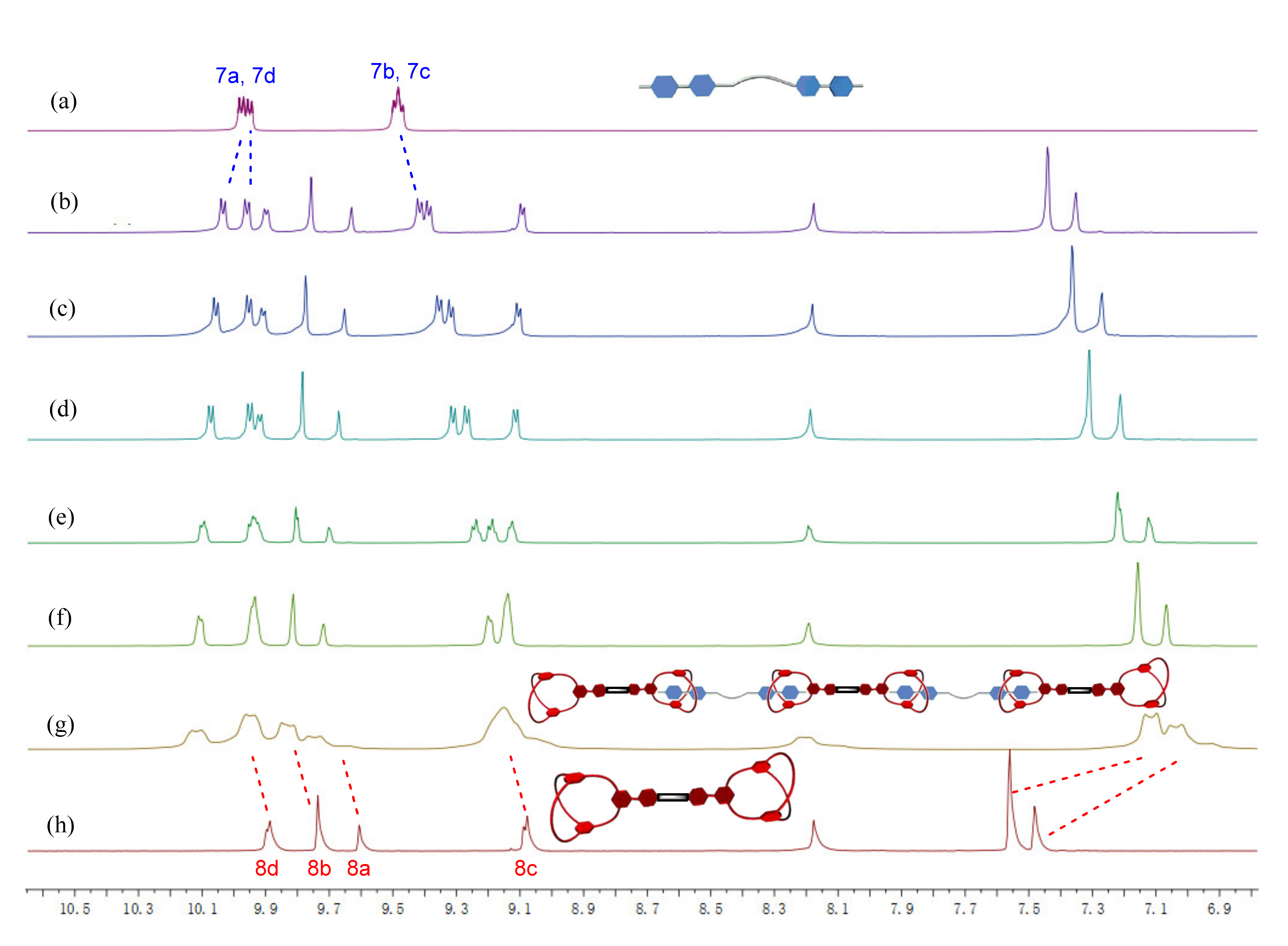
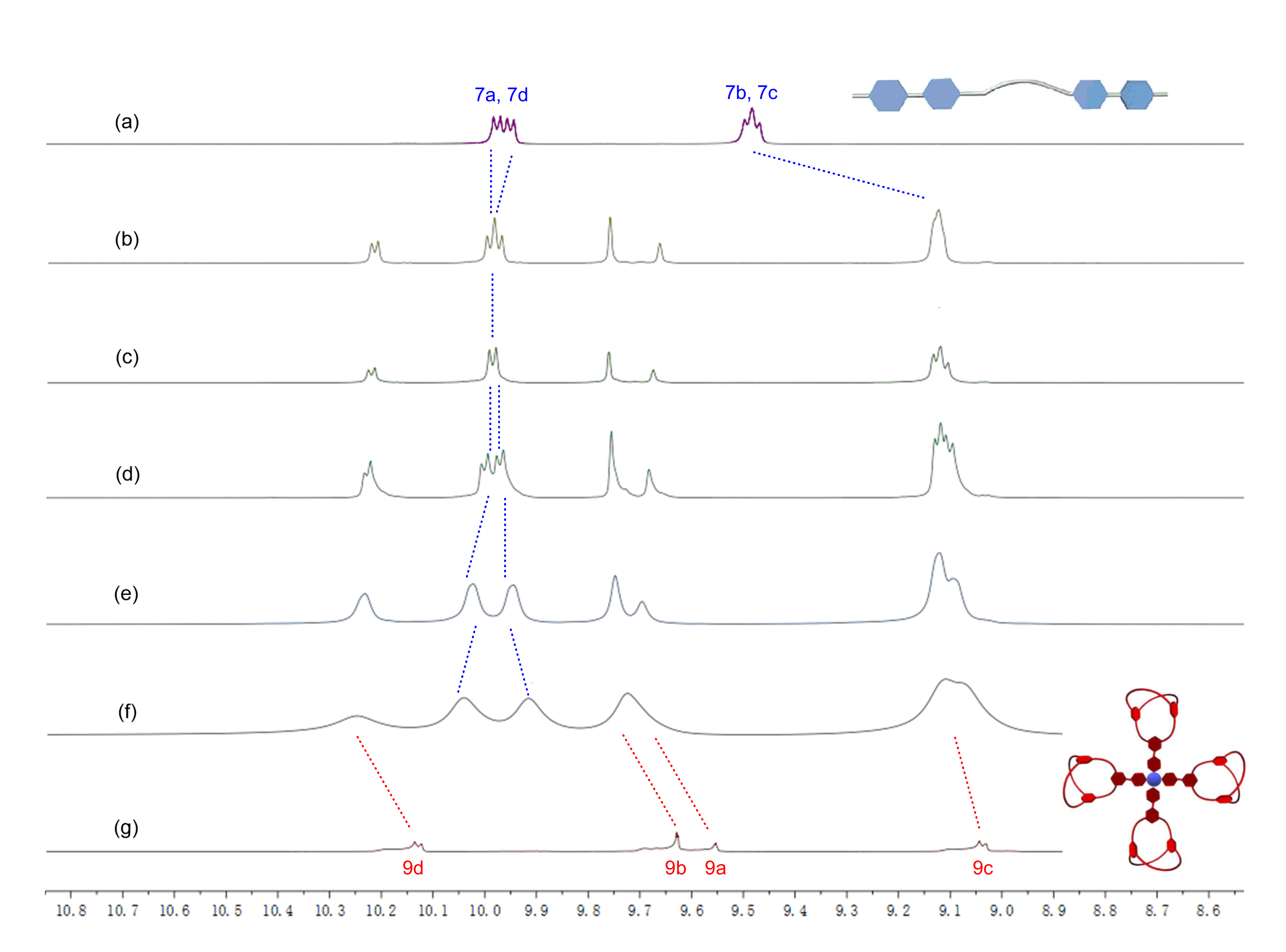
2.3. Preparation and Characterization of Di-Cryptand 8 and Tetra-Cryptand 9
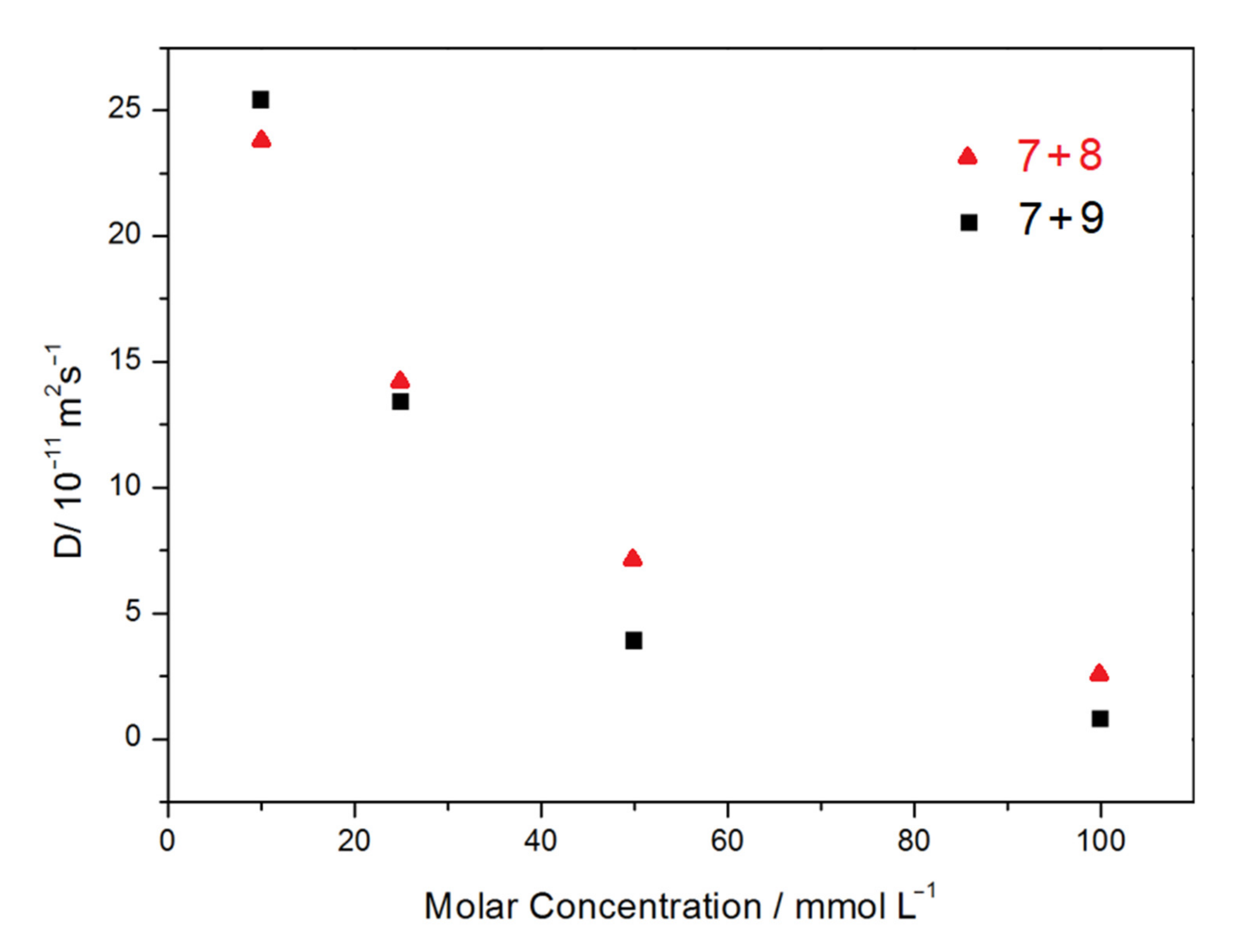
2.4. Formation and Characterization of Supramolecular Polymers
3. Materials and Methods
3.1. Synthesis of 1
3.2. Synthesis of 2
3.3. Synthesis of 3
3.4. Synthesis of 4
3.5. Synthesis of 8
3.6. Synthesis of 9
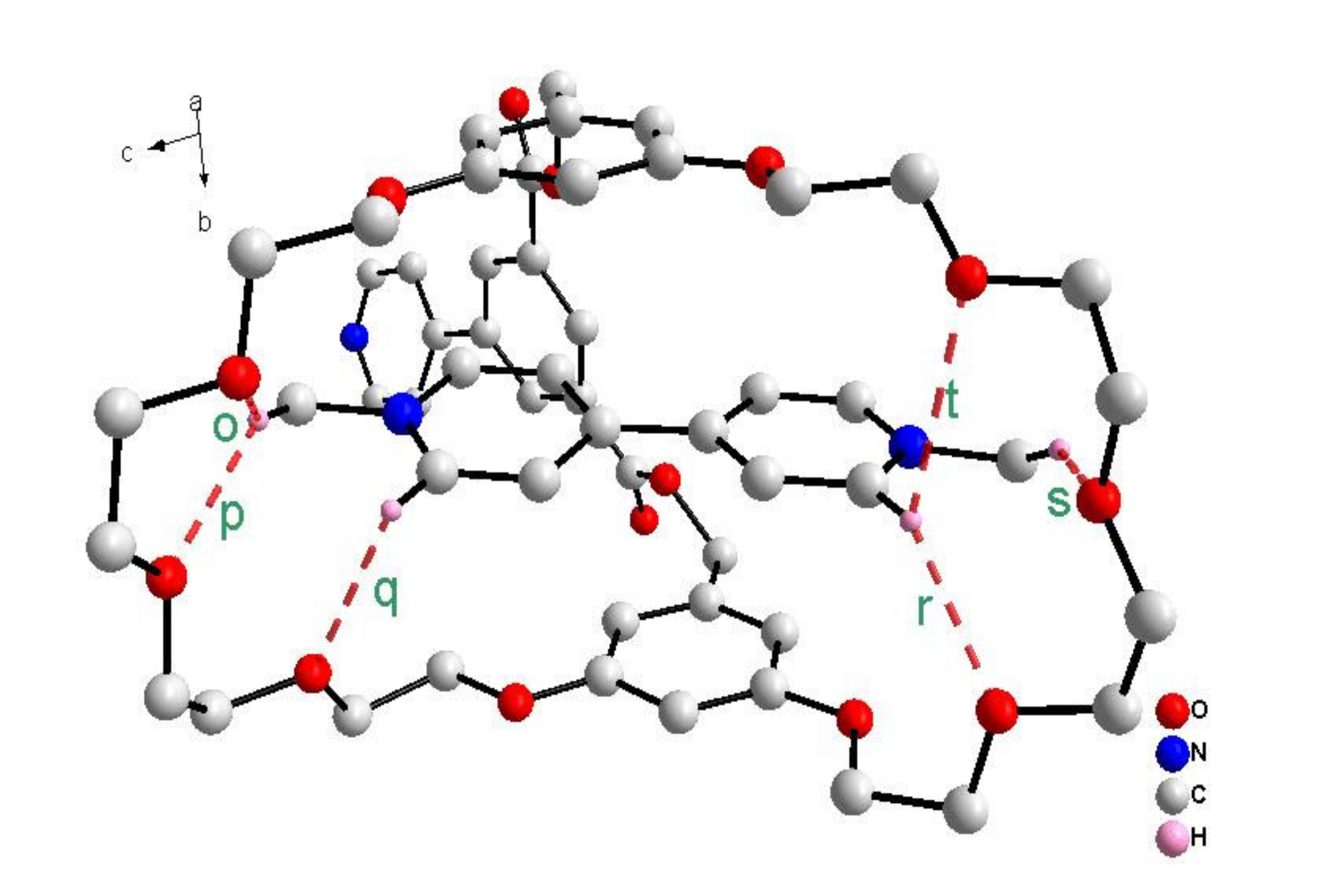
3.7. X-ray Crystal Data for 4⊃6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Huang, F. Stimuli-Responsive Supramolecular Polymeric Materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Zhang, X. Characterization of Supramolecular Polymers. Chem. Soc. Rev. 2012, 41, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, H. Stimuli-Responsive Supramolecular Polymers in Aqueous Solution. Acc. Chem. Res. 2014, 47, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Goor, O.J.G.M.; Hendrikse, S.I.S.; Dankers, P.Y.W.; Meijer, E.W. From Supramolecular Polymers to Multi-Component Biomaterials. Chem. Soc. Rev. 2017, 46, 6621–6637. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Wang, X.-Q.; Chen, L.-J.; Hu, Y.-X.; Yin, G.-Q.; Xu, L.; Jiang, B.; Yang, H.-B. Supramolecular Polymer Cross-Linked by Discrete Tris-[2]Pseudorotaxane Metallacycles and Its Redox-Responsive Behavior. Inorg. Chem. 2018, 57, 15414–15420. [Google Scholar] [CrossRef]
- Ligthart, G.B.W.L.; Ohkawa, H.; Sijbesma, R.P.; Meijer, E.W. Complementary Quadruple Hydrogen Bonding in Supramolecular Copolymers. J. Am. Chem. Soc. 2004, 127, 810–811. [Google Scholar] [CrossRef]
- Kolomiets, E.; Lehn, J.-M. Double Dynamers: Molecular and Supramolecular Double Dynamic Polymers. Chem. Commun. 2005, 1519–1521. [Google Scholar] [CrossRef]
- Park, T.; Zimmerman, S.C. Formation of a Miscible Supramolecular Polymer Blend through Self-Assembly Mediated by a Quadruply Hydrogen-Bonded Heterocomplex. J. Am. Chem. Soc. 2006, 128, 11582–11590. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Pollock, B.J.; Cook, T.R.; Chen, J.; Zhang, Y.; Ji, X.; Yu, Y.; Huang, F.; Stang, P.J. Supramolecular polymers with tunable topologies via hierarchical coordination-driven self-assembly and hydrogen bonding interfaces. Proc. Nat. Acad. Sci. USA 2013, 110, 15585–15590. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, J.A.; Mabesoone, M.F.J.; Iglesias, M.G.; Huizinga, A.; Meijer, E.W.; Palmans, A.R.A. Selenoamides Modulate Dipole-Dipole Interactions in Hydrogen Bonded Supramolecular Polymers of 1,3,5-Substituted Benzenes. Chem. Commun. 2019, 55, 14906–14909. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wu, H.; Sun, G.; Diao, K.; Wei, X.; Li, Z.-Y.; Suna, X.-Q.; Wang, L. An Efficient Artificial Light-Harvesting System with Tunable Emission in Water Constructed from a H-Bonded AIE Supramolecular Polymer and Nile Red. Chem. Commun. 2020, 56, 12021–12024. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Tan, X.; Wang, Z.; Zhang, X. Cucurbit[8]uril-Based Supramolecular Polymers: Promoting Supramolecular Polymerization by Metal-Coordination. Chem. Commun. 2013, 49, 5766–5768. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Yan, X.; Zhou, Z.; Saha, M.L.; Wang, Y.-C.; Stang, P.J. Fluorescent Metallacycle-Cored Polymers via Covalent Linkage and Their Use as Contrast Agents for Cell Imaging. Proc. Nat. Acad. Sci. USA 2016, 116, 11100–11105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, Y.; Liu, Y.; Zou, G.; Gao, Z.; Wang, F. Cooperative Supramolecular Polymerization of Fluorescent Platinum Acetylides for Optical Waveguide Applications. Angew. Chem. Int. Ed. 2017, 56, 12466–12470. [Google Scholar] [CrossRef] [PubMed]
- Abend, M.; Kunz, C.S.; Stumpf, S.; Gräf, S.; Zechel, S.; Müller, F.A.; Hager, M.D.; Schubert, U.S. Femtosecond Laser-Induced Scratch Ablation as an Efficient New Method to Evaluate the Self-Healing Behavior of Supramolecular Polymers. J. Mater. Chem. A 2019, 7, 2148–2155. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Zhao, J.; Yan, X. Engineering Orthogonality in the Construction of an Alternating Rhomboidal Copolymer with High Fidelity via Integrative Self-Sorting. Polym. Chem. 2020, 11, 367–374. [Google Scholar] [CrossRef]
- Wittmann, B.; Wenzel, F.A.; Wiesneth, S.; Haedler, A.T.; Drechsler, M.; Kreger, K.; Köhler, J.; Meijer, E.W.; Schmidt, H.-W.; Hildner, R. Enhancing Long-Range Energy Transport in Supramolecular Architectures by Tailoring Coherence Properties. J. Am. Chem. Soc. 2020, 142, 8323–8330. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host–Guest Interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, B.; Wang, F.; Huang, F. Supramolecular Polymers Constructed from Macrocycle-Based Host–Guest Molecular Recognition Motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Pillararene-Based Supramolecular Polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Ni, X.-L.; Zhang, H.; Wei, P.; Liu, J.; Xing, H.; Peng, H.-Q.; Lam, J.W.Y.; Zhang, P.; et al. Supramolecular Polymerization with Dynamic Self-Sorting Sequence Control. Macromolecules 2019, 52, 8814–8825. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Jiang, S.-T.; Zheng, W.; Ji, T.; Yin, G.-Q.; Li, X.; Liao, X. Supramolecular Metallacyclic Hydrogels with Tunable Strength Switched by Host-Guest Interactions. Polym. Chem. 2020, 11, 882–888. [Google Scholar] [CrossRef]
- Fang, R.; Liu, Y.; Wang, Z.; Zhang, X. Water-Soluble Supramolecular Hyperbranched Polymers Based on Host-Enhanced π-π Interaction. Polym. Chem. 2013, 4, 900–903. [Google Scholar] [CrossRef]
- Albano, G.; Pescitelli, G.; Bari, L.D. Chiroptical Properties in Thin Films of π-Conjugated Systems. Chem. Rev. 2020, 120, 10145–10243. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef]
- Yoshida, T.; Bera, M.K.; Narayana, Y.S.L.V.; Mondal, S.; Abe, H.; Higuchi, M. Electrochromic Os-Based Metallo-Supramolecular Polymers: Electronic State Tracking by in Situ XAFS, IR, and Impedance Spectroscopies. RSC Adv. 2020, 10, 24691–24696. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.; Yang, Y.; Li, H.; Xu, F.; Duan, Z.; Liang, T.; Wen, H.; Tian, W. Architecture Transition of Supramolecular Polymers through Hierarchical Self-Assembly: From Supramolecular Polymers to Fluorescent Materials. Polym. Chem. 2020, 11, 5642–5648. [Google Scholar] [CrossRef]
- Ji, X.; Dong, S.; Wei, P.; Xia, D.; Huang, F. A Novel Diblock Copolymer with a Supramolecular Polymer Block and a Traditional Polymer Block: Preparation, Controllable Self-Assembly in Water, and Application in Controlled Release. Adv. Mater. 2013, 25, 5725–5729. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, C.; Weder, C.; de Espinosa, L.M. Supramolecular Polymer Adhesives: Advanced Materials Inspired by Nature. Chem. Soc. Rev. 2016, 45, 342–358. [Google Scholar] [CrossRef]
- Sun, C.-L.; Peng, H.-Q.; Niu, L.-Y.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Artificial Light-Harvesting Supramolecular Polymeric Nanoparticles Formed by Pillar[5]arene-Based Host–Guest Interaction. Chem. Commun. 2018, 54, 1117–1120. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular Polymer Networks: Hydrogels and Bulk Materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef]
- Sun, X.-W.; Wang, Z.-H.; Li, Y.-J.; Yang, H.-L.; Gong, G.-F.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Lin, Q. Transparency and AIE Tunable Supramolecular Polymer Hydrogel Acts as TEA-HCl Vapor Controlled Smart Optical Material. Soft Matter 2020, 16, 5734–5739. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Zhou, Y.; Yu, G. Construction of a Pillar[5]arene-Based Linear Supramolecular Polymer and a Photo-Responsive Supramolecular Network. Polym. Chem. 2014, 5, 6645–6650. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, Y.; Yang, Z.; Wang, F. Responsive Supramolecular Polymers Based on the Bis[alkynylplatinum(II)] Terpyridine Molecular Tweezer/Arene Recognition Motif. Angew. Chem. Int. Ed. 2014, 53, 6090–6094. [Google Scholar] [CrossRef] [PubMed]
- Price, T.L.; Gibson, H.W. Supramolecular Pseudorotaxane Polymers from Biscryptands and Bisparaquats. J. Am. Chem. Soc. 2018, 140, 4455–4465. [Google Scholar] [CrossRef]
- Fu, H.-G.; Chen, Y.; Liu, Y. Multistimuli-Responsive and Photocontrolled Supramolecular Luminescent Gels Constructed by Anthracene-Bridged Bis(dibenzo-24-crown-8) with Secondary Ammonium Salt Polymer. ACS Appl. Mater. Interfaces 2019, 11, 16117–16122. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, K.; Jin, L.; Xie, C.; Li, S. Preparation of a Mechanically Interlocked Polymer from a Linear Supramolecular Polymer. Org. Chem. Front. 2020, 7, 1453–1462. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Ding, X.; Dong, S.; Liu, M.; Zheng, B.; Li, S.; Wu, L.; Yu, Y.; Gibson, H.W.; et al. Metal Coordination Mediated Reversible Conversion between Linear and Cross-Linked Supramolecular Polymer. Angew. Chem. Int. Ed. 2010, 49, 1090–1094. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, X.; Jiang, J. A Cross-Linked Supramolecular Polymer Constructed from Pillar[5]arene and Porphyrine via Host–Guest Interactions. Polym. Chem. 2015, 6, 5015–5020. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Deng, Y.; Luo, Z.; Zhang, Q.; Dong, S.; Han, C. Preparation of Cross-linked Supramolecular Polymers based on Benzo-21-crown-7/secondary Ammonium Salt Host-guest Interactions. Chem. Commun. 2018, 54, 12459–12462. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.; Li, G.; Liu, K.; Zhang, Z.; Li, P.; Dong, S.; Yu, W.; Huang, F.; Yan, X. A Self-Cross-Linking Supramolecular Polymer Network Enabled by Crown-Ether-Based Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Nagvekar, D.S.; Slebodnick, C.; Gibson, H.W. A Supramolecular Triarm Star Polymer from a Homotritopic Tris (Crown Ether) Host and a Complementary Monotopic Paraquat-Terminated Polystyrene Guest by a Supramolecular Coupling Method. J. Am. Chem. Soc. 2005, 127, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.M.; Zimmerman, S.C. Supramolecular Star Polymers. Increased Molecular Weight with Decreased Polydispersity through Self-Assembly. J. Am. Chem. Soc. 2007, 129, 14534–14535. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Yang, F.; Shen, H.; You, Y.; Wu, D. Self-Assembly Behavior of a Linear-Star Supramolecular Amphiphile Based on Host-Guest Complexation. Langmuir 2014, 30, 13014–13020. [Google Scholar] [CrossRef]
- Fernandez, G.; Perez, E.M.; Sanchez, L.; Martin, N. An Electroactive Dynamically Polydisperse Supramolecular Dendrimer. J. Am. Chem. Soc. 2008, 130, 2410–2411. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, B.; Chen, J.; Dong, S.; Ma, Z.; Huang, F.; Gibson, H.W. A Hyperbranched, Rotaxane-Type Mechanically Interlocked Polymer. J. Polym. Sci. Polym. Chem. 2010, 48, 4067–4073. [Google Scholar] [CrossRef]
- Dong, R.J.; Liu, Y.; Zhou, Y.F.; Yan, D.Y.; Zhu, X.Y. Photo-Reversible Supramolecular Hyperbranched Polymer Based on Host-Guest Interactions. Polym. Chem. 2011, 2, 2771–2774. [Google Scholar] [CrossRef]
- Ge, Z.S.; Liu, H.; Zhang, Y.F.; Liu, S.Y. Supramolecular Thermoresponsive Hyperbranched Polymers Constructed from Poly(N-Isopropylacrylamide) Containing One Adamantyl and Two β-Cyclodextrin Terminal Moieties. Macromol. Rapid Commun. 2011, 32, 68–73. [Google Scholar] [CrossRef]
- Niu, Z.; Gibson, H.W. Polycatenanes. Chem. Rev. 2009, 109, 6024–6046. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, F.; Dong, S.; Huang, F. Supramolecular Polymers Constructed by Crown Ether-Based Molecular Recognition. Chem. Soc. Rev. 2012, 41, 1621–1636. [Google Scholar] [CrossRef]
- Suzuki, S.; Ishiwari, F.; Nakazono, K.; Takata, T. Reversible Helix-Random Coil Transition of Poly(m-phenylenediethynylene) by a Rotaxane Switch. Chem. Commun. 2012, 48, 6478–6480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H.W. Stimuli-Responsive Host–Guest Systems Based on the Recognition of Cryptands by Organic Guests. Acc. Chem. Res. 2014, 47, 1995–2005. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, S.-P.; Zhu, B.; Cook, T.R.; Wu, J.; Li, S.; Stang, P.J. Self-Assembly of [3]Catenanes and a [4]Molecular Necklace Based on a Cryptand/Paraquat Recognition Motif. Org. Lett. 2015, 17, 2804–2807. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yao, C.; Cao, Y.; Wang, Q.; Pan, Y.; Jiang, J.; Wang, L. 4-Methylcoumarin-Bridged Fluorescent Responsive Cryptand: From [2+2] Photodimerization to Supramolecular Polymer. Chem. Commun. 2016, 52, 8715–8718. [Google Scholar] [CrossRef] [PubMed]
- Price, T.L.; Wessels, H.R., Jr.; Slebodnick, C.; Gibson, H.W. High-Yielding Syntheses of Crown Ether-Based Pyridyl Cryptands. J. Org. Chem. 2017, 82, 8117–8122. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.-Z.; Shao, Y.-G.; Zhang, Z.; Shen, Y.-F.; Huang, X.-C.; Zhang, P.-L.; Li, S. Synthesis of Catenanes from a BMP32C10-Based Cryptand Tuned by the Linkage Length of Paraquat Salts. Synthesis 2020. [Google Scholar] [CrossRef]
- Wei, P.; Yan, X.; Huang, F. Supramolecular Polymers Constructed by Orthogonal Self-Assembly Based on Host-Guest and Metal-Ligand Interactions. Chem. Soc. Rev. 2015, 44, 815–832. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Zhu, B.-Y.; Wang, S.-P.; Zhu, B.; Ye, Y.; Wu, J. Self-assembly directed and regulated by metal-ligand coordination. Science 2018, 360, 14–17. [Google Scholar]
- Qiu, S.; Gao, Z.; Yan, F.; Yuan, H.; Wang, J.; Tian, W. 1,8-Dioxapyrene-Based Electrofluorochromic Supramolecular Hyperbranched Polymers. Chem. Commun. 2020, 56, 383–386. [Google Scholar] [CrossRef]
- Jeyakkumar, P.; Liang, Y.; Guo, M.; Lu, S.; Xu, D.; Li, X.; Guo, B.; He, G.; Chu, D.; Zhang, M. Emissive Metallacycle-Crosslinked Supramolecular Networks with Tunable Crosslinking Densities for Bacterial Imaging and Killing. Angew. Chem. Int. Ed. 2020, 59, 15199–15203. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Q.; Li, D.; Dong, S.; Zhao, W.; Stang, P.J. Rational Design and Bulk Synthesis of Water-Containing Supramolecular Polymers. ACS Appl. Mater. Interfaces 2020, 12, 38700–38707. [Google Scholar] [CrossRef]
- Gibson, H.W.; Nagvekar, D.S. Difunctional Derivatives of Bis(m-phenylene)-32-Crown-10. Can. J. Chem. 1997, 75, 1375–1384. [Google Scholar] [CrossRef]
- Li, J.; Wei, P.; Wu, X.; Xue, M.; Yan, X. Three Protocols for the Formation of a [3]Pseudorotaxane via Orthogonal Cryptand-Based Host Guest Recognition and Coordination-Driven Self-Assembly. Org. Lett. 2013, 15, 4984–4987. [Google Scholar] [CrossRef]
- Datta, S.; Dey, N.; Bhattacharya, S. Electrochemical Probing of Hydrogelation Induced by the Self-Assembly of a Donor-Acceptor Complex Comprising Pyranine and Viologen. Chem. Commun. 2017, 53, 2371–2374. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, B.; Zhu, K.; Zhou, Q.; Zhai, C.; Li, S.; Li, N.; Huang, F. Formation of Linear Main-Chain Polypseudorotaxanes with Supramolecular Polymer Backbones via Two Self-Sorting Host-Guest Recognition Motifs. Chem. Commun. 2009, 45, 4375–4377. [Google Scholar] [CrossRef]
- Job, P. Job’s Method of Continuous Variation. Ann. Chim. 1928, 9, 113–203. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shao, Y.-G.; Yan, F.-Z.; Zhang, Z.; Li, S. Construction of Supramolecular Polymers with Different Topologies by Orthogonal Self-Assembly of Cryptand–Paraquat Recognition and Metal Coordination. Molecules 2021, 26, 952. https://doi.org/10.3390/molecules26040952
Wang K, Shao Y-G, Yan F-Z, Zhang Z, Li S. Construction of Supramolecular Polymers with Different Topologies by Orthogonal Self-Assembly of Cryptand–Paraquat Recognition and Metal Coordination. Molecules. 2021; 26(4):952. https://doi.org/10.3390/molecules26040952
Chicago/Turabian StyleWang, Kai, Yuan-Guang Shao, Feng-Zhi Yan, Zibin Zhang, and Shijun Li. 2021. "Construction of Supramolecular Polymers with Different Topologies by Orthogonal Self-Assembly of Cryptand–Paraquat Recognition and Metal Coordination" Molecules 26, no. 4: 952. https://doi.org/10.3390/molecules26040952
APA StyleWang, K., Shao, Y.-G., Yan, F.-Z., Zhang, Z., & Li, S. (2021). Construction of Supramolecular Polymers with Different Topologies by Orthogonal Self-Assembly of Cryptand–Paraquat Recognition and Metal Coordination. Molecules, 26(4), 952. https://doi.org/10.3390/molecules26040952





