Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties
Abstract
:1. Introduction
2. Bibliographic Search Criteria and Statistical Analyses
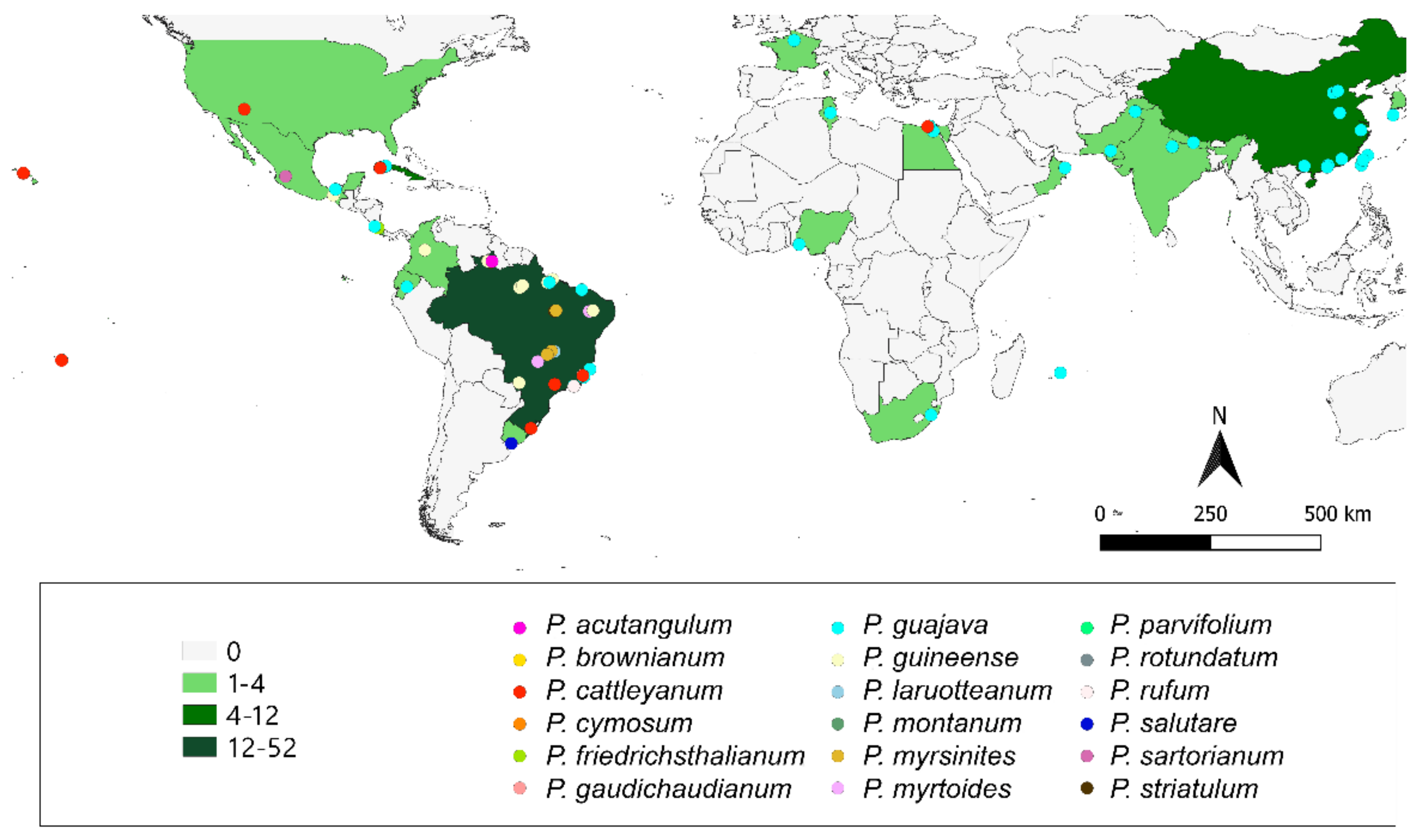
3. Plants Occurrence and the Bibliometric Network Data
4. Volatile Profiles
4.1. Psidium cattleyanum Sabine
4.2. Psidium friedrichsthalianum (O. Berg) Nied
4.3. Psidium guajava L.
4.4. Psidium guineense Sw.
4.5. Psidium laruotteanum Cambess
4.6. Psidium myrsinites DC
4.7. Psidium myrtoides O. Berg
4.8. Psidium salutare (Kunth) O. Berg
4.9. Psidium sartorianum (O.Berg) Nied
4.10. Psidium striatulum DC
4.11. Other Species
5. Seasonal Variation in the Essential Oils Composition
6. Biological Activities
6.1. Antioxidant Activity
6.2. Antifungal Activity
6.3. Antibacterial Activity
6.4. Phytotoxic Activity
6.5. Larvicidal Activity
6.6. Anti-Inflammatory
6.7. Cytotoxic
6.8. Other Activity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EO | Essential oil |
| FRAP | Ferric reducing antioxidant power |
| HD | Hydrodistillation |
| HS-SPME | HeadSpace Solid Phase Micro Extraction, |
| IC50 | Median inhibitory concentration |
| LC50 | Median lethal concentration |
| MDA | Malondialdehyde |
| MFC | Minimum Fungicide Concentration |
| MIC | Minimum inhibitory concentration |
| MMC | Minimum microbicide concentration |
| NO | Nitric oxide radical scavenging assay |
| ORAC | Oxygen radical absorbing capacity assay |
| SAFE | Solvent-assisted flavor evaporation |
| SD | Steam distillation |
| SDE | Simultaneous steam distillation-solvent extraction, |
| SE | Solvent extraction |
| SPME | Solid Phase Micro Extraction |
| TBARS | Thiobarbituric Acid Reactive Species |
| TLC | Thin Layer Chromatography |
| XO | Xanthine oxidase assay |
| TE/gEO | Trolox equivalent per gram of essential oil |
| µmol Fe+2/mg OE | Micromol of FE+2 per milligram of essential oil |
Appendix A
| Species | Occurrence | Plant Part/ Extraction Type | Primary Components (>5%) | Ref. |
|---|---|---|---|---|
| P. acutangulum | Boa vista-Bonfim Road, Roraima, Brazil | Leaf/stems (HD) | α-pinene (14.8%), 1,8-cineole (12.9%), and β-pinene (10.1%) | [56] |
| P. brownianum | Crato, CE, Brazil | Leaf (HD) | β-eudesmol (27.1%), 1,8-cineole (24.7%), α-elemol (11.8%), α-pinene (11.4%), guaiol (9.1%), and β-pinene (8.4%) | [103] |
| P. cattleyanum | Moorea Island, Haapiti, French Polynesia | Leaf (HD) | Profile I (caryophyllane): E-caryophyllene (31.5%) | [36] |
| P. cattleyanum | Arizona, USA | Leaf (SD) | Profile II (caryophyllane): E-caryophyllene (59.9%) and caryophyllene oxide (5.4%) | [37] |
| P. cattleyanum | Pelotas, RS, Brazil | Leaf (SD) | Profile III (caryophyllane): E-caryophyllene (59.6%), caryophyllene oxide (18.2%), and Z-caryophyllene (6.4%) | [38] |
| P. cattleyanum | Honolulu, Hawaii, USA | Leaf (HD) | Profile IV (caryophyllane/pinane/acyclic): E-caryophyllene (59.0%), α-pinene (13.2%), and myrcene (11.3%) | [39] |
| P. cattleyanum | Alegre, ES, Brazil | Leaf (HD) | Profile V (caryophyllane/pinane): E-caryophyllene (23.4%), caryophyllene oxide (11.5%), and α-pinene (11.3%) | [22] |
| P. cattleyanum | El-Behera, Egypt | Leaf (HD) | Profile VI (caryophyllane/pinane/acyclic): E-caryophyllene (28.8%), α-pinene (28.0%), myrcene (13.4%), and trans-β-ocimene (5.3%) | [40] |
| P. cattleyanum | Pelotas, RS, Brazil | Fruit (HD) | Profile VII (caryophyllane/eudesmane/aromadendrene): E-caryophyllene (22.5%), neo-intermedeol (14.2%), β-selinene (10.1%), trans-β-guaiene (9.1%), and α-humulene (7.5%) | [41] |
| P. cattleyanum | Pinar del Río, Cuba | Leaf (HD) | Profile VIII (cadinane/caryophyllane): epi-α-muurolol (21.9%), α-cadinol (20.0%), epi-α-cadinol (16.7%), caryophyllene oxide (13.6%), juniper camphor (9.4%), and 14-hydroxy-9-epi-E-caryophyllene (5.7%) | [42] |
| P. cattleyanum | Atlantic Forest, Brazil | Leaf (HD) | Profile IX (p-menthane/caryophyllane/eremophilane/acyclic): α-thujene (25.2%), 1,8-cineole (16.4%), E-caryophyllene (10.2%), valencene (8.0%), and myrcene (5.0%) | [43] |
| P. cattleyanum | Limeira, SP, Brazil | Leaf (HD) | Profile X (eudesmane/caryophyllane/p-menthane/aromadendrene) viridiflorol (17.9%), E-caryophyllene (11.8%), 1,8-cineole (10.8%), β-selinene (8.6%), α-humulene (6.0%), and aromadendrene (5.0%) | [44] |
| P. cymosum | Pinar del Rio, Cuba | Leaf (HD) | epi-α-cadinol (46.6%), 1,8-cineole (15.0%), α-muurolol (11.8%), α-terpineol (8.4%), and α-pinene (5.7%) | [99] |
| P. friedrichsthalianum | San Jose, Costa Rica | Leaf (SD) | Profile I (caryophyllane/elemane/pinane/germacrane/cadinane): E-caryophyllene (36.8%), β-elemene (12.86%), α-pinene (10.6%), bicyclogermacrene (8.3%), β-pinene (8.3%), and α-ylangene (7.8%) | [37] |
| P. friedrichsthalianum | Alegre, ES, Brazil | Leaf (HD) | Profile II (caryophyllane/cadinane): E-caryophyllene (24.6%), caryophyllene oxide (10.6%), α-humulene (9.2%), and α-copaene (5.9%) | [22] |
| P. gaudichaudianum | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (17.0%), limonene (16.2%), α-pinene (8.4%), caryophyllene oxide (7.5%), and α-humulene (5.8%) | [22] |
| P. guajava | Saint-Denis, France | Fruit (HD) | Profile I (caryophyllane/acyclic/aromadendrene): E-caryophyllene (24.6%), nerolidol (18.0%), and caryophyllene oxide (5.1%) | [49] |
| P. guajava | Sindh Province, Pakistan | Leaf (HD) | Profile I (caryophyllane/acyclic/aromadendrene): E-caryophyllene (20.3%), globulol (8.2%), and E-nerolidol (7.7%) | [50] |
| P. guajava | Kathmandu, Nepal | Leaf (HD) | Profile I (caryophyllane/acyclic/aromadendrene): E-nerolidol (35.6%), E-caryophyllene (15.8%), (2Z,6E)-farnesol (6.7%), and ledol (5.5%) | [51] |
| P. guajava | Guira de Melena, Havana, Cuba | Leaf (HD) | Profile II (caryophyllane/acyclic/eudesmane/aromadendrene): E-caryophyllene (21.6%), E-nerolidol (l9.2%), selin-ll-en-4α-ol (13.4%), viridiflorene (8.8%), α-selinene (8.3%), caryophyllene oxide (8.2%), and cedr-8(15)-en-9α-ol (7.9%) | [52] |
| P. guajava | Shijiazhuang, Hebei, China | Leaf (HD) | Profile III (caryophyllane/aromadendrene): E-caryophyllene (27.4%) and γ-gurjunene (13.5%) | [53] |
| P. guajava | Anguo, Hebei, China | Leaf (HD) | Profile III (caryophyllane/aromadendrene): E-caryophyllene (24.4%) and γ-gurjunene (12.7%) | [53] |
| P. guajava | Hangzhou, Zhejiang, China | Leaf (HD) | Profile III (caryophyllane/aromadendrene): E-caryophyllene (31.4%) and γ-gurjunene (14.0%), | [53] |
| P. guajava | Chenchou, Tunisia | Leaf (HD) | Profile III (caryophyllane/aromadendrene): viridiflorol (36.4%) and E-caryophyllene (5.9%) | [54] |
| P. guajava | Panyu, Guangdong, China | Leaf (HD) | Profile IV (caryophyllane/cadinane/aromadendrene): E-caryophyllene (25.7%), calamenene (7.4%), γ-gurjunene (9.5%), and epi-α-cadinol (6.4%) | [53] |
| P. guajava | Meizhou, Guangdong, China | Leaf (HD) | Profile IV (caryophyllane/cadinane/aromadendrene): E-caryophyllene (25.0%), γ-gurjunene (9.5%), epi-α-cadinol (6.1%), and calamenene (7.8%) | [53] |
| P. guajava | Taipei, Taiwan | Leaf (HD) | Profile IV (caryophyllane/cadinane/aromadendrene): E-caryophyllene (17.2), γ-gurjunene (9.3%), epi-α-cadinol (10.0%), and calamenene (6.7%) | [53] |
| P. guajava | Tainan, Taiwan | Leaf (HD) | Profile IV (caryophyllane/cadinane/aromadendrene): E-caryophyllene (21.4%), γ-gurjunene (9.2%), epi-α-cadinol (7.8%), and calamenene (6.6%) | [53] |
| P. guajava | Jiangmen, Guangdong, China | Leaf (HD) | Profile IV (caryophyllane/cadinane/aromadendrene): E-caryophyllene (25.0%), calamenene (7.1%), γ-gurjunene (9.5%), and epi-α-cadinol (6.0%) | [53] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | Profile V (caryophyllane/bisabolane/p-menthane/acyclic): E-caryophyllene (7.6%), β-bisabolol (19.5%), limonene (17.8%), 1,8-cineole (5.1%), and E-nerolidol (6.9%) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | Profile V (caryophyllane/bisabolane/p-menthane/acyclic): E-caryophyllene (9.4%), β-bisabolol (15.1%), limonene (6.5%), α-humulene (16.5%), E-nerolidol (7.4%), β-bisabolene (6.3%), and humulene epoxide II (6.0%) | [55] |
| P. guajava | Belém, PA, Brazil | Leaf/stems (HD) | Profile V (caryophyllane/bisabolane/p-menthane/acyclic): α-pinene (23.9%), 1,8-cineole (21.4%), β-bisabolol (9.2%), E-caryophyllene (5.2%), and E-nerolidol (5.0%) | [56] |
| P. guajava | Bozhou, Anhui, China | Leaf (HD) | Profile VI (caryophyllane/bisabolane/aromadendrene): E-caryophyllene (26.4%), β-bisabolene (5.23%), and γ-gurjunene (15.2%) | [53] |
| P. guajava | Cairo, Egypt | Leaf (HD) | Profile VII (caryophyllane/eudesmane/p-menthane/cadinane): E-caryophyllene (16.9%), selin-7(11)-en-4-α-ol (8.3%), α-selinene (6.5%), β-selinene (6.3%), 1,8-cineole (5.4%), and δ-cadinene (5.3%) | [57] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | Profile VIII (caryophyllane/eudesmane/aromadendrene): E-caryophyllene (26.6%), selin-11-en-4α-ol (6.7%), caryophyllene oxide (15.5%), aromadendrene epoxide (8.1%), β-selinene (7.6%), and α-selinene (6.5%) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | Profile IX (caryophyllane/eudesmane/p-menthane/aromadendrene): E-caryophyllene (19.4%), selin-11-en-4α-ol (7.4%), caryophyllene oxide (16.6%), aromadendrene epoxide (9.2%), 1,8-cineole (8.4%), and β-selinene (5.6%) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | Profile X (caryophyllane/eudesmane/p-menthane/aromadendrene/cadinane): E-caryophyllene (10.2%), selin-11-en-4α-ol (16.7%), caryophyllene oxide (8.0%), aromadendrene epoxide (9.5%), 1,8-cineole (7.6%), epi-α-cadinol (7.9%), β-selinene (8.2%), epi-cubenol (6.7%), and α-selinene (6.3%) | [55] |
| P. guajava | Moorea Island, Taravao, French Polynesia | Leaf (HD) | Profile X (caryophyllane/eudesmane/p-menthane/bisabolane/aromadendrene): E-caryophyllene (18.3%), selin-11-en 4α-ol (6.9%), 1,8-cineole (6.2%), and E-α-bisabolene (5.5%) | [36] |
| P. guajava | Parnaíba, PI, Brazil | Leaf (SD) | Profile X (caryophyllane/eudesmane/p-menthane/bisabolane): E-caryophyllene (39.0%), β-selinene (9.7%), α-selinene (9.7%), 1,8-cineole (6.9%), and aromadendrene (6.3%) | [58] |
| P. guajava | Cairo, Egypt | Fruit (SE) | Profile X (caryophyllane/eudesmane/p-menthane/bisabolane): E−caryophyllene (12.8%), α−selinene (8.4%), β−selinene (8.3%), and 3Z-hexenol (6.7%) | [59] |
| P. guajava | Chang-hua, Taiwan | Leaf (SD) | Profile XI (caryophyllane/aromadendrene/p-menthane): E-caryophyllene (27.7%), α-pinene (14.7%), 1,8-cineole (12.4%), and aromadendrene (6.6%) | [60] |
| P. guajava | Cairo, Egypt | Fruit (HD) | Profile XI (caryophyllane/eudesmane/p-menthane): E-caryophyllene (17.6%), limonene (11.0%), α-selinene (6.6%), and β-selinene (6.4%) | [57] |
| P. guajava | Guira de Melena, Havana, Cuba | Fruit (SDE) | Profile XI (caryophyllane/p-menthane/others): E-caryophyllene (12.2%), limonene (10.3%), hexadecanoic acid (8.7%), and 2E-hexenal (5.2%) | [61] |
| P. guajava | Mauritius | Leaf (HD) | Profile XI (caryophyllane/p-menthane): caryophyllene oxide (15.4%) and limonene (11.6%) | [62] |
| P. guajava | Lucknow, Uttar Pradesh, India | senescent leaves (HD) | Profile XI (caryophyllane/p-menthane): limonene (29.1%), E-caryophyllene (15.7%), caryophyllene oxide (8.8%), and caryophylla-4(12),8(13)-dien-5β-ol (6.5%) | [63] |
| P. guajava | Jeju Island, South Korea | Leaf (SAFE) | Profile XI (caryophyllane/p-menthane): α-pinene (12.3%) and E-caryophyllene 6.8%) | [64] |
| P. guajava | Lagos, Nigeria | Leaf (HD) | Profile XI (caryophyllane/p-menthane): limonene (42.1%) and E-caryophyllen (21.3%) | [65] |
| P. guajava | KwaZulu, Natal, South Africa | Leaf (HD)a white fruit | Profile XII (caryophyllane/acyclic/cadinane): caryophyllene oxide (14.0%), E-caryophyllene (13.9%), 1H-cycloprop[e]azulene (11.7%), adamantane (9.5%), E-nerolidol (6.8%), and α-cubebene (6.7%) | [66] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | Profile XIII (caryophyllane/eudesmane/cadinane): α-humulene (15.0%), E-caryophyllene (12.0%), β-selinene (11.0%), α-selinene (10.0%), cedr-8-(15)-en-9-α-ol (7.6%), and α-muurolol (5.6%) | [67] |
| P. guajava | Chenchou, Tunisia | Stems (HD) | Profile XIV (caryophyllane/germacrane/cadinane): germacrene D (16.8%), valerianol (10.6%), caryophyllene oxide (5.1%), and α-cadinol (5.0%) | [54] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | Profile XIV (caryophyllane/eudesmane/cadinane): α-humulene (15.0%), E-caryophyllene (12.0%), α-selinene (10.0%), cedr-8(15)-en-9-α-ol (7.6%), and epi-α-muurolol (5.6%) | [104] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | Profile XIV (caryophyllane/eudesmane/cadinane): caryophylla-4(12),8(13)-dien-5-β-ol (15.0%), α-humulene (13.0%), α-muurolol (9.6%), cedr-8-(15)-en-9-α-ol (7.4%), E-caryophyllene (7.2%), β-selinene (6.7%), humulene epoxide II (6.6%), and caryophyllene oxide (5.0%) | [67] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | Profile XV (caryophyllane/eudesmane): α-humulene (37.0%), E-caryophyllene (24.0%), β-selinene (14.0%), and α-selinene (12.0%) | [67] |
| P. guajava | Nanning, China | Leaf (SD) | Profile XVI (p-menthane/aromadendrene): α-pinene (37.8%), 1,8-cineole (18.9%), globulol (6.8%), and cedrenol (5.6%) | [69] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | Profile XVII (p-menthane/acyclic/caryophyllane/aromadendrene): α-pinene (25.5%), E-nerolidol (16.7%), E-caryophyllene (15.7%), δ-3-carene (8.8%), and cedran-8-ol (8.8%) | [70] |
| P. guajava | Alsharquia, Oman | Leaf (HD) | Profile XVII (p-menthane/acyclic/caryophyllane/aromadendrene): E-caryophyllene (33.5%), viridiflorene (13.0%), farnesene (11.7%), and limonene (9.8%) | [71] |
| P. guajava | Macas, Ecuador | Leaf (HD) | Profile XVIII (p-menthane): limonene (33.3%), α-pinene (29.5%), and carvotacetone acetate (8.2%) | [72] |
| P. guajava | El-Behera, Egypt | Leaf (HD) | Profile XVIII (p-menthane): limonene (54.7%) and 1, 8-cineole (32.1%) | [40] |
| P. guajava | Guira de Melena, Havana, Cuba | Fruit (SDE) | Profile XIX (p-menthane/others): limonene (8.3%), and 3-phenylpropyl acetate (6.2%) | [61] |
| P. guajava | Mauritius | Leaf (HD) | Profile XX (bisabolane/caryophyllane/p-menthane/cadinane): santalol (50.6%), caryophyllene oxide (15.4%), limonene (11.6%), and cycloisosativene (6.1%) | [73] |
| P. guajava | Monteverde, Costa Rica | Leaf (SDE) | Profile XXI (others/p-menthane/aromadendrene/acyclic): 2E-hexenal (28.4%), benzaldehyde (16.5%), 1,8-cineole (15.9%), globulol (10.3%), E-nerolidol (6.9%) | [74] |
| P. guajava | Saint-Denis, France | Fruit (HS-SPME) | Profile XXII (others): hexanal (65.9%), γ-butyrolactone (7.6%), 2E-hexenal (7.4%) | [49] |
| P. guajava | Guira de Melena, Havana, Cuba | Fruit (SDE) | Profile XXIII (others): 3Z-hexenyl acetate (5.0%) | [61] |
| P. guajava | Villahermosa, Mexico | Leaf (SD) | Profile XXIV (bisabolane/caryophyllane): β-bisabolene (19.2%), β-sesquiphellandrene (14.8%), E-caryophyllene (6.0%), E-γ-bisabolene (5.3%), and α-curcumene (5.1%) | [37] |
| P. guajava | Guira de Melena, Havana, Cuba | Fruit (SDE) | Profile XXV (others): E-cinnamyl acetate (5.6%) | [61] |
| P. guajava | Mimoso do Sul, ES, Brazil | Leaf (HD) | Profile XXVI: (caryophyllane/bisabolane/acyclic/p-menthane/cadinane/eudesmane/aromadendrene): E-caryophyllene (5.1–30.0%), α-humulene (2.0–24.4%), 14-hydroxy-epi-E-caryophyllene (1.3–19.3%), β-bisabolol (1.2–20.1%), E-nerolidol (0.5–19.9%), 14-hydroxy-epi-E-caryophyllene (0–14.7%), limonene (0.2–11.7%), γ-muurolene (1.5–6.4%), α-selinene (0.4–12.4%), β-selinene (0.5–13.3%), β-bisabolene (3.1–9.7%), hinesol (0.9–10.0%), epi-α-cadinol (0–6.4%), α-bisabolol (1.0–5.9%), selina-6-en-4-ol (0.6–9.1%), aromadendrene (0.3–7.4%), and 1,8-cineole (0.7–5.3%) | [24] |
| P. guajava | Linhares, ES, Brazil | Leaf (HD) | Profile XXVI (caryophyllane/bisabolane/acyclic/p-menthane/cadinane/eudesmane/aromadendrene): E-caryophyllene (5.1–32.3%), caryophyllene oxide (1.8–20.9%), α-humulene (1.7–19.9%), β-bisabolol (2.2–19.4%), E-nerolidol (2.1–13.7%), hinesol (3.2–12.4%), α-selinene (0.5–11.2%), β-selinene (0.5–12.8%), epi-α-cadinol (1.1–12.0%), limonene (0.1–11.0%), β-bisabolene (2.3–9.7%), α-bisabolol (0.5–7.3%), epi-β-cubenol (2.2.–7.1%), humulene epoxide (0.6–6.3%), selina-6-en-4-ol (3.3–6.1%), aromadendrene (3.1–5.6%), γ-muurolene (1.7–5.4%), and δ-cadinene (0.5–5.1%) | [24] |
| P. guajava | Abbottabad, Pakistan | Fruit (SD) | Profile XXVII (others): hexanol (13.9%), cinnamyl alcohol (10.9%), butanol (10.7%), 3-methyl glutaric anhydride (9.5%), hexene (7.7%), butanoic acid methyl ester (7.2%), and 3-hexenal (6.6%) | [75] |
| P. guajava | KwaZulu, Natal, South Africa | Leaf (HD)a pink fruit | Profile XXVIII (others/caryophyllane): tetracyclo[6,3,2,0(2,5).0(1,8)] tridecan-9-ol,4,4-dimethyl (13.0%), E-caryophyllene (9.6%), 1H-cycloprop[e]azulene (8.1%) | [66] |
| P. guineense | Santarém, PA, Brazil | Leaf (HD) | Profile I (p-menthane): limonene (47.4%) | [21] |
| P. guineense | Curuçá, PA, Brazil | Leaf (HD) | Profile II (p-menthane/pinane): limonene (30.7%) and α-pinene (26.1%) | [21] |
| P. guineense | Curuçá, PA, Brazil | Leaf (HD) | Profile II (p-menthane/pinane): limonene (30.4%) and α-pinene (17.7%) | [21] |
| P. guineense | Curuçá, PA, Brazil | Leaf (HD) | Profile II (p-menthane/pinane): limonene (37.2%) and α-pinene (34.0%) | [21] |
| P. guineense | Curuçá, PA, Brazil | Leaf (HD) | Profile III (p-menthane/pinane): limonene (26.5%), α-pinene (13.7%), and α-copaene (7.2%) | [21] |
| P. guineense | Monte Alegre, PA, Brazil | Leaf (HD) | Profile IV (p-menthane/bisabolane): limonene (9.6%) and epi-β-bisabolol (6.5%) | [21] |
| P. guineense | Santarém, PA, Brazil | Leaf (HD) | Profile V (p-menthane/bisabolane): limonene (23.4%), epi-β-Bisabolol (9.5%), and β-bisabolene (6.4%), | [21] |
| P. guineense | Crato, CE, Brazil | Leaf (SD) | Profile VI (p-menthane/germacrane/pinane/elemane): 1,8-cineole (40.5%), β-eudesmol (19.5%), α-pinene (13.9%), β-pinene (8.6%), elemol (7.7%), and γ-eudesmol (5.2%) | [83] |
| P. guineense | Curuçá, PA, Brazil | Leaf (HD) | Profile VII (pinane/cadinane/caryophyllane): α-pinene (35.6%), α-copaene (8.1%), E-caryophyllene (6.1%), and muurola-4,10(14)-dien-1-β-ol (5.8%) | [21] |
| P. guineense | Santarém, PA, Brazil | Leaf (HD) | Profile VIII (pinane/p-menthane/caryophyllane): α-pinene (26.4%), limonene (14.0%), and E-caryophyllene (5.2%) | [21] |
| P. guineense | Monte Alegre, PA, Brazil | Leaf (HD) | Profile IX (bisabolane): β-bisabolene (8.9%) and α-curcumene (5.0%) | [21] |
| P. guineense | Ocozocoautla, Chiapas, Mexico | Leaf (SD) | Profile X (bisabolane/pinane/acyclic/p-menthane): β-bisabolene (13.2%), α-pinene (12.5%), Z-nerolidol (5.5%), β-sesquiphellandrene (5.2%), and limonene (5.1%) | [37] |
| P. guineense | Ponta de Pedras, PA, Brazil | Leaf (HD) | Profile XI (caryophyllane/p-menthane): E-caryophyllene (24.0%) and limonene (5.4%) | [21] |
| P. guineense | Boa Vista do Alto Alegre, RR, Brazil | Leaf/stems (HD) | Profile XII (bisabolane/p-menthane): β-bisabolol (17.4%), limonene (6.8%), and epi-α-bisabolol (6.7%) | [56] |
| P. guineense | Santarém, PA, Brazil | Leaf (HD) | Profile XIII (bisabolane): epi-β-bisabolol (18.1%) and β-bisabolol (5.6%) | [21] |
| P. guineense | Dourados, MS, Brazil | Leaf (HD) | Profile XIV (germacrane): spathulenol (80.7%) | [84] |
| P. guineense | La Palma, Cundinamarca, Colombia | Fruits (SDE) | Profile XV (caryophyllane/eudesmane/others): E-caryophyllene (8.6%), butanol (7.4%), ethyl butyrate (7.4%), and selin-11-en-4α-ol (5.9%) | [85] |
| P. guineense | La Palma, Cundinamarca, Colombia | Fruits (HS-SPME) | Profile XVI (others): ethyl butyrate (30.3%) and ethyl hexanoate (23.8%) | [85] |
| P. laruotteanum | Brasilia, Brazil | Leaves (HD) | Profile I (p-menthane/pinane): p-cymene (24.8%), 1,8-cineole (19.2%), α-pinene (13.4%), and terpinen-4-ol (6.3%) | [86] |
| P. laruotteanum | Brasilia, Brazil | Leaves (HD) | Profile II (p-menthane/pinane): p-cymene (19.4%), γ-terpinene (14.0%), α-pinene (11.6%), limonene (10.2%), 1,8-cineole (6.9%), terpinen-4-ol (5.8%), terpinolene (5.1%) | [86] |
| P. laruotteanum | Brasilia, Brazil | Leaves (HD) | Profile III (p-menthane/pinane): p-cymene (34.8%), 1,8-cineole (12.5%), α-pinene (9.2%), limonene (7.9%), γ-terpinene (6.9%), and α-terpineol (6.0%) | [86] |
| P. montanum | West region, Cuba | Leaf (HD) | Profile p-menthane/pinane: 1,8-cineole (46.9%), α-terpineol (9.2%), and α-pinene (8.9%) | [101] |
| P. myrsinites | Anápolis, GO, Brazil | Leaf (HD) | Profile I (caryophyllane): E-caryophyllene (31.0%), α-humulene (12.3%), and caryophyllene oxide (7.3%) | [88] |
| P. myrsinites | Chapada das Mesas National Park, MA, Brazil | Leaf (HD) | Profile II (caryophyllane): E-caryophyllene (26.1%), 𝛼-humulene (23.9%), caryophyllene oxide (10.1%), humulene epoxide II (6.4%), and Caryophylla-4(12),8(13)-dien- 5-β-ol (5.7%) | [89] |
| P. myrsinites | Brasilia, Brazil | Leaf/flower (HD) | Profile III (caryophyllane/acyclic): caryophyllene oxide (26.1%), humulene epoxide II (8.8%), E-caryophyllene (7.4%), Z-caryophyllene (5.4%), and myrcene (5.4%) | [90] |
| P. myrtoides | Brasilia, Brazil | Leaf (HD) | Profile I (caryophyllane/acyclic) E-caryophyllene (22.4%), caryophyllene oxide (19.7%), α-humulene (8.4%), and myrcene (5.4%) | [23] |
| P. myrtoides | Chapada, do Araripe, CE, Brazil | Leaf (HD) b | Profile II (p-menthane/germacrane/pinane/elemane) 1,8-cineole (29.5–48.1%), α-eudesmol (11.7–20.0%), α-pinene (5.0–12.8%), elemol (3.3–6.7%), and γ-eudesmol (2.5–5.8%) | [93] |
| P. myrtoides | Rio verde, GO, Brazil | Leaf (HD) | Profile III (caryophyllane/cadinane/bisabolane): E-caryophyllene (30.9%), α-humulene (15.9%), α-copaene (7.8%), caryophyllene oxide (7.3%), and α-bisabolol (7.3%) | [91] |
| P. myrtoides | Alegre, ES, Brazil | Leaf (HD) | Profile III (caryophyllane/cadinane/bisabolane): E-caryophyllene (19.4%), α-bisabolol (10.4%), α-humulene (10.4%), α-copaene (6.3%), and caryophyllene oxide (5.3%) | [22] |
| P. parvifolium | Pinar del Río, Cuba | Leaf (HD) | viridiflorol (31.9%), α-terpineol (8.2%), cubenol (7.3%), borneol (7.2%), epi-α-muurolol (6.6%), and trans-sabinol (5.5%) | [42] |
| P. rotundatum | Pinar del Rio, Cuba | Leaf/Stalks (SDE) | 1,8-cineole (28.0%), α-pinene (18.3%), α-terpineol (9.2%), E-nerolidol (8.7%), and linalool (5.1%) | [102] |
| P. salutare | Punta Espinillo, Montevideo, Uruguay | Leaf/Twig (SD) | Profile I (p-menthane/acyclic): 1,8-cineole (31.1%), linalool (11.5%), and α-terpineol (7.0%) | [96] |
| P. salutare | Punta Espinillo, Montevideo, Uruguay | Leaf/Twig (SD) | Profile I (p-menthane/acyclic): 1,8-cineole (36.6%), linalool (12.4%), and α-terpineol (6.7%) | [96] |
| P. salutare | Crato, CE, Brazil | Leaf (SD) | Profile II (p-menthane/germacrane): 1,8-cineole (63.3%), p-cymene (14.1%), α-terpinyl acetate (7.2%), and β-eudesmol (8.8%) | [83,97] |
| P. salutare | Chapada do Araripe, CE, Brazil | Leaf (HD) b | Profile III (p-menthane/cadinane/acyclic): p-cymene (5.1–17.8%), terpinolene (6.9–17.0%), γ-terpinene (10.3–17.1%), epi-α-cadinol (10.4–12.8%), linalool (4.7–7.3%), and δ-cadinene (3.8–5.3%) | [25] |
| P. salutare | Pinar del Rio, Cuba | Leaf (HD) | Profile IV (caryophyllane/bisabolane/eudesmane/pinane): caryophyllene oxide (39.8%), ar-turmerone (17.3%), β-gurjunene (6.7%), β-selinene (6.0%), and α-pinene (5.6%) | [98] |
| P. sartorianum | Pinar del Rio, Cuba | Leaf (HD) | Profile I (p-menthane/pinane): limonene (43.0%), α-pinene (39.5%), and β-pinene (5.6%) | [99] |
| P. sartorianum | Guadalajara, Mexico | Leaf (SD) | Profile II (pinane/caryophyllane/p-menthane/acyclic): α-pinene (16.7%), E-caryophyllene (12.4%), α-phellandrene (9.8%), and Z-nerolidol (5.2%) | [37] |
| P. striatulum | Boa Vista, RR, Brazil | Fruits (HD) | Profile I (pinane/caryophyllane/cadinane/aromadendrene): α-pinene (12%), α-humulene (10.4%), α-copaene (7.1%), globulol (5.7%), and aromadendrene (5.1%) | [100] |
| P. striatulum | Carolina, MA, Brazil | Leaf/stems (HD) | Profile II (caryophyllane/eudesmane) E-caryophyllene (28.6%), α-selinene (7.7%), caryophyllene oxide (7.6%), β-selinene (7.4%), selin-11-en-4-α-ol (6.0%) | [56] |
| P. rufum | Rio de Janeiro, RJ, Brazil | Leaf (HD) | E-caryophyllene (21.0%), α-pinene (14.0%), γ-eudesmol (8.5%), 1,8-cineole (8.4%), α-eudesmol (8.2%), and β-eudesmol (6.8%) | [104] |
Appendix B
| Species | Occurrence | Plant Part/ Extraction Type | Primary Components (>5%) | Essential Oil Bioactivity | Ref. |
|---|---|---|---|---|---|
| P. brownianum | Crato, CE, Brazil | Leaf (HD) | β-eudesmol (27.1%), 1,8-cineole (24.7%), α-elemol (11.8%), α-pinene (11.4%), guaiol (9.1%), and β-pinene (8.4%) | Antinociceptive effect (doses 100 and 200 mg/kg) | [103] |
| P. cattleyanum | Pelotas, RS, Brazil | Leaf (SD) | E-caryophyllene (59.6%), caryophyllene oxide (18.2%), and Z-caryophyllene (6.4%) | Antioxidant in vitro, DPPH assay (inactive at 10–500 mg/mL); Antioxidant in vitro, ABTS assay (inactive at 10–500 mg/mL); Antioxidant in vitro, linoleic acid oxidation assay (IC50 56.41 μg/mL) Toxicity, mouse model oral administration (LD50 > 500mg/Kg) Antifungal, broth microdilution method (Candida albicans, MIC 166.7 µg/mL; Candida lipolytica, MIC 125 µg/mL; Candida guilhermondii, MIC 125 µg/mL; Candida parapsilosis, MIC 104.2 µg/mL; Trichosporon asahii, MIC 41.76 µg/mL) | [38] |
| P. cattleyanum | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (23.4%), caryophyllene oxide (11.5%), and α-pinene (11.3%) | Phytotoxic, dose 3000 µg/mL (Lactuca sativa, Germination inhibition 74.6%, Germination Speed Index 3.4 mm; Sorghum bicolor, Germination inhibition 92.6%, Germination Speed Index, 6.9 mm) | [22] |
| P. cattleyanum | El-Behera, Egypt | Leaf (HD) | E-caryophyllene (28.8%), α-pinene (28.0%), myrcene (13.4%), and trans-β-ocimene (5.3%) | Antibacterial, disk diffusion method (Neisseria gonorrhoeae, MIC 13.01 µg/mL) | [40] |
| P. cattleyanum | Pelotas, RS, Brazil | Fruit (HD) | E-caryophyllene (22.5%), neo-intermedeol (14.2%), β-selinene (10.1%), trans-β-guaiene (9.1%), and α-humulene (7.5%) | Antioxidant, DPPH assay on TLC plate (1:250 dilution) | [41] |
| P. cattleyanum | Limeira, SP, Brazil | Leaf (HD) | Viridiflorol (17.9%), E-caryophyllene (11.8%), 1,8-cineole (10.8%), β-selinene (8.6%), α-humulene (6.0%), and aromadendrene (5.0%) | Antibacterial, broth microdilution assay (Porphyromonas gingivalis, MIC 20 µg/mL; Prevotella nigrescens, MIC 62.5 µg/mL; Fusobacterium nucleatum, MIC 12.5 µg/mL; Bacteroides fragilis, MIC 12.5 µg/mL; Actinomyces naeslundii, MIC 50 µg/mL; Peptostreptococcus anaerobius, MIC 62.5 µg/mL; Aggregatibacter actinomycetemcomitans, MIC 6.25 µg/mL) | [44] |
| P. friedrichsthalianum | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (24.6%), caryophyllene oxide (10.6%), α-humulene (9.2%), α-copaene (5.9%) | Phytotoxic, dose 375 µg/mL (Lactuca sativa, Germination inhibition 92.8%, Germination Speed Index 5.7 mm; Sorghum bicolor, Germination inhibition 91.7%, Germination Speed Index, 8.4 mm) | [22] |
| P. gaudichaudianum | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (17.0%), limonene (16.2%), α-pinene (8.4%), caryophyllene oxide (7.5%), and α-humulene (5.8%) | Phytotoxic, dose 1500 µg/mL (Lactuca sativa, Germination inhibition 90.7%, Germination Speed Index 5.2 mm; Sorghum bicolor, Germination inhibition 91.1%, Germination Speed Index, 8.1 mm) | [22] |
| P. guajava | Mauritius | Leaf (HD) | Caryophyllene oxide (15.4%) and limonene (11.6%) | Antioxidant, DPPH assay (IC50 5.19 µg/mL); Antioxidant, ABTS assay (IC50 3.09 µg/mL); Antioxidant, XO assay (IC50 2.51 µg/mL); Antioxidant, OH assay (IC50 1.90 µg/mL); Antioxidant, NO assay (IC50 2.71 µg/mL); Antioxidant, ORAC assay (0.275 TE/gEO); Antioxidant, FRAP assay (44.41 µmol Fe+2/mg OE); | [62] |
| P. guajava | Mauritius | Leaf (HD) | Santalol (50.6%), caryophyllene oxide (15.4%), limonene (11.6%), and cycloisosativene (6.1%) | Antibacterial, disc diffusion assay (Enterococcus faecalis, 16.5 mm; Escherichia coli, 19.4 mm, Methicillin Resistant Staphylococcus aureus, 7.6 mm; Pseudomonas aeruginosa, 8.0 mm; Staphylococcus aureus, 18.6 mm; Staphylococcus epidermidis, 18.2 mm) | [73] |
| P. guajava | Macas, Ecuador | Leaf (HD) | Limonene (33.3%), α-pinene (29.5%), and carvotacetone acetate (8.2%) | Antifungal, disk diffusion assay (Candida albicans, MIC 0.14 mg/mL; Rhodotorula glutinis, MIC 0.09 mg/mL; Schizosaccharomyces pombe, MIC 0.09 mg/mL; Saccharomyces cerevisiae, MIC 0.06 mg/mL; Yarrowia lypolitica, MIC 0.23 mg/mL) | [72] |
| P. guajava | Bozhou, Anhui, China | Leaf (HD) | E-caryophyllene (26.4%), β-bisabolene (5.23%), and γ-gurjunene (15.2%) | Antioxidant activity: DPPH assay (IC50 23.39 mg/mL); ABTS assay (IC50 18.34 mg/mL); FRAP assay (6.57 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 9.24 mm; Alcaligenes faecalis, 11.46 mm; Bacillus aryabhattai, 18.18 mm; Arthrobacter creatinolyticus, 11.35 mm; Bacillus megaterium, 19.18 mm; Bacillus subtilis, 17.69 mm; Saccharomyces cerevisiae, 18.76 mm; Rhodotorula sp., 19.35 mm) | [53] |
| P. guajava | Panyu, Guangdong, China | Leaf (HD) | E-caryophyllene (25.7%), calamenene (7.4%), γ-gurjunene (9.5%%), and epi-α-cadinol (6.4%) | Antioxidant activity: DPPH assay (IC50 18.52 mg/mL); ABTS assay (IC50 13.12 mg/mL); FRAP assay (9.13 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 10.54 mm; Alcaligenes faecalis, 16.54 mm; Bacillus aryabhattai, 23.15 mm; Arthrobacter creatinolyticus, 15.27 mm; Bacillus megaterium, 22.98 mm; Bacillus subtilis, 19.34 mm; Saccharomyces cerevisiae, 20.13 mm; Rhodotorula sp., 26.36 mm) | [53] |
| P. guajava | Jiangmen, Guangdong, China | Leaf (HD) | E-caryophyllene (25.0%), calamenene (7.1%), γ-gurjunene (9.5%), and epi-α-cadinol (6.0%) | Antioxidant activity: DPPH assay (IC50 19.42 mg/mL); ABTS assay (IC50 15.31 mg/mL); FRAP assay (7.68 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 11.23 mm; Alcaligenes faecalis, 14.14 mm; Bacillus aryabhattai, 22.79 mm; Arthrobacter creatinolyticus, 14.97 mm; Bacillus megaterium, 21.45 mm; Bacillus subtilis, 18.89 mm; Saccharomyces cerevisiae, 21.23 mm; Rhodotorula sp., 26.71 mm) | [53] |
| P. guajava | Shijiazhuang, Hebei, China | Leaf (HD) | E-caryophyllene (27.4%) and γ-gurjunene (13.5%) | Antioxidant activity: DPPH assay (IC50 23.44 mg/mL); ABTS assay (IC50 19.13 mg/mL); FRAP assay (6.92 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 8.76 mm; Alcaligenes faecalis, 9.26 mm; Bacillus aryabhattai, 17.34 mm; Arthrobacter creatinolyticus, 10.24 mm; Bacillus megaterium, 17.39 mm; Bacillus subtilis, 18.27 mm; Saccharomyces cerevisiae, 18.69 mm; Rhodotorula sp., 19.35 mm) | [53] |
| P. guajava | Anguo, Hebei, China | Leaf (HD) | E-caryophyllene (24.4%) and γ-gurjunene (12.7%) | Antioxidant activity: DPPH method (IC50 24.07 mg/mL); ABTS method (IC50 20.34 mg/mL); FRAP method (6.91 mmol Vc/g DM) Antimicrobial, disc diffusion method (Escherichia coli, 8.35 mm; Alcaligenes faecalis, 9.78 mm; Bacillus aryabhattai, 17.26 mm; Arthrobacter creatinolyticus, 10.57 mm; Bacillus megaterium, 17.08 mm; Bacillus subtilis, 18.34 mm; Saccharomyces cerevisiae, 18.76 mm; Rhodotorula sp., 19.78 mm) | [53] |
| P. guajava | Taipei, Taiwan | Leaf (HD) | E-caryophyllene (17.2), γ-gurjunene (9.3%), epi-α-cadinol (10.0%), and calamenene (6.7%) | Antioxidant activity: DPPH assay (IC50 31.12 mg/mL); ABTS assay (IC50 24.15 mg/mL); FRAP assay (2.36 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 7.89 mm; Alcaligenes faecalis, 10.23 mm; Bacillus aryabhattai, 15.34 mm; Arthrobacter creatinolyticus, 9.12 mm; Bacillus megaterium, 16.89 mm; Bacillus subtilis, 17.02 mm; Saccharomyces cerevisiae, 16.89 mm; Rhodotorula sp., 20.23 mm) | [53] |
| P. guajava | Hangzhou, Zhejiang, China | Leaf (HD) | E-caryophyllene (31.4%) and γ-gurjunene (14.0%), | Antioxidant activity: DPPH assay (IC50 20.36 mg/mL); ABTS assay (IC50 19.39 mg/mL); FRAP assay (7.12 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 8.98 mm; Alcaligenes faecalis, 11.13 mm; Bacillus aryabhattai, 18.79 mm; Arthrobacter creatinolyticus, 10.79 mm; Bacillus megaterium, 19.09 mm; Bacillus subtilis, 18.01 mm; Saccharomyces cerevisiae, 17.11 mm; Rhodotorula sp., 18.34 mm | [53] |
| P. guajava | Tainan, Taiwan | Leaf (HD) | E-caryophyllene (21.4%), γ-gurjunene (9.2%), epi-α-cadinol (7.8%), and calamenene (6.6%) | Antioxidant activity, DPPH assay (IC50 33.71 mg/mL); Antioxidant activity, ABTS assay (IC50 25.35 mg/mL), Antioxidant activity, FRAP assay (2.29 mmol Vc/g DM); Antimicrobial, disc diffusion assay (Escherichia coli, 7.90 mm; Alcaligenes faecalis, 10.15 mm; Bacillus aryabhattai, 15.76 mm; Arthrobacter creatinolyticus, 9.34 mm; Bacillus megaterium, 17.02 mm; Bacillus subtilis, 18.10 mm; Saccharomyces cerevisiae, 18.98 mm; Rhodotorula sp., 19.90 mm) | [53] |
| P. guajava | Meizhou, Guangdong, China | Leaf (HD) | E-caryophyllene (25.0%), γ-gurjunene (9.5%), epi-α-cadinol (6.1%), and calamenene (7.8%) | Antioxidant activity: DPPH assay (IC50 20.26 mg/mL); ABTS assay (IC50 16.18 mg/mL); FRAP assay (7.34 mmol Vc/g DM) Antimicrobial, disc diffusion assay (Escherichia coli, 10.38 mm; Alcaligenes faecalis, 14.65 mm; Bacillus aryabhattai, 21.97 mm; Arthrobacter creatinolyticus, 14.65 mm; Bacillus megaterium, 21.22 mm; Bacillus subtilis, 18.97 mm; Saccharomyces cerevisiae, 20.57 mm; Rhodotorula sp., 25.98 mm) | [53] |
| P. guajava | Kathmandu, Nepal | Leaf (HD) | E-nerolidol (35.6%), E-caryophyllene (15.8%), (2Z,6E)-farnesol (6.7%), and ledol (5.5%) | Larvicidal activity against Chaoborus plumicornis (LC50 63.3 μg/mL); Insecticidal activity against Drosophila melanogaster (LC50 327 μg/mL) Nematicidal activity against Caenorhabditis elegans with (LC50 of 142 μg/mL). | [51] |
| P. guajava | Alsharquia, Oman | Leaf (HD) | E-caryophyllene (33.5%), viridiflorene (13.0%), farnesene (11.7%), and limonene (9.8%) | Antibacterial activity, disc diffusion assay (Enterococcus faecales, 6 mm; Staphylococcus aureus, 9 mm; Haemophilus influenzae, 12 mm; Pseudomonas aeruginosa, 6 mm; Escherichia coli, 13 mm) | [71] |
| P. guajava | El-Behera, Egypt | Leaf (HD) | limonene (54.7%) and 1, 8-cineole (32.1%) | Antibacterial activity, disc diffusion assay (Staphylococcus aureus, MIC 6.75 µg/mL) | [40] |
| P. guajava | Lucknow, Uttar Pradesh, India | senescent leaves (HD) | Limonene (29.1%), E-caryophyllene (15.7%), caryophyllene oxide (8.8%), caryophylla-4(12),8(13)-dien-5β-ol (6.5%) | Antibacterial activity, disc diffusion and microdilution broth assays (Staphylococcus aureus methicillin-resistant, 65 µg/mL; S. aureus, 65–261 µg/mL; Staphylococcus epidermidis, 130 µg/mL; S. epidermidis methicillin-resistant, 65 µg/mL; Mycobacterium smegmatis, 261 µg/mL) Antifungal activity, disc diffusion and microdilution broth assays (Candida krusei, 16.71 mg/mL) | [63] |
| P. guajava | Parnaíba, PI, Brazil | Leaf (SD) | E-caryophyllene (39.0%), β-selinene (9.7%), α-selinene (9.7%), 1,8-cineole (6.9%), and aromadendrene (6.3%) | Acaricidal activity (females of RhipicephalusMicroplus, adult immersion test, 99.95% of efficiency on engorged at 12.5 mg/L; larval packet test, larvae mortality 5.8% at 12.5 mg/L) | [58] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | α-humulene (15.0%), E-caryophyllene (12.0%), β-selinene (11.0%), α-selinene (10.0%), cedr-8-(15)-en-9-α-ol (7.6%), and α-muurolol (5.6%) | Anti-inflammatory activity, Pleurisy induced by lipopolysaccharide model (inhibition in migration of eosinophils 76% at 100 mg/kg) | [67] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | α-humulene (37.0%), E-caryophyllene (24.0%), β-selinene (14.0%), and α-selinene (12.0%) | Anti-inflammatory activity, Pleurisy induced by lipopolysaccharide model (inhibition in migration of eosinophils 67% at 100 mg/kg) | [67] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | caryophylla-4(12),8(13)-dien-5-β-ol (15.0%), α-humulene (13.0%), α-muurolol (9.6%), cedr-8-(15)-en-9-α-ol (7.4%), E-caryophyllene (7.2), β-selinene (6.7%), humulene epoxide II (6.6%), and caryophyllene oxide (5.0%) | Anti-inflammatory, Pleurisy induced by LPS model (migration inhibition of eosinophils 74% at 100 mg/kg) | [67] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (26.6%), selin-11-en-4α-ol (6.7%), caryophyllene oxide (15.5%), aromadendrene epoxide (8.1%), β-selinene (7.6%), and α-selinene (6.5%) | Larvicidal activity against Aedes aegypti (LC50 39.48 μg/mL; LC90 57.34 μg/mL) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (7.6%), β-bisabolol (19.5%), limonene (17.8%), 1,8-cineole (5.1%), and E-nerolidol (6.9%) | Larvicidal activity against Aedes aegypti (LC50 51.11 μg/mL; LC90 71.56 μg/mL) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (9.4%), β-bisabolol (15.1%), limonene (6.5%), α-humulene (16.5%), E-nerolidol (7.4%), β-bisabolene (6.3%), and humulene epoxide II (6.0%) | Larvicidal activity against Aedes aegypti (LC50 53.47 μg/mL; LC90 73.84 μg/mL) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (19.4%), selin-11-en-4α-ol (7.4%), caryophyllene oxide (16.6%), aromadendrene epoxide (9.2%), 1,8-cineole (8.4%), and β-selinene (5.6%) | Larvicidal activity against Aedes aegypti (LC50 63.35 μg/mL; LC90 82.44 μg/mL) | [55] |
| P. guajava | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (10.2%), selin-11-en-4α-ol (16.7%), caryophyllene oxide (8.0%), aromadendrene epoxide (9.5%), 1,8-cineole (7.6%), epi-α-cadinol (7.9%), β-selinene (8.2%), epi-cubenol (6.7%), and α-selinene (6.3%) | Larvicidal activity against Aedes aegypti (LC50 64.25 μg/mL; LC90 86.00 μg/mL) | [55] |
| P. guajava | Rio de Janeiro, RJ, Brazil | Leaf (HD) | α-pinene (25.5%), E-nerolidol (16.7%), E-caryophyllene (15.7%), δ-3-carene (8.8%), and cedran-8-ol (8.8%) | Inseticidal activity (Tribolium castaneum, Fumigation LC50 6.1 µg/L air after 24 h of treatment and < 2 µg/L air after 72 h Inseticidal activity (Culex pipiens, Fumigation LC50 > 50 µg/L) Larvicidal activity (Culex pipiens, Fumigation LC50 > 100 µg/L) | [70] |
| P. guajava | Abbottabad, Pakistan | Fruit (SD) | Hexanol (13.9%), cinnamyl alcohol (10.9%), Butanol (10.7%), 3-methyl glutaric anhydride (9.5%), hexene (7.7%), butanoic acid methyl ester (7.2%), and 3-hexenal (6.6%) | Vasorelaxant effect, rabbit aorta preparations against pre-concentrations of K+ (EC50 5.52 mg/mL); Vasorelaxant effect, rabbit aorta preparations against phenylephrine (EC50 6.23 mg/mL); Spasmolytic effect, isolated rabbit jejunum against contractions spontaneous (EC50 0.84 mg/mL) Spasmolytic effect, isolated rabbit jejunum against induced contractions by K+ (EC50 0.71 mg/mL) | [75] |
| P. guajava | Cairo, Egypt | Fruit (HD) | E-caryophyllene (17.6%), limonene (11.0%), α-selinene (6.6%), and β-selinene (6.4%) | Antioxidant activity: DPPH assay (IC50 8.11 mg/mL); deoxyribose degradation (IC50 42.78 μg/mL); Anti-inflammatory activity: inhibition of 5-lipoxygenase (IC50 49.76 μg/mL); Cytotoxic activity (HepG2 hepatic cancer, IC50 196.45 µg/mL; MCF-7 breast cancer, IC50 544.38 µg/mL) | [57] |
| P. guajava | Cairo, Egypt | Leaf (HD) | E-caryophyllene (16.9%), selin-7(11)-en-4-α-ol (8.3%), α-selinene (6.5%), β-selinene (6.3%), 1,8-cineole (5.4%), and δ-cadinene (5.3%) | Antioxidant activity: DPPH assay (IC50 3.59 mg/mL); deoxyribose degradation (IC50 12.64 μg/mL); Anti-inflammatory activity: inhibition of 5-lipoxygenase (IC50 32.53 μg/mL); Cytotoxic activity: HepG2 hepatic cancer, IC50 130.69 µg/mL; MCF-7 breast cancer, IC50 351.00 µg/mL) | [57] |
| P. guineense | Dourados, MS, Brazil | Leaf (HD) | Spathulenol (80.7%) | Antioxidant activity: DDPH assay (IC50 63.08 µg/mL); ABTS assay (IC50 780.13 µg/mL); MDA assay (IC50 37.91 µg/mL) Anti-inflammatory activity: carrageenan-induced mice paw oedema model, inhibition of 59.46% after second and fourth hour at 300 mg/kg); pleurisy model, reduction in the increase in total leukocytes of 45.33% at 30 mg/kg and 77.70% at 100 mg/kg); reduction in the rise in protein levels of 49.72%, at 30 mg/kg, and 78.40%, at 100 mg/kg;Cytotoxic activity: U251 glioma, GI50 9.84 µg/mL; MCF-7 breast, GI50 7.90 µg/mL; NCI/ADR-RES ovarian expressing the phenotype of multiple drug resistance, GIC50 9.25 µg/mL; 786–0 renal, GI50 2.57 µg/mL; NCI-H460 lung, GI50 4.57 µg/mL; PCO-3 prostate, GI50 9.18 µg/mL; OVCAR-3 ovarian, GI50 0.89 µg/mL; HT-29 colon, GI50 5.62 µg/mL; K-562 leukemia, GI50 5.03 µg/mL; HaCaT keratinocytes, GI50 7.98 µg/mL) | [84] |
| P. myrsinites | Anápolis, GO, Brazil | Leaf (HD) | E-caryophyllene (31.0%), α-humulene (12.3%), and caryophyllene oxide (7.3%) | Larvicidal activity against Artemia salina (LC50 95.3 µg/mL) | [88] |
| P. myrsinites | Chapada das Mesas National Park, MA, Brazil | Leaf (HD) | E-caryophyllene (26.1%), 𝛼-humulene (23.9%), caryophyllene oxide (10.1%), humulene epoxide II (6.4%), and Caryophylla-4(12),8(13)--ien-5-β-ol (5.7%) | Larvicidal activity against Aedes aegypti (LC50 292 mg/mL) | [89] |
| P. myrtoides | Chapada, do Araripe, CE, Brazil | Leaf (HD) b | 1,8-cineole (29.5–48.1%), α-eudesmol (11,7–20.0%), α-pinene (5.0–12.8%), elemol(3.3–6.7%), and γ-eudesmol (2.5–5.8%) | Antifungal activity: broth microdilution assay (Candida albicans, MFC 1.02–4.10 µg/mL, IC50 103.3–963.8 µg/mL; C. krusei, MFC 8.19–16.38 µg/mL, IC50 1235.9–3564.5 µg/mL; C. tropicalis, MFC > 16.384 µg/mL, IC50 1671.1–2535.1 µg/mL) | [93] |
| P. myrtoides | Rio verde, GO, Brazil | Leaf (HD) | E-caryophyllene (30.9%), α-humulene (15.9%), α-copaene (7.8%), caryophyllene oxide (7.3%), and α-bisabolol (7.3%) | Antibacterial activity, broth microdilution assay: Streptococcus mitis, MIC100 μg/mL; S. sanguinis, MIC 100 μg/mL; S. sobrinus, MIC 250 μg/mL; S. salivarius, MIC 250 μg/mL; S. mutans MIC, 62.5 μg/mL; Cytotoxic activity: MCF-7 human breast adenocarcinoma, IC50 254.5 μg/mL; HeLa, human cervical adenocarcinoma, IC50 324.2 μg/mL; M059J human glioblastoma, IC50 289.3 μg/mL) | [91] |
| P. myrtoides | Alegre, ES, Brazil | Leaf (HD) | E-caryophyllene (19.4%), α-bisabolol (10.4%), α-humulene (10.4%), α-copaene (6.3%), and caryophyllene oxide (5.3%) | Phytotoxic activity (dose 3000 µg/mL): Lactuca sativa, Germination inhibition 47.4%, Germination Speed Index 3.4 mm; Sorghum bicolor, Germination inhibition 90.4%, Germination Speed Index, 7.8 mm) | [22] |
| P. salutare | Crato, CE, Brazil | Leaf (SD) | 1,8-cineole (63.3%), p-cymene (14.1%), α-terpinyl acetate (7.2%), and β-eudesmol (8.8%) | Antinociceptive effect (dose 400 mg/kg in mouse model) | [83,97] |
| P. salutare | Chapada do Araripe, CE, Brazil | Leaf (HD) b | p-cymene (5.1–17.8%), terpinolene (6.9–17.0%), γ-terpinene (10.3–17.1%), epi-α-cadinol (10.4–12.8%), linalool (4.7–7.3%), and δ-cadinene(3.8–5.3%) | Antifungal activity: Candida albicans, MFC 1.02–4.10 µg/mL; Candida krusei, MFC 8.19–16.38 µg/mL; Candida tropicalis, MFC > 16.38 mg/mL) | [25] |
| P. striatulum | Boa Vista, RR, Brazil | Fruits (HD) | α-pinene (12%), α-humulene (10.4%), α-copaene (7.1%), globulol (5.7%), and aromadendrene (5.1%) | Antibacterial activity: Staphylococcus aureus, IC50 28.62 μg/mL; Bacillus cereus, IC50 24.74 μg/mL; Salmonella typhimurium, IC50 18.69 μg/mL); Enzyme acetylcholinesterase, inhibition 44.42%) | [100] |
| P. rufum | Rio de Janeiro, RJ, Brazil | Leaf (HD) | E-caryophyllene (21.0%), α-pinene (14.0%), γ-eudesmol (8.5%), 1,8-cineole (8.4%), α-eudesmol (8.2%), and β-eudesmol (6.8%) | Anti-inflammatory activity: zymosan induced inflammatory model (reduction in eosinophil migration 70% at 100 mg/kg), in vitro nitric oxide production (moderate effect, 51% at 100 mg/kg) | [104] |




References
- Soares-Silva, L.H.; Proença, C.E.B. A new species of Psidium L. (Myrtaceae) from southern Brazil. Bot. J. Linn. Soc. 2008, 158, 51–54. [Google Scholar] [CrossRef] [Green Version]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Psidium, L. Available online: https://www.gbif.org/species/3187232 (accessed on 10 July 2020).
- Tuler, A.C.; Carrijo, T.T.; Ferreria, M.F.S.; Peixoto, A.L. Flora of Espírito santo: Psidium (Myrtaceae). Rodriguésia 2017, 68, 1791–1805. [Google Scholar] [CrossRef] [Green Version]
- Gressler, E.; Pizo, M.A.; Morellato, L.P.C. Polinização e dispersão de sementes em Myrtaceae do Brasil. Rev. Bras. Botânica 2006, 29, 509–530. [Google Scholar] [CrossRef]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; Da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Tuler, A.C.; Carrijo, T.T.; Peixoto, A.L.; Garbin, M.L.; Ferreira, M.F.D.S.; Carvalho, C.R.; Spadeto, M.S.; Clarindo, W.R. Diversification and geographical distribution of Psidium (Myrtaceae) species with distinct ploidy levels. Trees 2019, 33, 1101–1110. [Google Scholar] [CrossRef]
- Bezerra, J.E.F.; Lederman, I.E.; Silva-Junior, J.F.; da Franzon, R.C.; Sousa-Silva, J.C.; Campos, L.Z.O.; Proença, C.E.B. Psidium spp.: Araçá. In Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas Para o Futuro—Região Centro-Oeste; Vieira, R.F., Camillo, J., Coradin, L., Eds.; Ministério do Meio Ambiente, Secretaria de Biodiversidade: Bloco DF, Brazil, 2016; pp. 294–314. ISBN 9788577383092. [Google Scholar]
- Silva, P.T.M.; Silva, M.A.F.; Silva, L.; Seca, A.M.L. Ethnobotanical Knowledge in Sete Cidades, Azores Archipelago: First Ethnomedicinal Report. Plants 2019, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Vázquez, M.D.C.; Carranza-Álvarez, C.; Alonso-Castro, A.J.; González-Alcaraz, V.F.; Bravo-Acevedo, E.; Chamarro-Tinajero, F.J.; Solano, E. Ethnobotany of medicinal plants used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013, 148, 521–527. [Google Scholar] [CrossRef]
- Moraes, L.L.C.; Freitas, J.L.; Matos Filho, J.R.; Silva, R.B.L.; Borges, C.H.A.; Santos, A.C. Ethno-knowledge of medicinal plants in a community in the eastern Amazon. Rev. Ciências Agrárias 2019, 42, 565–573. [Google Scholar]
- Costa, J.C.; Marinho, M.G.V. Etnobotânica de plantas medicinais em duas comunidades do município de Picuí, Paraíba, Brasil. Rev. Bras. Plantas Med. 2016, 18, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Penido, A.B.; De Morais, S.M.; Ribeiro, A.B.; Silva, A.Z. Ethnobotanical study of medicinal plants in Imperatriz, State of Maranhão, Northeastern Brazil. Acta Amaz. 2016, 46, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Shruthi, S.D.; Roshan, A.; Timilsina, S.S.; Sunita, S. A review on the medicinal plant Psidium guajava Linn. (Myrtaceae). J. Drug Deliv. Ther. 2011, 3, 162–168. [Google Scholar]
- Da Costa, J.S.; da Cruz, E.d.N.S.; Setzer, W.N.; Da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; de Aguiar Andrade, E.H.; das Graças Bichara Zoghbi, M.; da Silva Santos, L. Constituents and pharmacological activities of Myrcia (Myrtaceae): A review of an aromatic and medicinal group of plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef] [Green Version]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Zouari, N. Essential Oils Chemotypes: A Less Known Side. Med. Aromat. Plants 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Chalchat, J.-C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and Quantitative Variation in Monoterpene Co-Occurrence and Composition in the Essential Oil of Thymus vulgaris Chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.L.; Silva, R.C.; Da Silva, J.K.R.; Suemitsu, C.; Mourão, R.H.V.; Maia, J.G.S. Chemical variability in the essential oil of leaves of Araçá (Psidium guineense Sw.), with occurrence in the Amazon. Chem. Central J. 2018, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, L.C.; de Souza Santos, E.; de Oliveira Bernardes, C.; da Silva Ferreira, M.F.; Ferreira, A.; Tuler, A.C.; Carvalho, J.A.M.; Pinheiro, P.F.; Praça-Fontes, M.M. Phytochemical analysis and effect of the essential oil of Psidium L. species on the initial development and mitotic activity of plants. Environ. Sci. Pollut. Res. 2019, 26, 26216–26228. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.O.; De Morais, S.M.; Silveira, E.R. Volatile Constituents of Psidium myrsinoides O. Berg. J. Essent. Oil Res. 2002, 14, 364–365. [Google Scholar] [CrossRef]
- Souza, T.S.; Ferreira, M.F.D.S.; Menini, L.; Souza, J.R.C.D.L.; Bernardes, C.D.O.; Ferreira, A. Chemotype diversity of Psidium guajava L. Phytochem. 2018, 153, 129–137. [Google Scholar] [CrossRef] [PubMed]
- De Macêdo, D.G.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; dos Santos, A.T.L.; da Cruz, R.P.; da Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-junior, L.J.; da Silva Almeida, J.R.G.; et al. Effect of seasonality on chemical profile and antifungal activity of essential oil isolated from leaves Psidium salutare (Kunth). O. Berg. Peer J. 2018, 6, e5476. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, S.S.; Croteau, R. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Siani, A.C.; de Azevedo, M.B.M.; Ramos, M.F.S.; Trigo, J.R. Monoterpenes and sesquiterpenes of Neotropical Myrtaceae. In Current Trends in Phytochemistry; Epifano, F., Ed.; Research Signpost: Kerala, India, 2008; pp. 223–251. ISBN 978-81-308-0277-0. [Google Scholar]
- Franzon, R.C.; Campos, L.Z.O.; Proença, C.E.B.; Sousa-Silva, J.C. Araçás do Gênero Psidium: Principais Espécies, Ocorrência, Descrição e Usos, 1st ed.; Embrapa Cerrados: Planaltina, Brazil, 2009; ISBN 2176-5081. [Google Scholar]
- Patel, S. Exotic tropical plant Psidium cattleianum: A review on prospects and threats. Rev. Environ. Sci. Bio/Technol. 2012, 11, 243–248. [Google Scholar] [CrossRef]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies da Flora do Brasil. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 11 November 2020).
- Govaerts, R.; Sobral, M.; Ashton, P.; Barrie, F.; Holst, B.K.; Landrum, L.L.; Matsumoto, K.; Mazine, F.F.; Nic Lughadha, E.; Proenca, C.; et al. World Checklist of Myrtaceae; Royal Botanic Gardens, Kew: Richmond, UK, 2018. [Google Scholar]
- Goncalves, R.C.; Faleiro, J.H.; Faleiro Naves, P.L.; dos Santos, M.N.; Malafaia, G. Pharmacognostic Characterization, Bioactive Com-pounds and Powder Antioxidant Action of Leaves of Araca (Psidium cattleianum Myrtaceae). Gen. Med. Open Access 2016, 4. [Google Scholar]
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Biegelmeyer, R.; Andrade, J.M.M.; Aboy, A.L.; Apel, M.A.; Dresch, R.R.; Marin, R.; Raseira, M.D.C.B.; Henriques, A.T. Comparative Analysis of the Chemical Composition and Antioxidant Activity of Red (Psidium cattleianum) and Yellow (Psidium cattleianum var. lucidum) Strawberry Guava Fruit. J. Food Sci. 2011, 76, C991–C996. [Google Scholar] [CrossRef] [PubMed]
- Raseira, M.D.C.B.; Raseira, A. Contribuição ao Estudo do Araçazeiro (Psidium cattleyanum); EMBRAPA: Pelotas, Brazil, 1996. [Google Scholar]
- Adam, F.; Vahirua-Lechat, I.; Deslandes, E.; Menut, C. Aromatic Plants of French Polynesia. V. Chemical Composition of Essential Oils of Leaves of Psidium guajava L. and Psidium cattleyanum Sabine. J. Essent. Oil Res. 2011, 23, 98–101. [Google Scholar] [CrossRef]
- Tucker, O.; Maciarelloa, M.J.; Landrumb, L.R. Volatile leaf oils of American Myrtaceae. III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., P. guineense Sw., and P. sartorianum (Berg) Niedenzu. J. Essent. Oil Res. 1995, 7. [Google Scholar] [CrossRef]
- Castro, M.R.; Victoria, F.N.; Oliveira, D.H.; Jacob, R.G.; Savegnago, L.; Alves, D. Essential oil of Psidium cattleianum leaves: Antioxidant and antifungal activity. Pharm. Biol. 2015, 53, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Lichwa, J.; Ray, C. Essential Oils of Selected Hawaiian Plants and Associated Litters. J. Essent. Oil Res. 2007, 19, 276–278. [Google Scholar] [CrossRef]
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Saber, F.R. Comparative study of the volatile oil content and antimicrobial activity of Psidium guajava L. and Psidium cattleianum Sabine leaves. Bull. Fac. Pharmacy, Cairo Univ. 2016, 54, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Marin, R.; Apel, M.A.; Limberger, R.P.; Raseira, M.C.B.; Pereira, J.F.M.; Zuanazzi, J.Â.S.; Henriques, A.T. Volatile Components and Antioxidant Activity from some Myrtaceous Fruits cultivated in Southern Brazil. Lat. Am. J. Pharm. 2008, 27, 172–177. [Google Scholar]
- Pino, J.A.; Bello, A.; Urquiola, A.; Marbot, R.; Martí, M.P. Leaf Oils of Psidium parvifolium Griseb. And Psidium cattleianum Sabine from Cuba. J. Essent. Oil Res. 2004, 16, 370–371. [Google Scholar] [CrossRef]
- Marques, F.A.; Wendler, E.P.; Maia, B.H.L.N.S.; Coffani-Nunes, J.V.; Campana, J.; Guerrero, P.G. Volatile Oil ofPsidium cattleianumSabine from the Brazilian Atlantic Forest. J. Essent. Oil Res. 2008, 20, 519–520. [Google Scholar] [CrossRef]
- Chrystal, P.; Pereira, A.C.; Alves, C.C.F.; De Souza, J.M.; Martins, C.H.; Potenza, J.; Crotti, A.E.M.; Miranda, M.L.D. Essential Oil from Psidium cattleianum Sabine (Myrtaceae) Fresh Leaves: Chemical Characterization and in vitro Antibacterial Activity Against Endodontic Pathogens. Braz. Arch. Biol. Technol. 2020, 63, 63. [Google Scholar] [CrossRef]
- Gentil, D.F.D.O.; Ferreira, S.A.D.N.; Rebouças, E.R. Germination of Psidium friedrichsthalianum (O. Berg) Nied. seeds under different temperature and storage conditions. J. Seed Sci. 2018, 40, 246–252. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants, 1st ed.; Springer: London, UK, 2012; ISBN 9789400725331. [Google Scholar]
- Flores, G.; Dastmalchi, K.; Wu, S.-B.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D′Armiento, J.M.; Kennelly, E.J. Phenolic-rich extract from the Costa Rican guava (Psidium friedrichsthalianum) pulp with antioxidant and anti-inflammatory activity. Potential for COPD therapy. Food Chem. 2013, 141, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Diaz-De-Cerio, E.; Verardo, V.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Health Effects of Psidium guajava L. Leaves: An Overview of the Last Decade. Int. J. Mol. Sci. 2017, 18, 897. [Google Scholar] [CrossRef] [Green Version]
- Paniandy, J.-C.; Chane-Ming, J.; Pieribattesti, J.-C. Chemical Composition of the Essential Oil and Headspace Solid-Phase Microextraction of the Guava Fruit (Psidium guajava L.). J. Essent. Oil Res. 2000, 12, 153–158. [Google Scholar] [CrossRef]
- Arain, A.; Sherazi, S.T.H.; Mahesar, S.A. Essential Oil from Psidium guajava Leaves: An Excellent Source of β-Caryophyllene. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2015, 3, 11–14. [Google Scholar]
- Pino, J.A.; Agüero, J.; Marbot, R.; Fuentes, V. Leaf Oil of Psidium guajava L. from Cuba. J. Essent. Oil Res. 2001, 13, 61–62. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Huang, T.; Shi, K.; Wu, Z. Chemical Compositions, Antioxidant and Antimicrobial Activities of Essential Oils of Psidium guajava L. Leaves from Different Geographic Regions in China. Chem. Biodivers. 2017, 14, e1700114. [Google Scholar] [CrossRef]
- Khadhri, A.; El Mokni, R.; Almeida, C.; Nogueira, J.; Araújo, M.E.M. Chemical composition of essential oil of Psidium guajava L. growing in Tunisia. Ind. Crops Prod. 2014, 52, 29–31. [Google Scholar] [CrossRef]
- Mendes, L.A.; Martins, G.F.; Valbon, W.R.; Souza, T.D.S.D.; Menini, L.; Ferreira, A.; Ferreira, M.F.D.S. Larvicidal effect of essential oils from Brazilian cultivars of guava on Aedes aegypti L. Ind. Crop. Prod. 2017, 108, 684–689. [Google Scholar] [CrossRef]
- Da Silva, J.D.; Luz, A.I.R.; Silva, M.H.L.; Andrade, E.H.A.; Zoghbi, M.G.B.; Maia, J.G.S. Essential oils of leaves and stems of four Psidium spp. Flavour Fragr. J. 2003, 18. [Google Scholar]
- El-Ahmady, S.H.; Ashour, M.L.; Wink, M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013, 25, 475–481. [Google Scholar] [CrossRef]
- Castro, K.N.C.; Costa-Júnior, L.M.; Lima, D.F.; Canuto, K.M.; Sousa de Brito, E.; De Andrade, I.M.; Teodoro, M.S.; Oi-ram-Filho, F.; Dos Santos, R.C.; Mayo, S.J. Acaricidal activity of cashew nut shell liquid associated with essential oils from Cordia verbenacea and Psidium guajava on Rhipicephalus microplus. J. Essent. Oil Res. 2019, 31, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Clery, R.A.; Hammond, C.J. New Sulfur Components of Pink Guava Fruit (Psidium guajava L.). J. Essent. Oil Res. 2008, 20, 315–317. [Google Scholar] [CrossRef]
- Chen, H.-C.; Sheu, M.-J.; Lin, L.-Y.; Wu, C.-M. Chemical Composition of the Leaf Essential Oil of Psidium guajava L. from Taiwan. J. Essent. Oil Res. 2007, 19, 345–347. [Google Scholar] [CrossRef]
- Pino, J.A.; Ortega, A.; Rosado, A. Volatile Constituents of Guava (Psidium guajava L.) Fruits from Cuba. J. Essent. Oil Res. 1999, 11, 623–628. [Google Scholar] [CrossRef]
- Mahomoodally, F.; Aumeeruddy-Elalfi, Z.; Venugopala, K.N.; Hosenally, M. Antiglycation, comparative antioxidant potential, phenolic content and yield variation of essential oils from 19 exotic and endemic medicinal plants. Saudi J. Biol. Sci. 2019, 26, 1779–1788. [Google Scholar] [CrossRef]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Km, Y.-S.; Choi, H.-K.; Cho, S.K. Determination of the Volatile Components in the Fruits and Leaves of Guava Plants (Psidium guajava L.) Grown on Jeju Island, South Korea. J. Essent. Oil Res. 2011, 23, 52–56. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Ekundayo, O.; Koenig, W.A. Chemical composition of the leaf volatile oil of Psidium guajavaL. growing in Nigeria. Flavour Fragr. J. 2003, 18, 136–138. [Google Scholar] [CrossRef]
- Chalannavar, R.K.; Venugopala, K.N.; Baijnath, H.; Odhav, B. The Chemical Composition of Leaf Essential Oils ofPsidium guajavaL. (White and Pink fruit forms) from South Africa. J. Essent. Oil Bear. Plants 2014, 17, 1293–1302. [Google Scholar] [CrossRef]
- Siani, A.C.; Souza, M.C.; Henriques, M.G.M.O.; Ramos, M.F.S. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013, 51, 881–887. [Google Scholar] [CrossRef]
- Ramos, A.R.; Falcão, L.L.; Barbosa, G.S.; Marcellino, L.H.; Gander, E.S. Neem (Azadirachta indica a. Juss) components: Candidates for the control of Crinipellis perniciosa and Phytophthora ssp. Microbiol. Res. 2007, 238–243. [Google Scholar] [CrossRef]
- Ji, X.-D.; Pu, Q.-L.; Garraffo, H.M.; Pannell, L.K. The Essential Oil of the Leaves ofPsidium guajavaL. J. Essent. Oil Res. 1991, 3, 187–189. [Google Scholar] [CrossRef]
- El-Sabrout, A.M.; Salem, M.Z.M.; Bin-Jumah, M.; Allam, A.A. Toxicological activity of some plant essential oils against Tribo-lium castaneum and Culex pipiens larvae. Processes 2019, 7, 933. [Google Scholar] [CrossRef] [Green Version]
- Weli, A.; Al-Kaabi, A.; Al-Sabahi, J.; Said, S.; Hossain, M.A.; Al-Riyami, S.; Hossain, A. Chemical composition and biological activities of the essential oils of Psidium guajava leaf. J. King Saud Univ. Sci. 2019, 31, 993–998. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. J. Herb. Med. 2016, 6, 88–95. [Google Scholar] [CrossRef]
- Cole, R.A.; Setzer, W.N. Chemical Composition of the Leaf Essential Oil of Psidium guajava from Monteverde, Costa Rica. J. Essent. Oil Bear. Plants 2007, 10, 365–373. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Khan, T.; Wahid, F.; Khan, R.; Shah, A.J. Chemical composition and vascular and intestinal smooth muscle relaxant effects of the essential oil from Psidium guajava fruit. Pharm. Biol. 2016, 54, 2679–2684. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.G.; Ferreira, P.R.B.; Mendes, C.S.O.; Reis, R.; Valerio, H.M.; Brandi, I.V.; de Oliveira, D.A. Antibacterial activity of tannins from Psidium guineense Sw. (Myrtaceae). J. Med. Plants Res. 2014, 8, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, P.B. Frutas Comestíveis na Amazônia, 7th ed.; Museu Paraense Emilio Goeldi: Belém, Brazil, 2010. [Google Scholar]
- Dos Santos, M.A.C.; Rego, M.M.D.; De Queiroz, M.A.; Dantas, B.F.; Otoni, W.C. Synchronizing The In Vitro Germination Of Psidium Guineense Sw. Seeds By Means of Osmotic Priming. Revista Árvore 2016, 40, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Padilha, M.D.R.F.; Shinohara, N.K.S.; Pereira, E.D.P.R.; Pimentel, R.M.M.; Andrade, S.A.C.; Portela, F.H.; Bernardino, A.V.S. Physical, physicochemical and taxonomic characterization of Psidium araça Raddi. J. Environ. Anal. Prog. 2016, 1, 106. [Google Scholar] [CrossRef]
- Dos Santos, M.A.C.; De Queiroz, M.A.; Bispo, J.D.S.; Dantas, B.F. Seed germination of Brazilian guava (Psidium guineense Swartz.). J. Seed Sci. 2015, 37, 214–221. [Google Scholar] [CrossRef]
- Dos Santos, M.A.C.; do Rêgo, M.M.; de Queiróz, M.A.; Caproni, D.T.R.; Dietrich, O.H.S.; Santos, A.F.; Rocha, D.I.; Batista, D.S.; Otoni, W.C. In vitro growth performance of Psidium guajava and P. guineense plantlets as affected by culture medium formu-lations. Vegetos 2020, 33, 435–445. [Google Scholar] [CrossRef]
- Fernandes, T.G.; De Mesquita, A.R.C.; Randau, K.P.; Franchitti, A.A.; Ximenes, E.A. In Vitro Synergistic Effect of Psidium guineense (Swartz) in Combination with Antimicrobial Agents against Methicillin-Resistant Staphylococcus aureus Strains. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Neto, M.A.; De Alencar, J.W.; Cunha, A.N.; Silveira, E.R.; Batista, T.G. Volatile Constituents ofPsidium pohlianumBerg, andPsidium guyanensisPers. J. Essent. Oil Res. 1994, 6, 299–300. [Google Scholar] [CrossRef]
- Nascimento, K.F.D.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Ruiz, A.L.T.G.; Foglio, M.A.; De Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Peralta-Bohórquezo, A.F.; Parada, F.; Quijano, C.E.; Pino, J.A. Analysis of Volatile Compounds of Sour Guava (Psidium guin-eense Swartz) Fruit. J. Essent. Oil Res. 2010, 22, 493–498. [Google Scholar] [CrossRef]
- Medeiros, F.C.M.; Del Menezzi, C.H.S.; Vieira, R.F.; Fernandes, Y.F.M.; Santos, M.C.S.; Bizzo, H.R. Scents from Brazilian Cerrado: Chemical composition of the essential oil from Psidium laruotteanum Cambess (Myrtaceae). J. Essent. Oil Res. 2018, 30, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Fagg, C.W.; Lughadha, E.N.; Milliken, W.; Nicholas Hind, D.J.; Brandão, M.G.L. Useful Brazilian plants listed in the manu-scripts and publications of the Scottish medic and naturalist George Gardner (1812–1849). J. Ethnopharmacol. 2015, 161, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Durães, E.R.B.; Clementino, C.D.O.; Fari, L.R.; Ramos, L.M.; Oliveira, M.S.; De Paula, J.A.M.; Naves, P.L.F. Phytochemical study, toxicity and antimicrobial activity of Psidium myrsinites DC. (Myrtaceae) leaves. Biosci. J. 2017, 33, 1305–1313. [Google Scholar] [CrossRef]
- Dias, C.N.; Alves, L.P.L.; Rodrigues, K.A.D.F.; Brito, M.C.A.; Rosa, C.D.S.; Amaral, F.M.M.D.; Monteiro, O.D.S.; Andrade, E.H.D.A.; Maia, J.G.S.; Moraes, D.F.C. Chemical Composition and Larvicidal Activity of Essential Oils Extracted from Brazilian Legal Amazon Plants againstAedes aegyptiL. (Diptera: Culicidae). Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, F.C.M.; Del Menezzi, C.H.S.; Bizzo, H.R.; Vieira, R.F. Scents from Brazilian Cerrado: Psidium myrsinites DC. (Myrtaceae) leaves and inflorescences essential oil. J. Essent. Oil Res. 2015, 27, 289–292. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Batista, H.R.F.; Estevam, E.B.B.; Alves, C.C.F.; Forim, M.R.; Nicolella, H.D.; Furtado, R.A.; Tavares, D.C.; Silva, T.S.; Martins, C.H.G.; et al. Chemical composition and in vitro antibacterial and antiproliferative activities of the essential oil from the leaves of Psidium myrtoides O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 33, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.D.; Felfili, J.M. Uso de plantas medicinais na região de Alto Paraíso de Goiás, GO, Brasil. Acta Bot. Bras. 2006, 20, 135–142. [Google Scholar] [CrossRef]
- De Macêdo, D.G.; de Almeida Souza, M.M.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; dos Santos, A.T.L.; Machado, A.J.T.; Rodrigues, F.F.G.; da Costa, J.G.M.; de Menezes, I.R.A. Seasonality influence on the chemical composition and antifungal activity of Psidium myrtoides O. Berg. South Afr. J. Bot. 2020, 128, 9–17. [Google Scholar] [CrossRef]
- Vieira, R.F.; Agostini-Costa, T.D.; Silva, D.B.; Ferreira, F.R.; Sano, S.M. Frutas Nativas da Região Centro-Oste do Brasil, 1st ed.; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2006; ISBN 9788587697448. [Google Scholar]
- Landrum, L.R. A revision of the Psidium salutare complex (Myrtaceae). SIDA Contrib. Bot. 2003, 20, 1449–1469. [Google Scholar]
- Rossini, C.; Dellacassa, E.; Moyna, P. Comparative Study of the Leaf Oils of Psidium luridum and Psidium incanum. J. Essent. Oil Res. 1994, 6, 513–515. [Google Scholar] [CrossRef]
- Santos, F.; Rao, V.; Silveira, E. Naloxone-resistant antinociceptive activity in the essential oil of Psidium pohlianum Berg. Phytomedicine 1996, 3, 197–201. [Google Scholar] [CrossRef]
- Pino, J.A.; Bello, A.; Urquiola, A.; Agüero, J. Leaf Oil of Psidium salutare (HBK) Berg. from Cuba. J. Essent. Oil Res. 2003, 15, 19–20. [Google Scholar] [CrossRef]
- Pino, J.A.; Bello, A.; Urquiola, A.; Aguero, J.; Marbot, R. Leaf Oils of Psidium cymosum Urb. and Psidium sartorianum Niedz. from Cuba. J. Essent. Oil Res. 2003, 15, 187–188. [Google Scholar] [CrossRef]
- Moniz, A.M.H.; De Carvalho Neto, M.F.; Goncalves Reis De Melo, A.C.; Takahashi, J.A.; Ferraz, V.P.; Chagas, E.A.; Chagas Cardoso, P.; De Melo Filho, A.A. Biological Evaluation of Essential Oil from Green Fruits of Psidium Striatulum of the Roraima State, Brazil. Chem. Eng. Trans. 2019, 75, 379–384. [Google Scholar]
- Pino, J.A.; Marbot, R.; Payo, A.; Chao, D.; Herrera, P. Aromatic Plants from Western Cuba VII. Composition of the Leaf Oils of Psidium wrightii Krug et Urb., Lantana involucrata L., Cinnamomum montanum (Sw.) Berchtold et J. Persl. And Caesalpinia violacea (Mill.) Standley. J. Essent. Oil Res. 2006, 18, 170–174. [Google Scholar] [CrossRef]
- Pino, J.A.; Rosado, A.; Bello, A.; Urquiobi, A.; Garcia, S. Essential Oil of Psidium rotundatum Griseb. from Cuba. J. Essent. Oil Res. 1999, 11, 783–784. [Google Scholar] [CrossRef]
- Sampaio, R.S.; Petícia do Nascimento, E.; Alencar de Menezes, I.R.; Sales, V.S.; Brito Pereira, A.O.; Mendes de Lacerda, G.; Santos, E.S.; Pereira Lopes, M.J.; Gomes da Silva, L.; de Araújo Delmondes, G.; et al. Antinociceptive activity of the Psidium brownianum Mart ex DC. leaf essential oil in mice. Food Chem. Toxicol. 2020, 135, 111053. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.F.S.; Siani, A.C.; Souza, M.C.; Rosas, E.C.; Henriques, M.G.M.O. Avaliação da atividade antiinflamatória dos óleos essenciais de cinco espécies de Myrtaceae. Rev. Fitos 2006, 2, 58–66. [Google Scholar]
- Nunes, X.P.; Silva, F.S.; Almeida, J.R.G.S.; de Lima, J.T.; Ribeiro, L.A.A.; Quintans Júnior, L.J.; Barbosa Filho, J.M. Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals—Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Di Santo, R. Natural products as antifungal agents against clinically relevant pathogens. Nat. Prod. Rep. 2010, 27, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D. From natural products to clinically useful antifungals. Biochim. Biophys. Acta Mol. Basis Dis. 2002, 1587, 224–233. [Google Scholar] [CrossRef] [Green Version]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of Antifungal Activity of Terpenoid Phenols Resembles Calcium Stress and Inhibition of the TOR Pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, S.; Kumar, A.; Bawankule, D.U.; Tandon, S.; Singh, A.K.; Verma, R.S.; Saikia, D. Chemical composition, bactericidal kinetics, mechanism of action, and anti-inflammatory activity of Isodon melissoides (Benth.) H. Hara essential oil. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Gavarić, N.; Kovač, J.; Kretschmer, N.; Kladar, N.; Možina, S.S.; Bucar, F.; Bauer, R.; Božin, B. Natural Products as Antibacterial Agents—Antibacterial Potential and Safety of Post-distillation and Waste Material from Thymus vulgaris L., Lamiaceae. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; IntechOpen: London, UK, 2016; p. 31. [Google Scholar]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Identification of Phytotoxic Substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; Amaral, F.M.M.; Coutinho, D.F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Trop. 2020, 212, 105705. [Google Scholar] [CrossRef]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar]
- Romano, B.; Iqbal, A.J.; Maione, F. Natural Anti-Inflammatory Products/Compounds: Hopes and Reality. Mediators Inflamm. 2015, 2015, 374239. [Google Scholar] [PubMed] [Green Version]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.C.e.; Costa, J.S.d.; Figueiredo, R.O.d.; Setzer, W.N.; Silva, J.K.R.d.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. https://doi.org/10.3390/molecules26040965
Silva RCe, Costa JSd, Figueiredo ROd, Setzer WN, Silva JKRd, Maia JGS, Figueiredo PLB. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules. 2021; 26(4):965. https://doi.org/10.3390/molecules26040965
Chicago/Turabian StyleSilva, Renan Campos e, Jamile S. da Costa, Raphael O. de Figueiredo, William N. Setzer, Joyce Kelly R. da Silva, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2021. "Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties" Molecules 26, no. 4: 965. https://doi.org/10.3390/molecules26040965
APA StyleSilva, R. C. e., Costa, J. S. d., Figueiredo, R. O. d., Setzer, W. N., Silva, J. K. R. d., Maia, J. G. S., & Figueiredo, P. L. B. (2021). Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules, 26(4), 965. https://doi.org/10.3390/molecules26040965









