Removal of Cu(II) Contamination from Aqueous Solution by Ethylenediamine@β-Zeolite Composite
Abstract
1. Introduction
2. Experimental
2.1. Materials
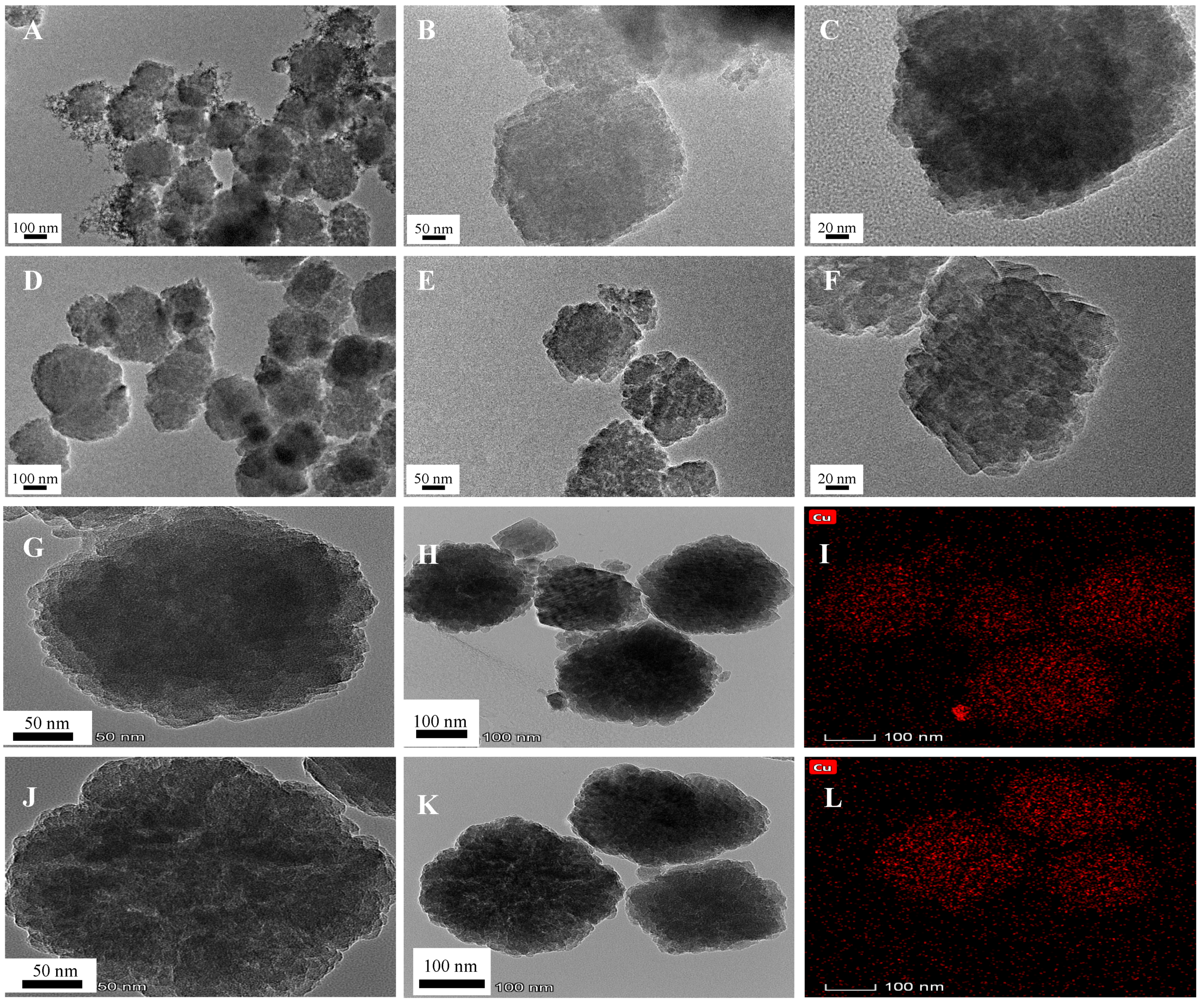
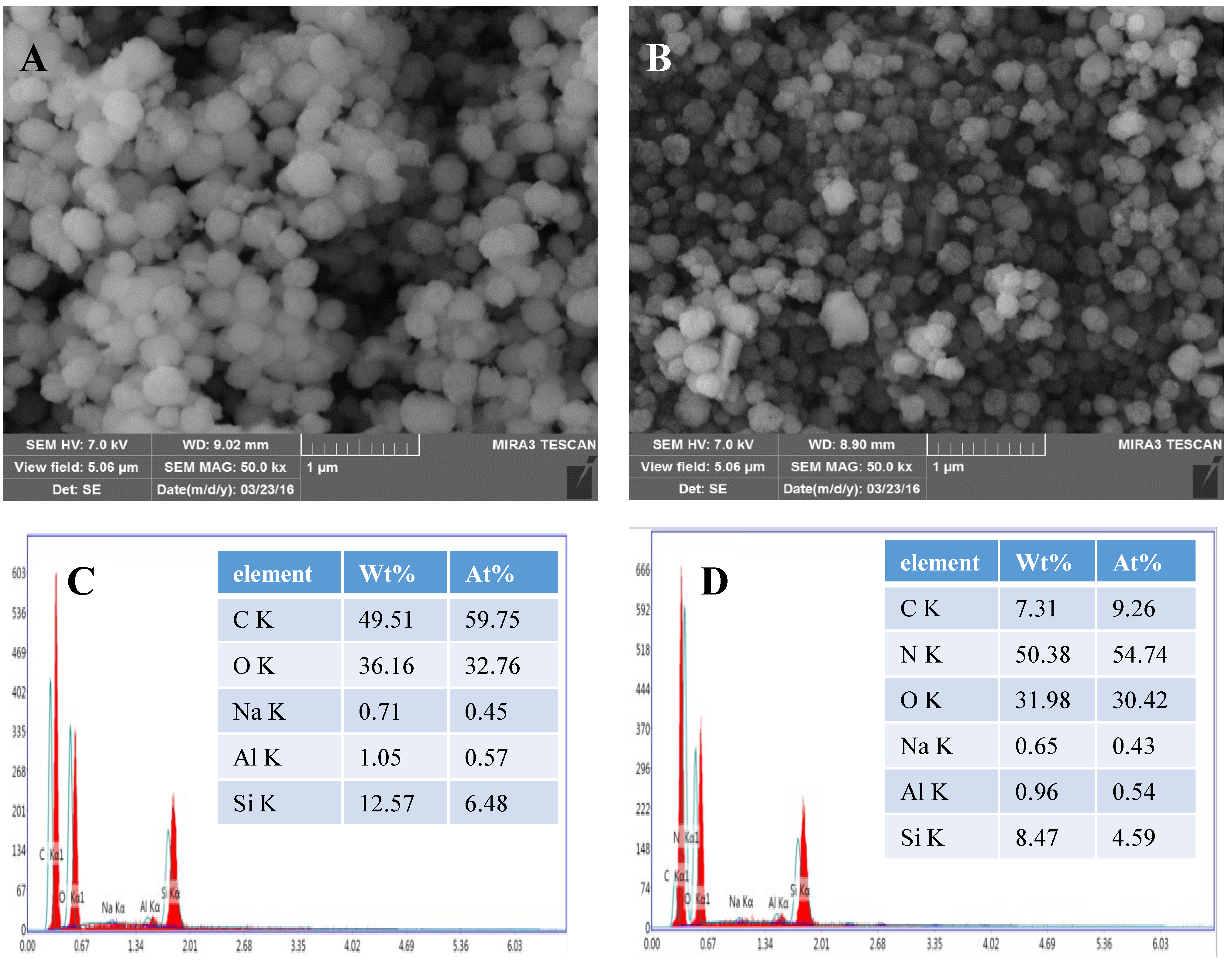
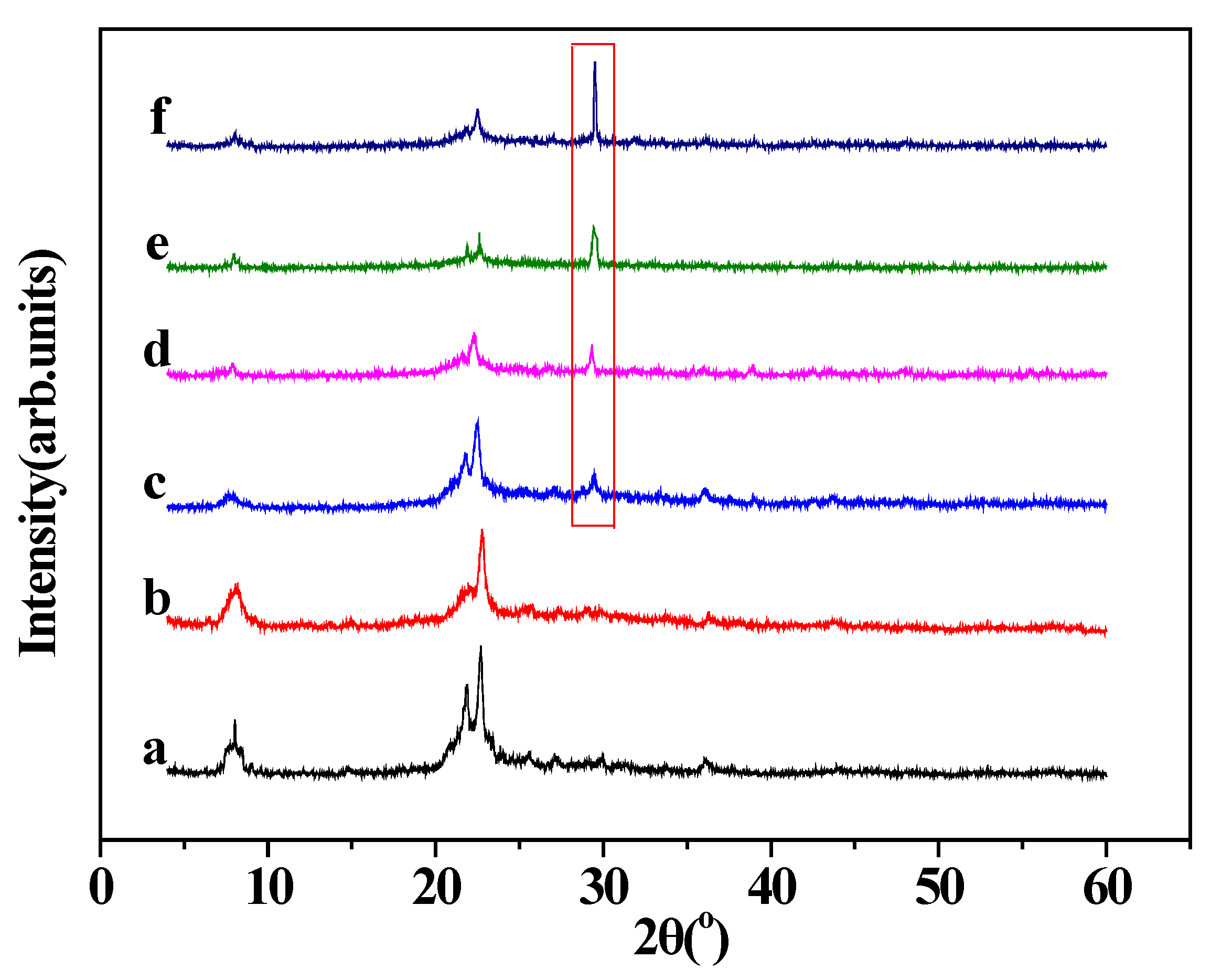
2.2. Characterization
2.3. Batch Experiments
3. Results and Discussion
3.1. Effect of pH on Cu(II) Removal
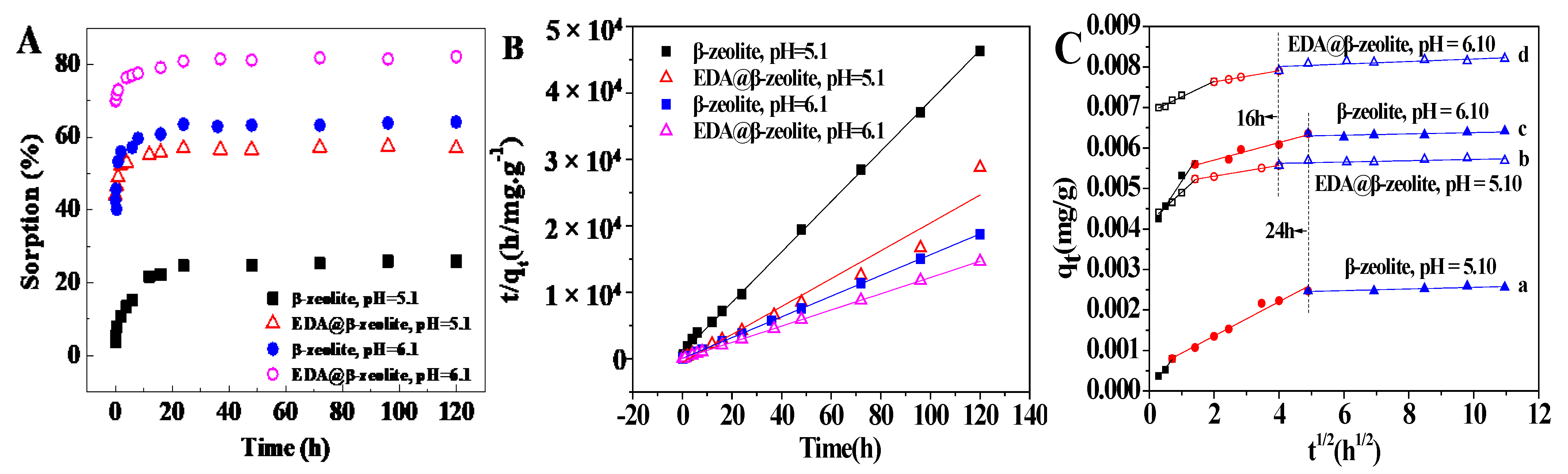
3.2. Removal Kinetics
3.3. Thermodynamic Estimation
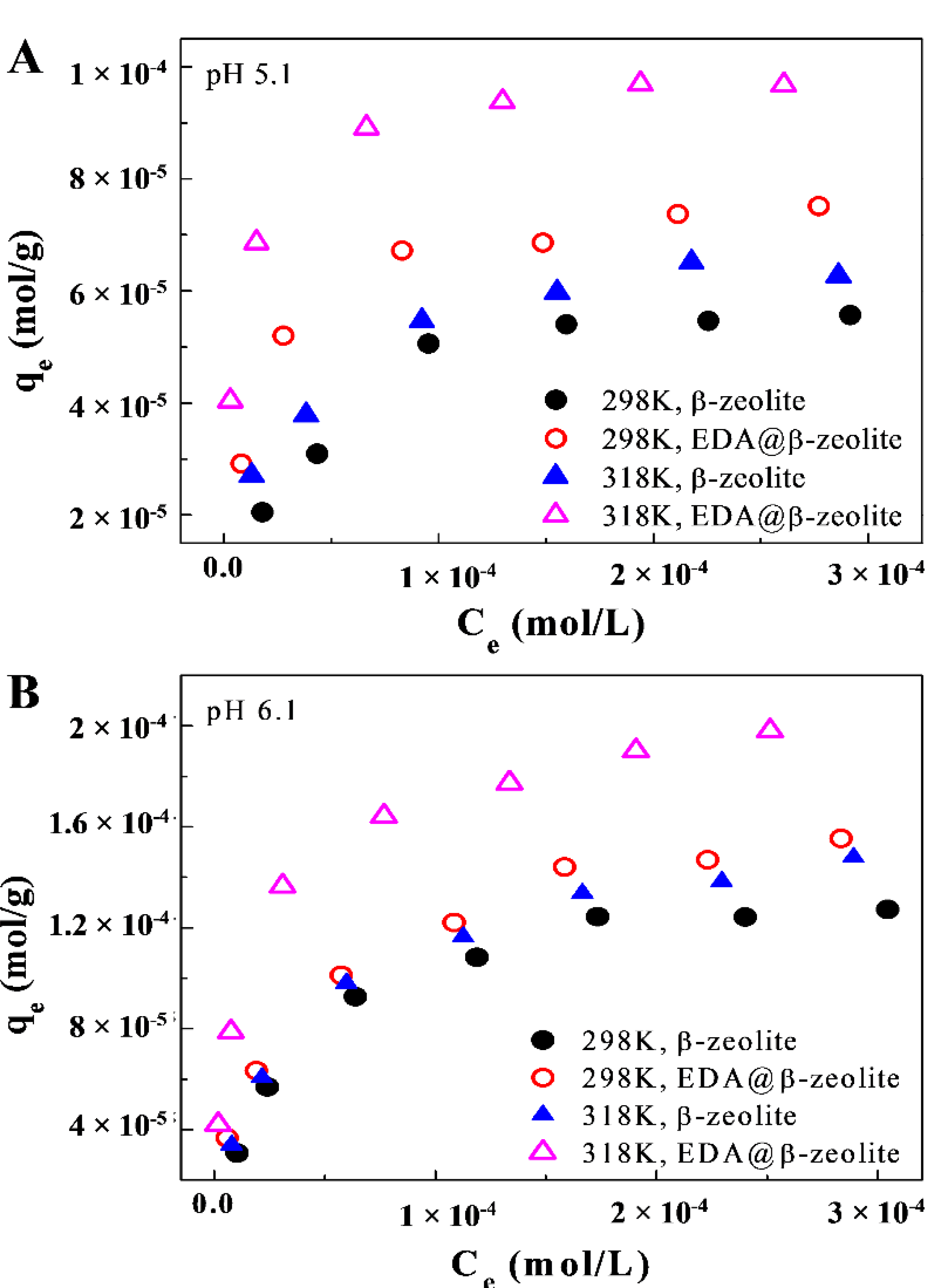
3.4. Removal Isotherms
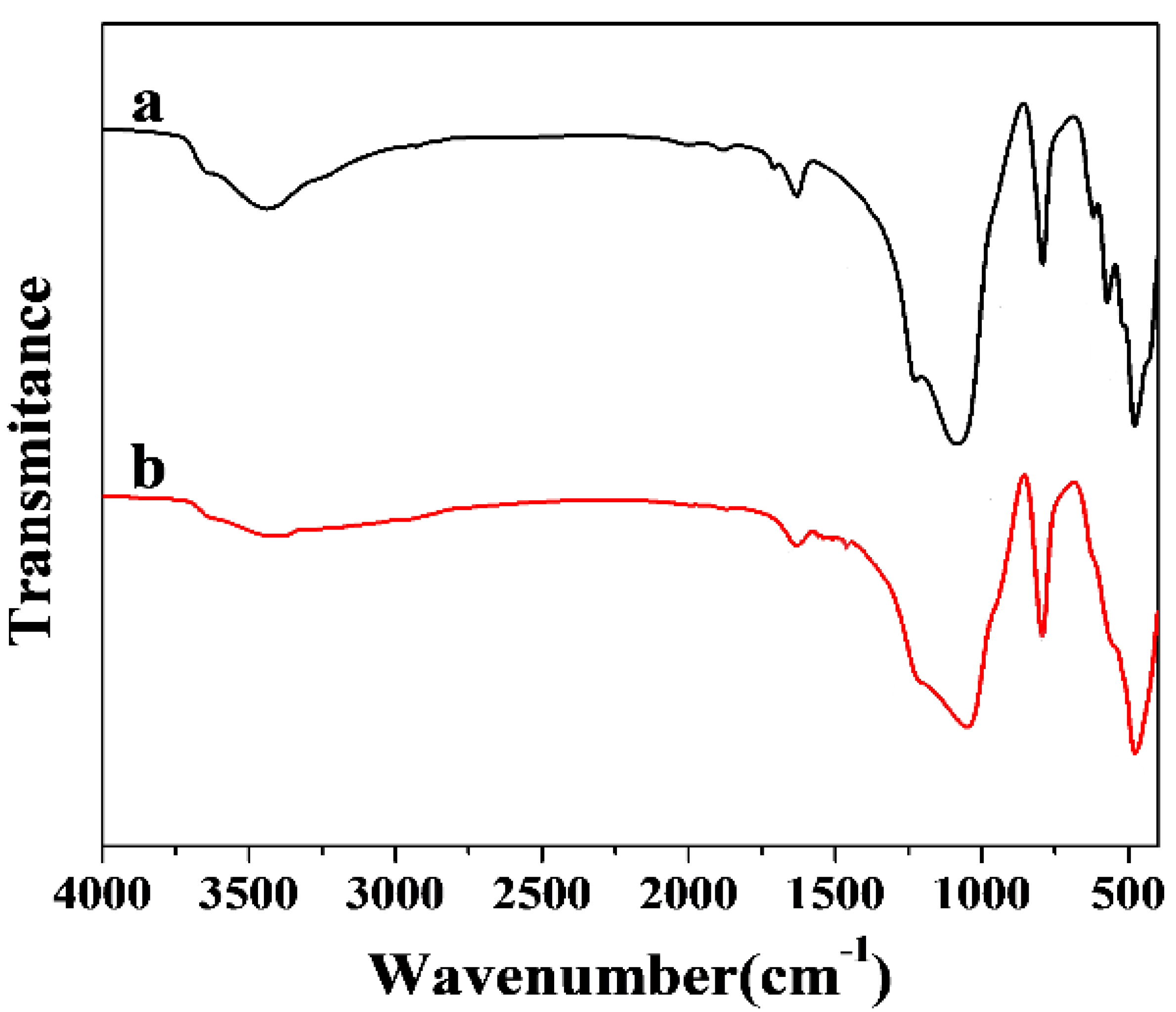
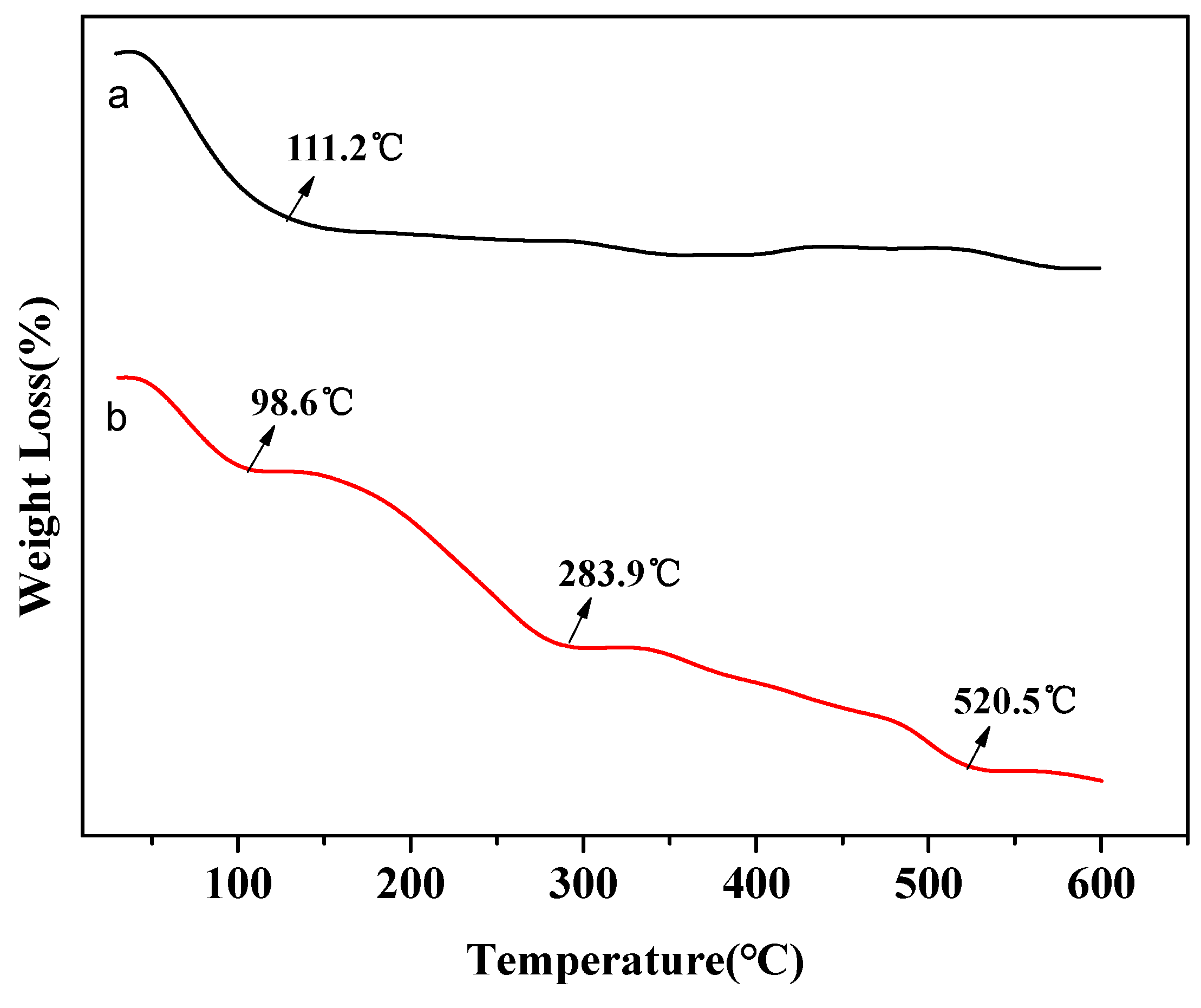
3.5. Removal Mechanism
3.6. Regeneration
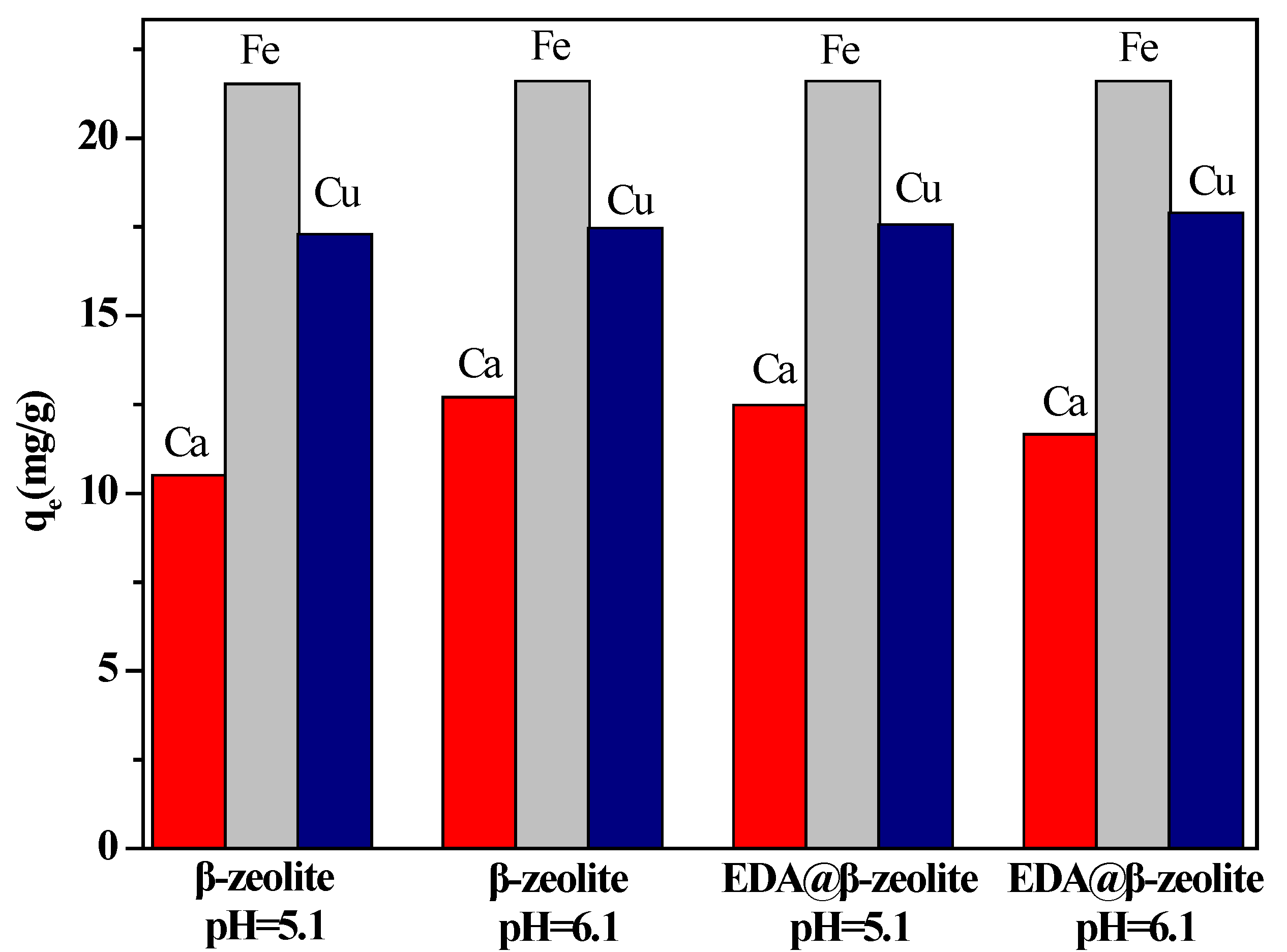
3.7. Effect of Coexisting Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Chen, M.; Yongmei, H. Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2013, 218, 46–54. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Mahajan, S.; Guleria, L.K. Polymers from renewable resources: Sorption of Cu2+ ions by cellulose graft copolymers. Desalination 2000, 130, 85–88. [Google Scholar] [CrossRef]
- Zhu, J.; Cozzolino, V.; Fernandez, M.; Sánchez, R.M.T.; Pigna, M.; Huang, Q.; Violante, A. Sorption of Cu on a Fe-deformed montmorillonite complex: Effect of pH, ionic strength, competitor heavy metal, and inorganic and organic ligands. Appl. Clay Sci. 2011, 52, 339–344. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.; Fan, Q.; Ren, X.; Li, J.; Chen, Y.; Wang, X. Sorption of copper (II) onto super-adsorbent of bentonite–polyacrylamide composites. J. Hazard. Mater. 2010, 173, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, D.; Yang, S. Impact of solution chemistry conditions on the sorption behavior of Cu (II) on Lin’an montmorillonite. Desalination 2011, 269, 84–91. [Google Scholar] [CrossRef]
- Kim, M.S.; Hong, K.M.; Chung, J.G. Removal of Cu(II) from aqueous solutions by adsorption process with anatase-type titanium dioxide. Water Res. 2003, 37, 3524–3529. [Google Scholar] [CrossRef]
- Ren, Y.; Li, N.; Feng, J.; Luan, T.; Wen, Q.; Li, Z.; Zhang, M. Adsorption of Pb (II) and Cu (II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J. Colloid Interface Sci. 2012, 367, 415–421. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, F.; Xu, C.; Gao, J.; Chen, D.; Li, A. Enhanced removal of Cu (II) and Ni (II) from saline solution by novel dual-primary-amine chelating resin based on anion-synergism. J. Hazard. Mater. 2015, 287, 234–242. [Google Scholar] [CrossRef]
- Higgins, J.; LaPierre, R.B.; Schlenker, J.; Rohrman, A.; Wood, J.; Kerr, G.; Rohrbaugh, W. The framework topology of zeolite beta. Zeolites 1988, 8, 446–452. [Google Scholar] [CrossRef]
- Xia, Q.H.; Shen, S.C.; Song, J.; Kawi, S.; Hidajat, K. Structure, morphology, and catalytic activity of β zeolite synthesized in a fluoride medium for asymmetric hydrogenation. J. Catal. 2003, 219, 74–84. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Bala, V.S.S.; Thiruvengadaravi, K.; Nandagopal, J.; Palanichamy, M.; Sivanesan, S. The removal of copper ions from aqueous solution using phosphoric acid modified β-zeolites. Indian J. Sci. Tech. 2009, 2, 63–66. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Zou, W.; Han, R.; Chen, Z.; Jinghua, Z.; Shi, J. Kinetic study of adsorption of Cu(II) and Pb(II) from aqueous solutions using manganese oxide coated zeolite in batch mode. Colloid Surf. A 2006, 279, 238–246. [Google Scholar] [CrossRef]
- Liu, P.; Yuan, N.; Xiong, W.; Wu, H.; Pan, D.; Wu, W. Removal of Nickel(II) from Aqueous Solutions Using Synthesized β-Zeolite and Its Ethylenediamine Derivative. Ind. Eng. Chem. Res. 2017, 56, 3067–3076. [Google Scholar] [CrossRef]
- Esfahani, S.M.B.; Faghihian, H. Modification of synthesized β-zeolite by ethylenediamine and monoethanolamine for adsorption of Pb2+. J. Water Process Eng. 2014, 3, 62–66. [Google Scholar] [CrossRef]
- Peng, L.; Hanyu, W.; Ni, Y.; Zhuoxin, Y.; Duoqiang, P.; Wangsuo, W. β-Zeolite modified by ethylenediamine for sorption of Th(IV). Radiochim. Acta 2017, 105, 463–470. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Yuan, N; Liu, Y.; Pan, D.; Wu, W.S. Removal of U(VI) from aqueous solution using synthesized β-zeolite and its ethylenediamine derivative. J. Mol. Liq. 2017, 56, 40–48. [Google Scholar] [CrossRef]
- García-Sosa, I.; Solache-Ríos, M.; Hernández-Zarate, D. Effect of temperature and ethylenediamine on the sorption behavior of cobalt by zeolite ZSM-5. J. Radioanal. Nucl. Chem. 2000, 243, 817–819. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- Aydın, Y.A.; Aksoy, N.D. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Tan, T. Sorption equilibrium, mechanism and thermodynamics studies of 1, 3-propanediol on beta zeolite from an aqueous solution. Bioresour. Technol. 2013, 145, 37–42. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Shao, D.; Hu, J.; Wang, X. Adsorption of Ni(II) on oxidized multi-walled carbon nanotubes: Effect of contact time, pH, foreign ions and PAA. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Rengaraj, S.; Kim, Y.; Joo, C.K.; Yi, J. Removal of copper from aqueous solution by aminated and protonated mesoporous aluminas: Kinetics and equilibrium. J. Colloid Interface Sci. 2004, 273, 14–21. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Micropor. Mesopor. Mat. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Bartell, F.; Thomas, T.L.; Fu, Y. Thermodynamics of Adsorption from Solutions. IV. Temperature Dependence of Adsorption. J. Phys. Chem. 1951, 55, 1456–1462. [Google Scholar] [CrossRef]
- Ping, L.; Zhuoxin, Y.; Jianfeng, L.; Qiang, J.; Yaofang, D.; Qiaohui, F.; Wangsuo, W. The immobilization of U(VI) on iron oxyhydroxides under various physicochemical conditions. Environ. Sci. Proc. Imp. 2014, 16, 2278–2287. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem 1906, 57, e470. [Google Scholar]
- Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of textile dyes on pine cone from colored wastewater: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 268, 117–125. [Google Scholar] [CrossRef]
- Liu, P.; Qi, W.; Du, Y.; Li, Z.; Wang, J.; Bi, J.; Wu, W. Adsorption of thorium (IV) on magnetic multi-walled carbon nanotubes. Sci. China Chem. 2014, 57, 1483–1490. [Google Scholar] [CrossRef]
- Chen, C.L.; Li, X.L.; Zhao, D.L.; Tan, X.L.; Wang, X.K. Adsorption kinetic, thermodynamic and desorption studies of Th(IV) on oxidized multi-wall carbon nanotubes. Colloid Surf. A 2007, 302, 449–454. [Google Scholar] [CrossRef]
- Lin, S.H.; Wang, C.S. Treatment of high-strength phenolic wastewater by a new two-step method. J. Hazard. Mater. 2002, 90, 205–216. [Google Scholar] [CrossRef]
- Chen, L.; Gao, X. Thermodynamic study of Th(IV) sorption on attapulgite. Appl. Radiat. Isot. 2009, 67, 1–6. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, X.; Sun, L.; Liu, X. Adsorption separation of carbon dioxide, methane and nitrogen on monoethanol amine modified β-zeolite. J. Nat. Gas Chem. 2009, 18, 167–172. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. An investigation of Cu(II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef]



| pH | Sample | qe (mg/g) | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|---|---|
| qe1(mg/g) | R12 | qe2(mg/g) | R22 | |||
| 5.10 | β-zeolite | 0.00272 | 0.001939 | 0.8259 | 0.002636 | 0.9992 |
| 5.10 | EDA@β-zeolite | 0.00454 | 0.000694 | 0.8711 | 0.004515 | 0.9999 |
| 6.10 | β-zeolite | 0.00645 | 0.001462 | 0.7267 | 0.006412 | 0.9999 |
| 6.10 | EDA@β-zeolite | 0.00821 | 0.000726 | 0.8992 | 0.008203 | 0.9999 |
| pH | Sample | Parameters | R12 | |
|---|---|---|---|---|
| 5.10 | β-zeolite | Ka1 = 0.0010 | Ca1 = 0.0001 | 0.9432 |
| Ka2 = 0.0004 | Ca2 = 0.0005 | 0.9748 | ||
| Ka3 = 2 × 10−5 | Ca3 = 0.0024 | 0.7126 | ||
| 5.10 | EDA@β-zeolite | Kb1 = 0.0007 | Cb1 = 0.0042 | 0.9893 |
| Kb2 = 0.0001 | Cb2 = 0.0050 | 0.9958 | ||
| Kb3 = 2 × 10−5 | Cb3 = 0.0056 | 0.4200 | ||
| 6.10 | β-zeolite | Kc1 = 0.0012 | Cc1 = 0.0040 | 0.9605 |
| Kc2 = 0.0002 | Cc2 = 0.0053 | 0.9378 | ||
| Kc3 = 1 × 10−5 | Cc3 = 0.0062 | 0.5071 | ||
| 6.10 | EDA@β-zeolite | Kd1 = 0.0004 | Cd1 = 0.0069 | 0.9786 |
| Kd2 = 0.0001 | Cd2 = 0.0074 | 0.9980 | ||
| Kd3 = 3 × 10−5 | Cd3 = 0.0079 | 0.6320 | ||
| pH | T (K) | Sample | Ln Kd = A Ce + B | Thermodynamic Data | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | R | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/mol·K) | |||
| 5.10 | 298 | β-zeolite | −2194.71 | 2.92 | 0.9924 | −1.0716 | 0.1994 | 4.2649 |
| 5.10 | 298 | EDA@β-zeolite | −3944.03 | 3.39 | 0.9465 | −1.2208 | 0.3622 | 5.3123 |
| 5.10 | 318 | β-zeolite | −3233.53 | 3.18 | 0.9501 | −1.1569 | 0.1994 | |
| 5.10 | 318 | EDA@β-zeolite | −5381.72 | 3.77 | 0.9122 | −1.3271 | 0.3357 | |
| 6.10 | 298 | β-zeolite | −1885.44 | 3.21 | 0.9850 | −1.1663 | 0.3338 | 5.0339 |
| 6.10 | 298 | EDA@β-zeolite | −3418.45 | 3.58 | 0.9272 | −1.2754 | 0.4147 | 5.6715 |
| 6.10 | 318 | β-zeolite | −4138.52 | 3.55 | 0.9782 | −1.2670 | 0.3086 | |
| 6.10 | 318 | EDA@β-zeolite | −5032.34 | 4.01 | 0.9091 | −1.3888 | 0.3864 | |
| Element | β-Zeolite | Cu(II)-Loaded β-Zeolite (pH = 5.10) | Cu(II)-Loaded β-Zeolite (pH = 6.10) | Assignments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | FWHM | Area (%) | BE (eV) | FWHM | Area (%) | BE (eV) | FWHM | Area (%) | ||
| O 1S | 532.10 | 1.14 | 37.26 | 532.15 | 1.14 | 37.75 | 532.16 | 1.14 | 46.42 | Si-O-Al |
| 532.55 | 1.01 | 28.83 | 532.58 | 1.01 | 38.37 | 532.62 | 1.01 | 42.43 | -OH | |
| 533.01 | 1.03 | 33.91 | 533.05 | 1.03 | 42.14 | 533.07 | 1.03 | 11.15 | water | |
| Element | EDA@β-zeolite | Cu(II)-Loaded EDA@β-Zeolite (pH = 5.10) | Cu(II)-Loaded EDA@β-Zeolite (pH = 6.10) | Assignments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE(eV) | FWHM | Area(%) | BE(eV) | FWHM | Area(%) | BE(eV) | FWHM | Area(%) | ||
| O 1S | 531.54 | 1.14 | 32.94 | 531.86 | 1.14 | 35.47 | 531.97 | 1.14 | 35.88 | Si-O-Al |
| 532.05 | 1.01 | 37.86 | 532.27 | 1.01 | 24.72 | 532.48 | 1.01 | 38.31 | -OH | |
| 532.58 | 1.03 | 29.20 | 532.74 | 1.03 | 39.81 | 532.91 | 1.03 | 25.81 | water | |
| N 1S | 399.66 | 1.72 | 23.42 | 399.99 | 1.72 | 67.45 | 400.18 | 1.72 | 72.35 | -NH |
| 401.79 | 2.56 | 76.58 | 401.87 | 2.56 | 32.55 | 401.91 | 2.56 | 27.65 | -NH2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Ruan, H.; Li, T.; Chen, J.; Ma, F.; Pan, D.; Wu, W. Removal of Cu(II) Contamination from Aqueous Solution by Ethylenediamine@β-Zeolite Composite. Molecules 2021, 26, 978. https://doi.org/10.3390/molecules26040978
Liu P, Ruan H, Li T, Chen J, Ma F, Pan D, Wu W. Removal of Cu(II) Contamination from Aqueous Solution by Ethylenediamine@β-Zeolite Composite. Molecules. 2021; 26(4):978. https://doi.org/10.3390/molecules26040978
Chicago/Turabian StyleLiu, Peng, Hui Ruan, Tiantian Li, Jiaqi Chen, Fuqiu Ma, Duoqiang Pan, and Wangsuo Wu. 2021. "Removal of Cu(II) Contamination from Aqueous Solution by Ethylenediamine@β-Zeolite Composite" Molecules 26, no. 4: 978. https://doi.org/10.3390/molecules26040978
APA StyleLiu, P., Ruan, H., Li, T., Chen, J., Ma, F., Pan, D., & Wu, W. (2021). Removal of Cu(II) Contamination from Aqueous Solution by Ethylenediamine@β-Zeolite Composite. Molecules, 26(4), 978. https://doi.org/10.3390/molecules26040978






