Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films
Abstract
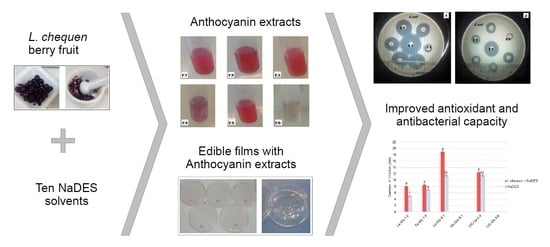
:1. Introduction
2. Results and Discussion
2.1. NADES Performance in Anthocyanin Extraction
2.2. Color and Anthocyanins
2.3. Antibacterial and Antioxidant Activity of Anthocyanin Extracts
2.4. Influence of L. chequen (Molina) A. Gray Extracts on Edible Films
3. Materials and Methods
3.1. Chemical Reagents and Solvents
3.2. Plant Material
3.3. NADES Synthesis
3.4. Extraction of Anthocyanins by Ultrasound
3.5. Chromatographic Profile of Anthocyanins by HPLC-DAD
3.6. Total Monomeric Anthocyanins
3.7. Polymeric Color of Anthocyanins
3.8. CIELab Color
3.9. Antibacterial Capacity of Extracts
3.9.1. Agar Diffusion Method
3.9.2. MIC
3.10. Antioxidant Capacity
3.11. Edible Films
3.12. Antioxidant Capacity of Edible Films
3.12.1. ABTS+ Radical Cation Method
3.12.2. DPPH Radical Method
3.13. Antibacterial Capacity of Edible Films
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, e00215. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Zareie, Z.; Yazdi, F.T.; Mortazavi, S.A. Development and characterization of antioxidant and antimicrobial edible films based on chitosan and gamma-aminobutyric acid-rich fermented soy protein. Carbohydr. Polym. 2020, 244, 116491. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the Extraction of Quercetin from Vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikov, A.N.; Kosman, V.M.; Flissyuk, E.V.; Smekhova, I.E.; Elameen, A.; Pozharitskaya, O.N. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola rosea L. Molecules 2020, 25, 1826. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Redovniković, I.R.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.D.L.Á.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; da Veiga, M.L.; Rocha, M.I.D.U.M.D.; Vizzotto, M.; Rodrigues, E.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.; Scenna, N. Comparison between conventional and ultrasound-assisted techniques for extraction of anthocyanins from grape pomace. Experimental results and mathematical modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Pauletto, R.; Cavalheiro, S.D.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.D.B.D.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.D.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC–DAD–ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Zhang, J.; Yang, B.; Yu, Y.; Liu, T.; Nie, B.; Song, B. Functional analysis of an anthocyanin synthase gene StANS in potato. Sci. Hortic. 2020, 272, 109569. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Gouveia, T.I.; Biernacki, K.; Castro, M.C.; Gonçalves, M.P.; Souza, H.K. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Lapeña, D.; Lomba, L.; Artal, M.; Lafuente, C.; Giner, B. The NADES glyceline as a potential Green Solvent: A comprehensive study of its thermophysical properties and effect of water inclusion. J. Chem. Thermodyn. 2019, 128, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. In Application of Ionic Liquids in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Itoh, T., Koo, Y.M., Eds.; Springer: Cham, Switzerland, 2018; Volume 168. [Google Scholar]
- el Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Cairone, F.; Carradori, S.; Locatelli, M.; Casadei, M.A.; Cesa, S. Reflectance colorimetry: A mirror for food quality—A mini review. Eur. Food Res. Technol. 2019, 246, 259–272. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Gómez-García, M.; Sol, C.; de Nova, P.J.G.; Puyalto, M.; Mesas, L.; Puente, H.; Mencía-Ares, Ó.; Miranda, R.; Argüello, H.; Rubio, P.; et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porc. Health Manag. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; Cadiz-Gurrea, M.D.L.L.; Mahmoodi-Khaledi, E.; Cadiz-Gurrea, M.L. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Lacombe, A.; Wu, V.C.; Tyler, S.; Edwards, K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010, 139, 102–107. [Google Scholar] [CrossRef]
- Das, Q.; Islam, R.; Marcone, M.F.; Warriner, K.; Diarra, M.S. Potential of berry extracts to control foodborne pathogens. Food Control. 2017, 73, 650–662. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Leyton, F.; Ascar, L.; Ramirez, O.; Giordano, A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CyTA J. Food 2020, 18, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Ulloa-Inostroza, E.M.; Ulloa-Inostroza, E.G.; Alberdi, M.; Peña-Sanhueza, D.; González-Villagra, J.; Jaakola, L.; Reyes-Díaz, M. Native Chilean fruits and the effects of their functional compounds on human health. In Superfood and Functional Food: An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; InTech: Rijeka, Croatia, 2017; pp. 99–130. [Google Scholar]
- Velásquez, P.; Montenegro, G. Chilean Endemic/Native Plants Resources as Functional and Superfoods. In Superfood and Functional Food: An Overview of their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-2920-2. [Google Scholar]
- Lillo, A.; Carvajal-Caiconte, F.; Nuñez, D.; Balboa, N.; Zamora, M.A. Cuantificación espectrofotométrica de compuestos fenólicos y actividad antioxidante en distintos berries nativos del Cono Sur de América. RIA. Rev. Investig. Agropecu. 2016, 42, 168–174. Available online: https://www.redalyc.org/pdf/864/86447075010.pdf (accessed on 20 July 2019).
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2019, 40, 12725. [Google Scholar] [CrossRef]
- De Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.M.; Hadi, R.S.; Mohammad, A.M.S.; Ahmad, S.Y.S.; Ghorbani, H.A. Improving antibacterial activity of edible films based on chitosan by incorporating thyme and clove essential oils and EDTA. J. Appl. Sci. 2008, 8, 2895–2900. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2014, 17, 1718–1727. [Google Scholar] [CrossRef]
- Fu, Y.; Sarkar, P.; Bhunia, A.K.; Yao, Y. Delivery systems of antimicrobial compounds to food. Trends Food Sci. Technol. 2016, 57, 165–177. [Google Scholar] [CrossRef] [Green Version]

| NADES (Code) | Monomeric Anthocyanins (mg/g dw) | Total Anthocyanins (mg/g dw) | Total Anthocyanins (mg Eq Cyanidin 3-Glucoside/100 g dw) |
|---|---|---|---|
| La-Gly 1:1 | 1.16 ± 0.02 e,f | 1.28 ± 0.02 c | 127.7 ± 2.1 c |
| La-Gly 1:2 | 2.22 ± 0.04 b,c,d | 2.40 ± 0.04 a,b,c | 240.4 ± 4.2 a,b,c |
| La-Gly 2:1 | 2.07 ± 0.04 c,d | 2.16 ± 0.51 b,c | 216.4 ± 5.1 b,c |
| Ta-Gly 1:2 | 0.66 ± 0.00 f | 0.81 ± 0.04 d | 81.1 ± 4.2 e |
| Ta-Gly 1:3 | 1.65 ± 0.04 d,e | 1.76 ± 0.02 c,d | 175.8 ± 2.1 c,d |
| Ta-Gly 1:4 | 2.90 ± 0.02 a,b | 3.05 ± 0.10 a,b | 305.0 ± 10.6 a,b |
| La-Glu 8:1 | 2.76 ± 0.42 a,b,c | 3.30 ± 0.04 a | 330.6 ± 4.2 a |
| Gly-Glu 8:1 | 2.94 ± 0.08 a | 3.06 ± 0.08 a,b | 306.5 ± 8.5 a,b |
| ClC-Ca 5:4 | 2.07 ± 0.08 c,d | 2.27 ± 0.08 a,b,c | 226.9 ± 2.0 a,b,c |
| ClC-Gly 4:6 | 3.13 ± 0.35 a | 3.28 ± 0.46 a,b | 327.6 ± 46.7 a,b |
| Ethanol | 0.98 ± 0.06 g | 1.16 ± 0.03 d | 116.2 ± 3.6 d |
| Solvents | Polymeric Color | Color Density | % Polymeric Color | L* | a* | b* | C* | H° |
|---|---|---|---|---|---|---|---|---|
| La-Gly 1:2 | 0.5 ± 0.1 c | 2.3 ± 0.4 a | 25.5 ± 0.0 e | 19.4 ± 2.0 c | 19.7 ± 3.7 a | 7.3 ± 0.2 b,c | 21.0 ± 3.4 a | 20.6 ± 4.2 b |
| Ta-Gly 1:4 | 1.0 ± 0.1 b | 3.6 ± 1.4 a | 28.4 ± 0.1 c | 18.8 ± 0.1 c | 18.1 ± 0.4 a | 7.9 ± 4.2 a,b | 19.9 ± 2.1 a,b | 22.9 ± 10.6 b |
| La-Glu 8:1 | 1.4 ± 0.4 a | 4.9 ± 0.9 a | 29.4 ± 0.0 c | 21.5 ± 0.3 c | 21.1 ± 1.4 a | 5.6 ± 2.5 b,c | 20.4 ± 1.5 a | 15.9 ± 6.2 c |
| Gly-Glu 8:1 | 1.7 ± 1.5 a | 3.0 ± 1.5 a | 55.2 ± 0.2 a | 23.9 ± 1.9 b | 8.9 ± 0.1 c | 2.1 ± 1.6 c | 9.2 ± 0.4 c | 13.3 ± 9.8 c |
| ClC-Ca 5:4 | 0.7 ± 0.2 c | 2.4 ± 1.0 a | 31.1 ± 0.1 c | 19.4 ± 0.3 c | 22.8 ± 0.0 a | 10.5 ± 2.9 a | 25.2 ± 1.2 a | 24.6 ± 5.8 b |
| ClC-Gly 4:6 | 0.8 ± 0.3 c | 1.9 ± 1.2 a | 40.0 ± 0.1 b | 27.5 ± 0.5 a | 7.1 ± 0.6 c | 8.9 ± 3.4 a,b | 11.5 ± 3.0 c | 50.6 ± 8.1 a |
| Ethanol | 0.6 ± 0.1 c | 2.2±0.9 a | 27.2 ± 0.1 d | 26.6± 0.3 a | 13.9 ± 0.1 b | 13.8 ± 1.5 a | 19.6 ± 1.6 a,b | 44.5 ± 5.1 a |
| Antioxidant Capacity | Antibacterial Capacity | ||||
|---|---|---|---|---|---|
| Edible Films | DPPH Inhibition (%) | ABTS Inhibition (%) | E. coli (mm) | S. typhi (mm) | S. aureus (mm) |
| Control (C) | 7.35 ± 2.04 a | 21.72 ± 0.74 b | ND | ND | ND |
| C + (La-Gly 1:2) | 7.38 ± 2.61 a | 30.50 ± 1.95 a | 5.0 ±0.1 b | ND | ND |
| C + (Ta-Gly 1:4) | 8.71 ± 4.65 a | 22.47 ± 0.00 b | 5.0 ± 0.1 b | ND | 6.0 ±0.1 b |
| C + (La-Glu 8:1) | 10.81±1.99 a | 21.73 ± 2.18 b | 6.0 ± 0.1 a | 6.0 ± 0.1 a | 6.0 ± 0.1 b |
| C + (Gly-Glu 8:1) | 7.44 ± 2.52 a | 23.85 ± 2.11 a,b | 5.0 ± 0.1 b | ND | 6.0 ± 0.2 b |
| C + (ClC-Gly 4:6) | 7.26 ± 2.77 a | 20.87 ± 2.11 b | 5.0 ± 0.1 b | ND | 7.0 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules 2021, 26, 984. https://doi.org/10.3390/molecules26040984
Velásquez P, Bustos D, Montenegro G, Giordano A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules. 2021; 26(4):984. https://doi.org/10.3390/molecules26040984
Chicago/Turabian StyleVelásquez, Patricia, Daniela Bustos, Gloria Montenegro, and Ady Giordano. 2021. "Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films" Molecules 26, no. 4: 984. https://doi.org/10.3390/molecules26040984
APA StyleVelásquez, P., Bustos, D., Montenegro, G., & Giordano, A. (2021). Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules, 26(4), 984. https://doi.org/10.3390/molecules26040984