Optimization and Validation of HS-GC/MS Method for the Controlled Release Study of Microencapsulated Specific Bioattractants for Target-Plaguicide Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microencapsulation Characterization.by Differential Scanning Calotimetry (DSC)
2.2. Optimization and Validation of HS-GC/MS Method for Bioattractants Analysis in the Microencapsulated Product
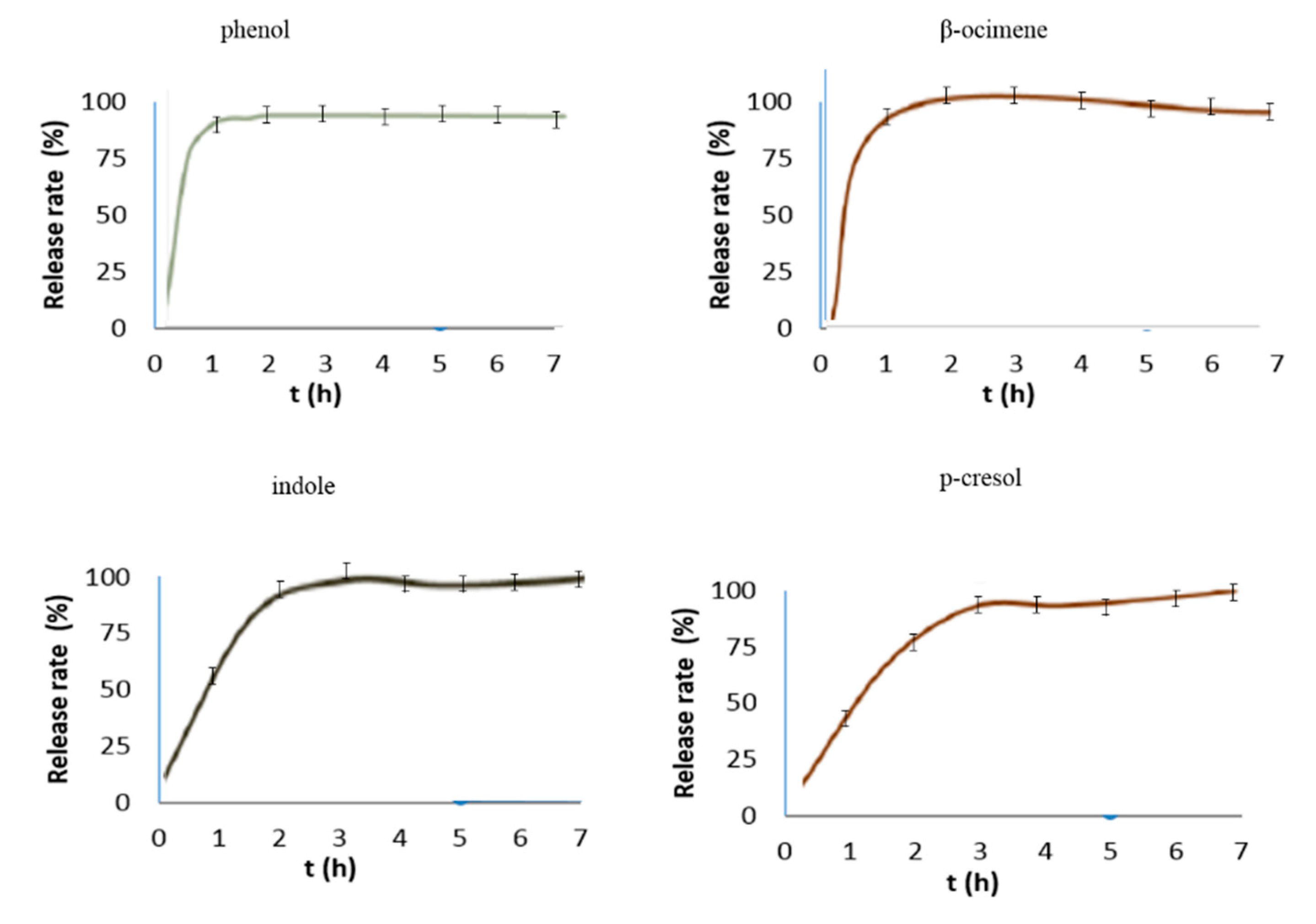
2.3. Microencapsulated Bioattractants Controlled Release Study
3. Material and Methods
3.1. Material
3.2. Methods
3.2.1. Microencapsulation Procedure
3.2.2. Microencapsulation Characterization.by Differential Scanning Calorimetry (DSC)
3.2.3. Optimization and Validation of HS-GC/MS Method for Bioattractants Analysis in the Microencapsulated Product
3.2.4. Microencapsulated Bioattractants Controlled Release Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO report. Manual for the Public Health Management of Chemical Incidents. Available online: http://www.who.int/environmental_health_emergencies/publications/FINAL-PHM-chemical-Incidents_web.pdf (accessed on 13 December 2020).
- Khalequzzaman, M.; Ara, H.; Zohura, F.; Nahar, J. Toxic, Repellent and Attractant Properties of Some Insecticides Towards the Housefly (Musca domestica L.). J. Biol. Sci. 2002, 2, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Tangtrakulwanich, K. Sensory Morphology and Chemical Ecology of the Stable Fly, Stomoxys Calcitrans: Host-Seeking and Ovipositional Selection. Available online: http://digitalcommons.unl.edu/entomologydiss (accessed on 13 December 2020).
- Baker, T.C.; Cosse, A.A. Fly Attractant Compositions. U.S. Patent 6106821, 22 August 2020. [Google Scholar]
- Rohlfs, M.; Churchill, A.C. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011, 48, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Etxaniz, A. Bioatrayentes Específicos Para el Control de Insectos Sinantrópicos. Master’s Thesis, Universidad del País Vasco (UPV/EHU), Leioa, Spain, 2014. [Google Scholar]
- EPA Programs. Biopesticides Registration Action Document. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-036604_01-Nov-10.pdf (accessed on 13 December 2020).
- Quinn, B.P.; Bernier, U.R.; Geden, C.J.; Hogsette, J.A.; Carlson, D.A. Analysis of extracted and volatile components in blackstrap molasses feed as candidate house fly attractants. J. Chromatogr. A 2007, 1139, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Appl. Clay Sci. 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Garrido, J.; Cagide, F.; Melle-Franco, M.; Borges, F.; Garrido, J.; Cagide-Fagín, F. Microencapsulation of herbicide MCPA with native β-cyclodextrin and its methyl and hydroxypropyl derivatives: An experimental and theoretical investigation. J. Mol. Struct. 2014, 1061, 76–81. [Google Scholar] [CrossRef]
- Scher, H.B. Microencapsulation of pesticides by Interfacial polymerization: Process and performance considerations from Pesticide Chemistry: Human Welfare and the Environment. In Agris, 2nd ed.; Greenhalgh, R., Drescher, N., Eds.; Pergamon Press: Oxford, UK, 1983; pp. 295–300. [Google Scholar]
- Calazans, S.H.C.; Americo, J.A.; Fernandes, F.D.C.; Aldridge, D.C.; Rebelo, M.D.F. Assessment of toxicity of dissolved and microencapsulated biocides for control of the Golden Mussel Limnoperna fortunei. Mar. Environ. Res. 2013, 91, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.; Alonso, M.; Bartolome, L.; Alonso, R. Headspace Gas Chromatography Methods and their Potential Industrial Applications. Curr. Chromatogr. 2014, 1, 100–121. [Google Scholar] [CrossRef]
- Alonso, M.L.; Recio, G.; Alonso, R.; Jiménez, R.; Laza, J.; Vilas-Vilela, J.L.; Fananas, R. Advantages of biocides:β-cyclodextrin inclusion complexes against active components for pesticide industry. Int. J. Environ. Anal. Chem. 2011, 92, 963–978. [Google Scholar] [CrossRef]
- Román, I.S.; Alonso, M.L.; Bartolomé, L.; Alonso, R.; Fananas, R. Analytical strategies based on multiple headspace extraction for the quantitative analysis of aroma components in mushrooms. Talanta 2014, 123, 207–217. [Google Scholar] [CrossRef]
- Alonso, M.L.; Laza, J.M.; Alonso, R.M.; Jiménez, R.M.; Vilas-Vilela, J.L.; Fañanás, R.; Alonso, R.M. Pesticides microencapsulation. A safe and sustainable industrial process. J. Chem. Technol. Biotechnol. 2013, 89, 1077–1085. [Google Scholar] [CrossRef]
- EPA Programs. Recognition and Management of Pesticide Poisonings. Available online: https://www.epa.gov/sites/production/files/2015-01/documents/rmpp_6thed_final_lowresopt.pdf (accessed on 13 December 2020).
- WHO report. Evaluation and Testing of Insecticides. Available online: http://apps.who.int/iris/bitstream/handle/10665/65962/CTD_WHOPES_IC_96.1.pdf?sequence=1&isAllowed=y (accessed on 13 December 2020).
- WHO report. Pesticides and Their Application: For the Control of Vectors and Pests of Public Health Importance. Available online: http://apps.who.int/iris/bitstream/handle/10665/69223/WHO_CDS_NTD_WHOPES_GCDPP_2006.1_eng.pdf?sequence=1&isAllowed=y (accessed on 13 December 2020).
- EPA Programs. List of Restricted Use Products (RUP). Available online: https://www.epa.gov/pesticide-worker-safety/restricted-use-products-rup-report (accessed on 13 December 2020).
- FAO and WHO report. Codex Pesticides 25 Residues in Food and Feed. Available online: http://www.fao.org/fao-who-codexalimentarius/standards/pesticide-mrls/ (accessed on 13 December 2020).
- EC No 1107. Active Substances Regulation. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 13 December 2020).
- EC No 396 Pesticides EU- MRLs Regulation. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0396 (accessed on 13 December 2020).
- Alonso, M.L. Tesis Doctoral en la Universidad del Pais Vasco–Euskal Herriko Unibertsitatea, Vitoria-Gasteiz, Spain (UPV/EHU). 2011. [Google Scholar]
- Del Valle, E. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Ho, T.M.; Howes, T.; Bhandari, B.R. Encapsulation of CO2 into amorphous and crystalline α-cyclodextrin powders and the characterization of the complexes formed. Food Chem. 2015, 187, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Martínez, G.; Gómez, M.A. Ciclodextrinas: Complejos de inclusión con polímeros. Revista Iberoamericana de Polímeros 2007, 8, 300–312. [Google Scholar]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Cabral-Marques, H. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced Thermal Stability of Eugenol by Cyclodextrin Inclusion Complex Encapsulated in Electrospun Polymeric Nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, J.; Manojlovic, V.; Levic, S.; Rajic, N.; Nedović, V.; Bugarski, B. Microencapsulation of Flavors in Carnauba Wax. Sensors 2010, 10, 901–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Ruellan, S.; Fourmentin, S. Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food Chem. 2014, 164, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Inclusion interactions of cyclodextrins and crosslinked cyclodextrin polymers with linalool and camphor in Lavandula angustifolia essential oil. Carbohydr. Polym. 2012, 87, 1963–1970. [Google Scholar] [CrossRef]
- Bahadir, M. Safe formulations of agrochemicals. Chemosphere 1987, 16, 615–621. [Google Scholar] [CrossRef]
- Dailey, O.D. Volatilization of Alachlor from Polymeric Formulations. J. Agric. Food Chem. 2004, 52, 6742–6746. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.S. An experimental study of relative humidity effect on VOCs’ effective diffusion coefficient and partition coefficient in a porous medium. Build. Environ. 2011, 46, 1785–1796. [Google Scholar] [CrossRef]
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, A.K. Controlled pesticide release from biodegradable polymers. Open Chem. 2014, 12, 453–469. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. Discuss. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Feigenbrugel, V.; Le Calvé, S.; Mirabel, P.; Louis, F. Henry’s law constant measurements for phenol, o-, m-, and p-cresol as a function of temperature. Atmos. Environ. 2004, 38, 5577–5588. [Google Scholar] [CrossRef]
- Huynh, C.T.; Lee, D.S. Controlled Release, from Encyclopedia of Polymeric Nanomaterials. In Encyclopedia of Polymeric Nanomaterials, 2nd ed.; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin, Germany, 2015; pp. 439–449. [Google Scholar]
- Zhou, H.-Y.; Jiang, L.-J.; Zhang, Y.-P.; Li, J. β-Cyclodextrin inclusion complex: Preparation, characterization, and its aspirin release in vitro. Front. Mater. Sci. 2012, 6, 259–267. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins and their inclusion complexes. In Cyclodextrin Technology, 2nd ed.; Szejtli, J., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1988; pp. 1–296. [Google Scholar]
- Harrison, M.A.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmospheric Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Allen, S.K.; Allen, C.W. Phenol concentrations in air and rain water samples collected near a wood preserving facility. Bull. Environ. Contam. Toxicol. 1997, 59, 702–707. [Google Scholar] [CrossRef] [PubMed]
- The Portable Unscrambler v. 9.7 CAMO 2005, Norway. 2005.
- Liu, H.; Tang, Q.; Markovich, R.J.; Rustum, A.M. A general static-headspace gas chromatographic method for determination of residual benzene in oral liquid pharmaceutical products. J. Pharm. Biomed. Anal. 2011, 54, 417–421. [Google Scholar] [CrossRef]
- Operating Manual, Agilent 7694 Headspace Sampler. Wilmington, DE, USA. 2000.
- EPA Programs. Calibration Curves: Program Use/Needs. Available online: https://www.epa.gov/measurements-modeling/calibration-curves-program-useneeds (accessed on 13 December 2020).
- SANTE/12682/2019. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commission Directorate General for Health and Food Safety: Brussel, Belgium, 2019; pp. 1–42. [Google Scholar]

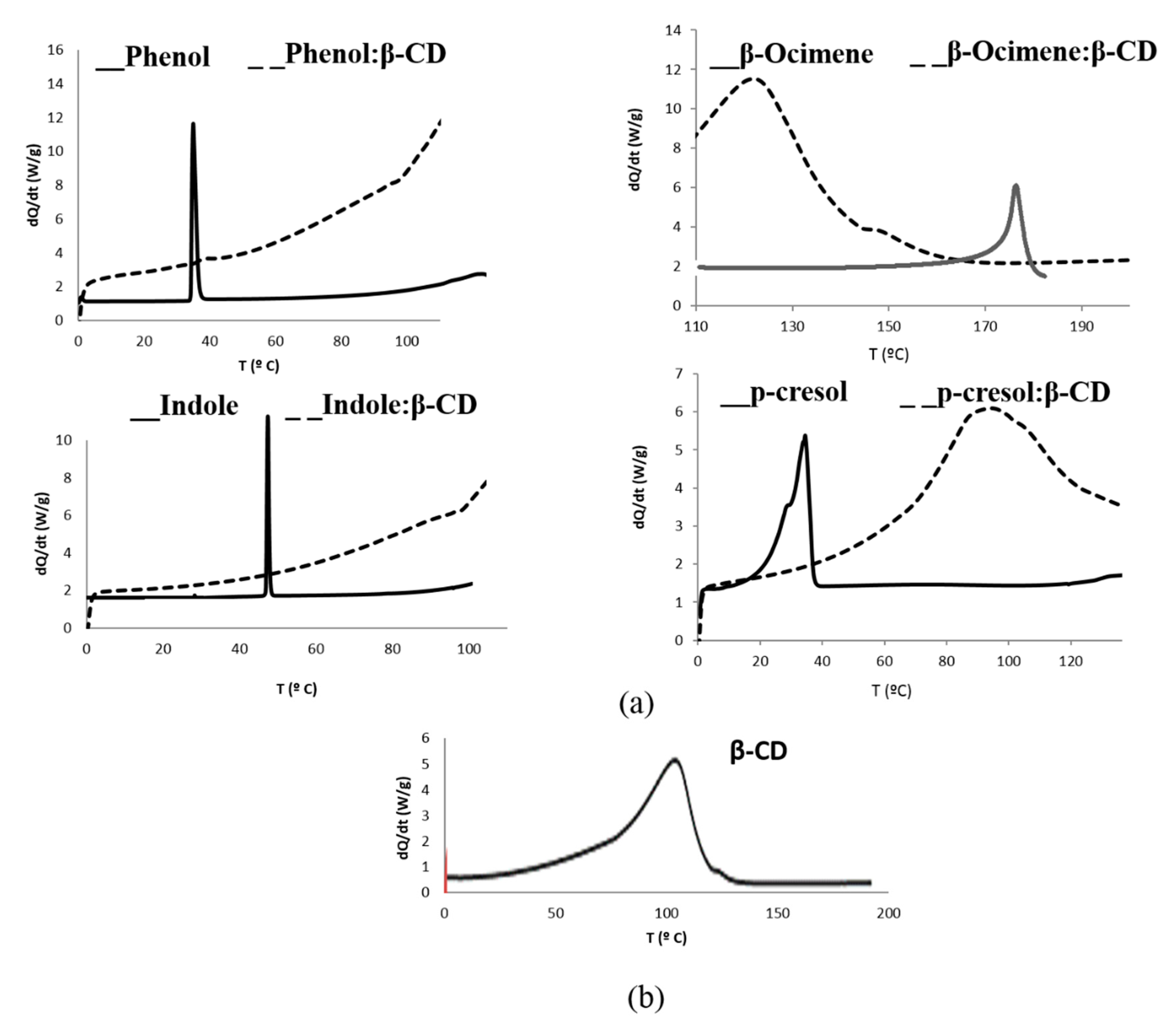
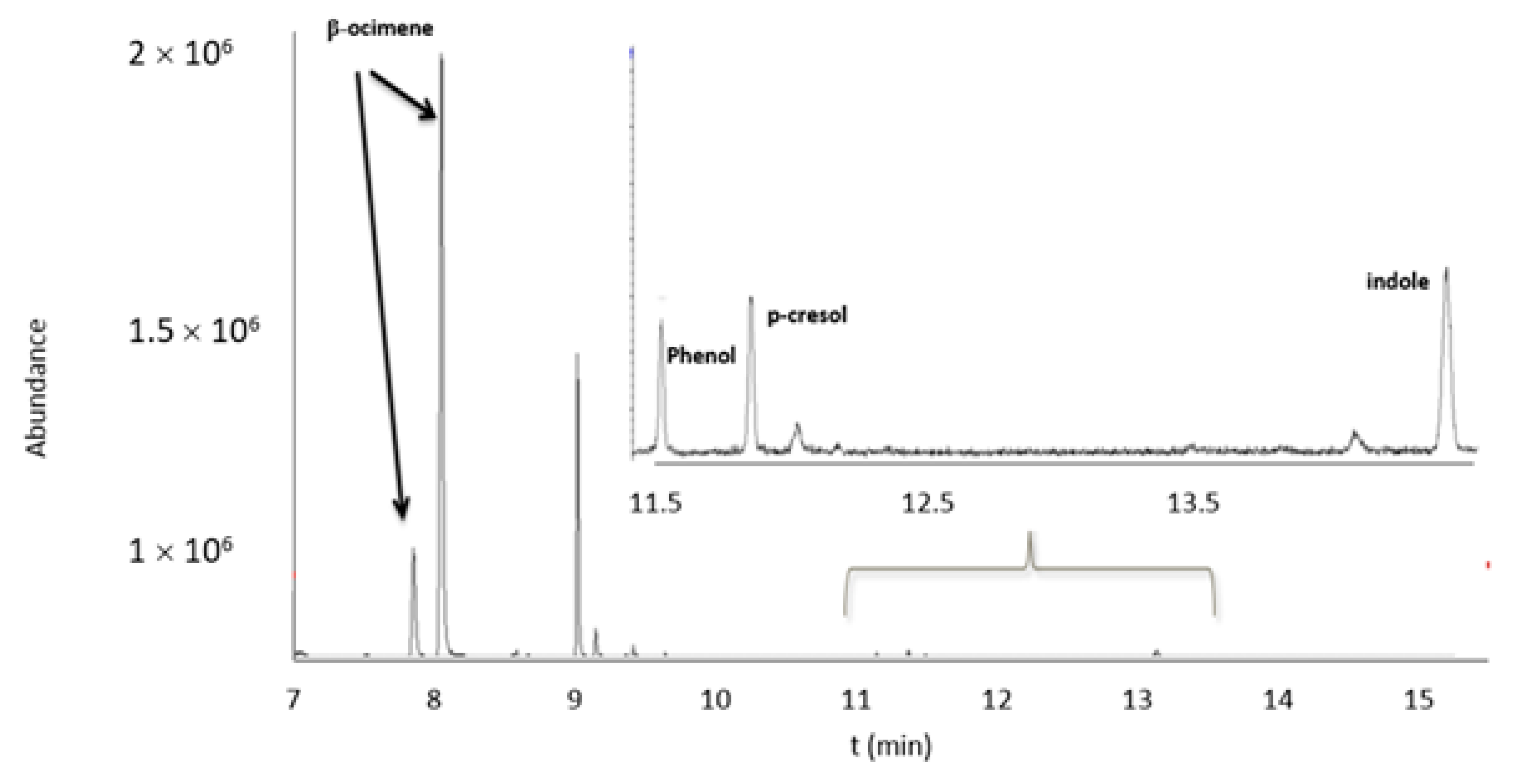
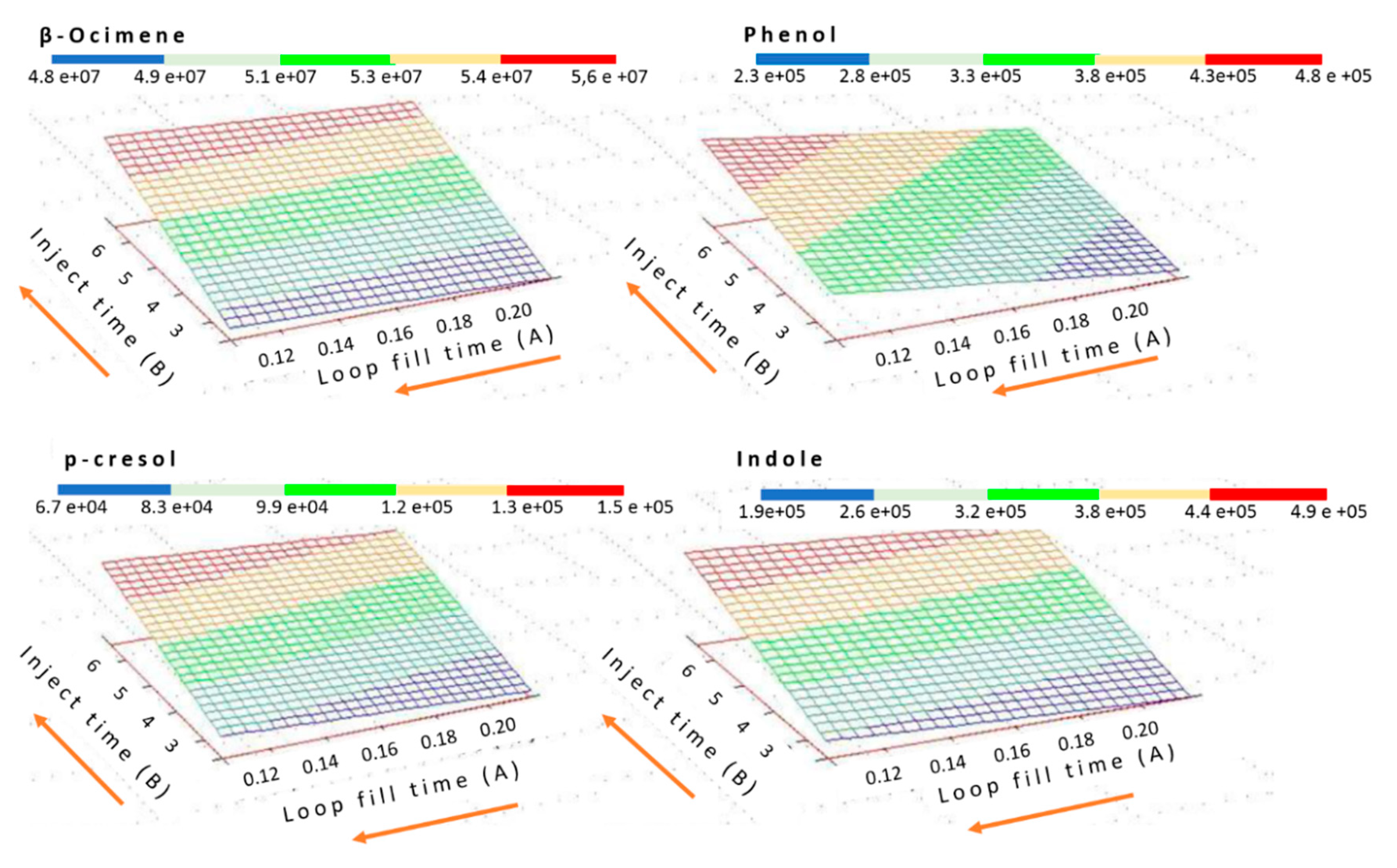

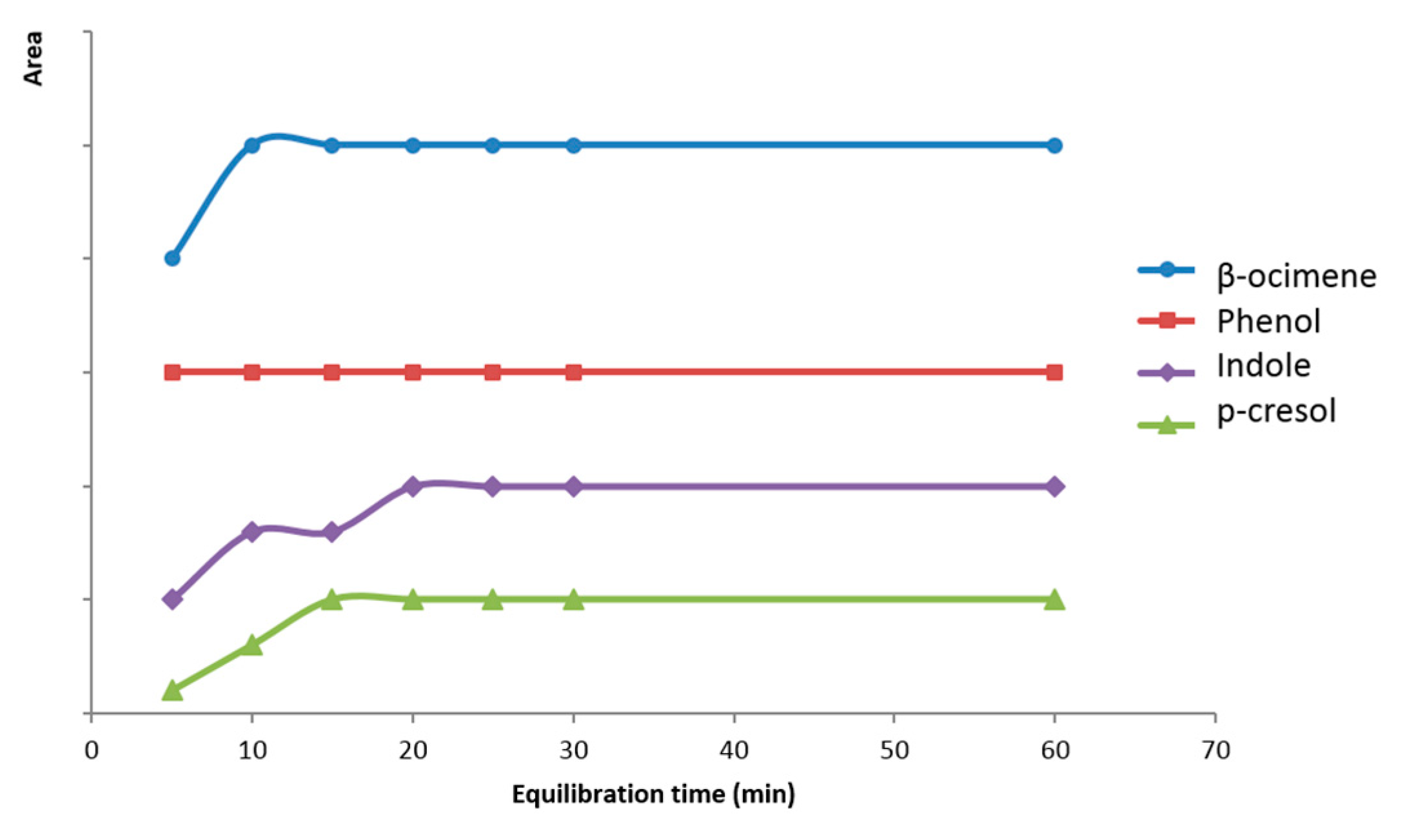

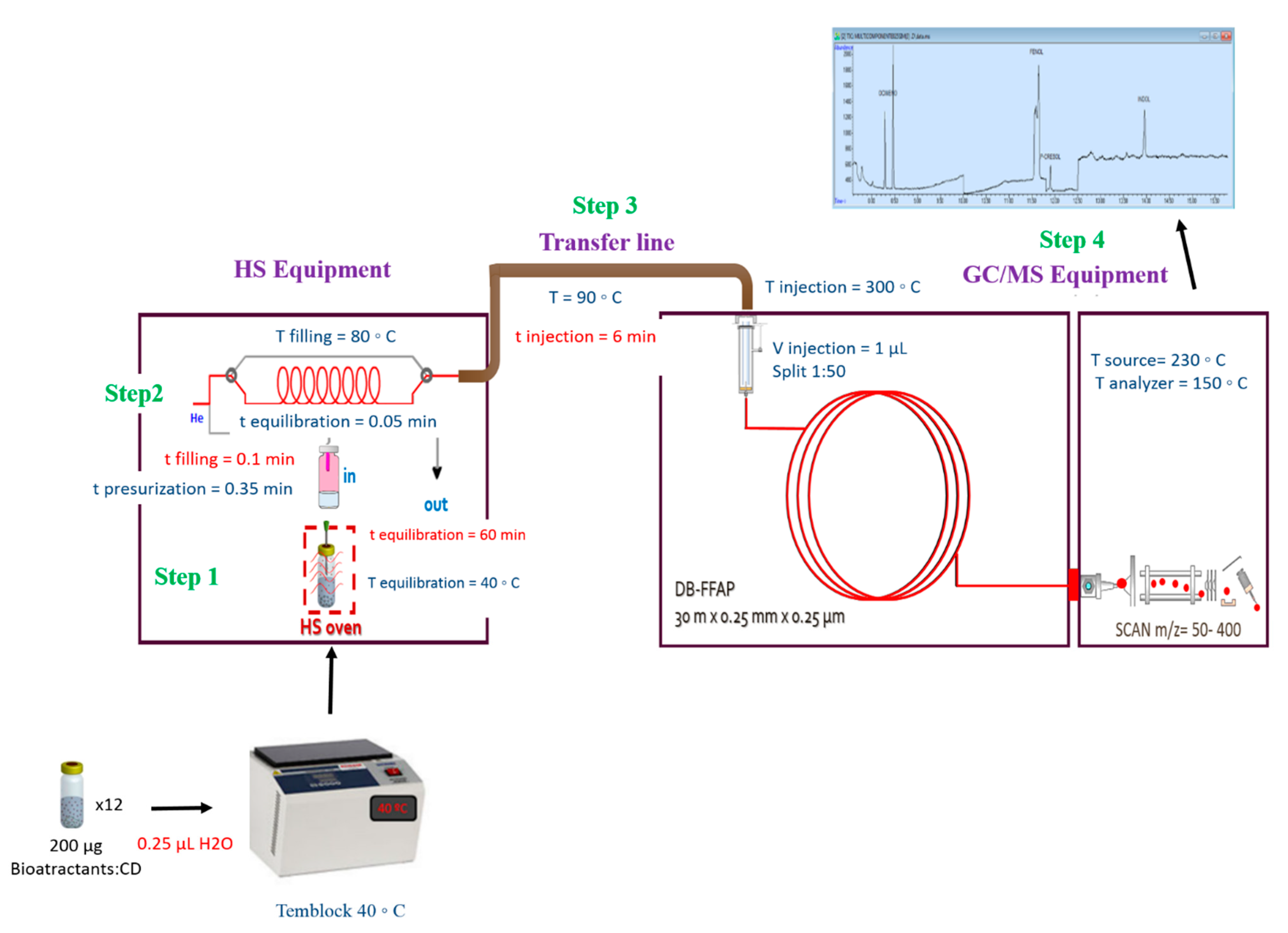
| Compound | Retention Time (min) | m/z |
|---|---|---|
| β-ocimene | 8.02/8.23 | 79, 91, 93 |
| Phenol | 11.47 | 39, 66, 94 |
| p-cresol | 11.71 | 107, 108 |
| Indole | 13.56 | 90, 117 |
| Experiment | Injection Time (min) | Loop Fill Time (min) |
|---|---|---|
| 1 | 4.50 | 0.10 |
| 2 | 4.50 | 0.22 |
| 3 | 2.40 | 0.16 |
| 4 | 6.60 | 0.16 |
| 5 | 3.00 | 0.12 |
| 6 | 3.00 | 0.20 |
| 7 | 6.00 | 0.12 |
| 8 | 6.00 | 0.20 |
| 9 | 4.50 | 0.16 |
| 10 | 4.50 | 0.16 |
| 11 | 4.50 | 0.16 |
| Compund | Concentration (mg/L) | Recovery % | RSD % | ||
|---|---|---|---|---|---|
| Trueness Inter-Day | Trueness Intra-Day | Repeatability Inter-Day | Repeatability Intra-Day | ||
| β-ocimene | 0.2 (LOQ) 0.5 1 | 103.2 116.1 109.9 | 105.9 115.1 109.1 | 13.0 9.8 13.5 | 13.4 7.8 13.0 |
| Phenol | 0.15 (LOQ) 0.5 1 | 98.2 98.4 91.3 | 98.9 99.1 94.4 | 8.6 9.1 8.5 | 3.3 10.2 7.5 |
| p-cresol | 0.15 (LOQ) 0.5 1 | 91.1 94.3 95.6 | 90.5 92.7 89.3 | 8.4. 7.9 16.7 | 2.4 9.0 10.7 |
| Indole | 0.1 (LOQ) 0.5 1 | 106.9 106.4 97.6 | 107.7 106.8 94.0 | 9.2 6.5 13.4 | 9.5 6.2 14.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, M.L.; González, O.; Alonso, R.M. Optimization and Validation of HS-GC/MS Method for the Controlled Release Study of Microencapsulated Specific Bioattractants for Target-Plaguicide Production. Molecules 2021, 26, 996. https://doi.org/10.3390/molecules26040996
Alonso ML, González O, Alonso RM. Optimization and Validation of HS-GC/MS Method for the Controlled Release Study of Microencapsulated Specific Bioattractants for Target-Plaguicide Production. Molecules. 2021; 26(4):996. https://doi.org/10.3390/molecules26040996
Chicago/Turabian StyleAlonso, María Luz, Oskar González, and Rosa María Alonso. 2021. "Optimization and Validation of HS-GC/MS Method for the Controlled Release Study of Microencapsulated Specific Bioattractants for Target-Plaguicide Production" Molecules 26, no. 4: 996. https://doi.org/10.3390/molecules26040996






