Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia
Abstract
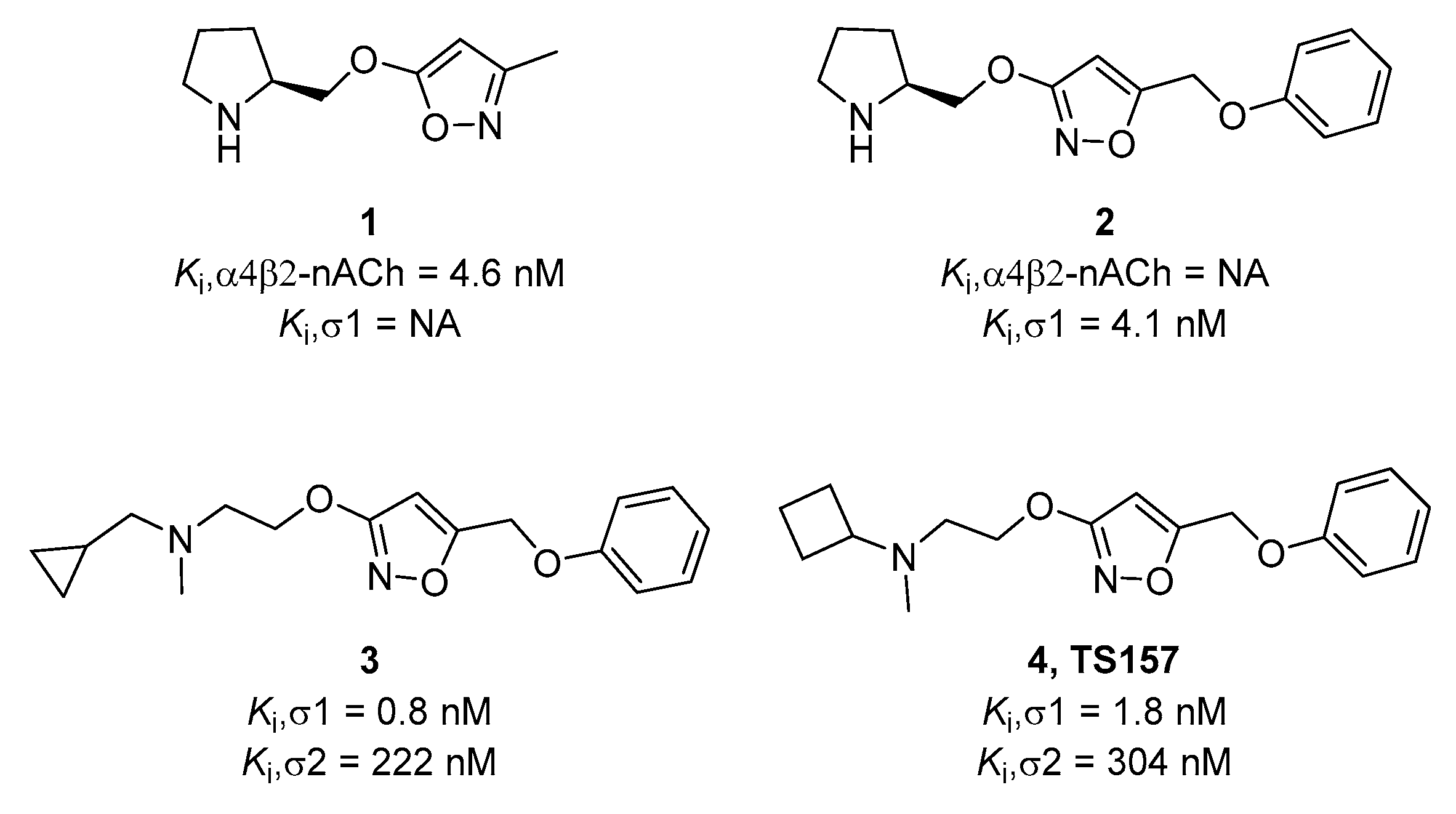
1. Introduction
2. Results
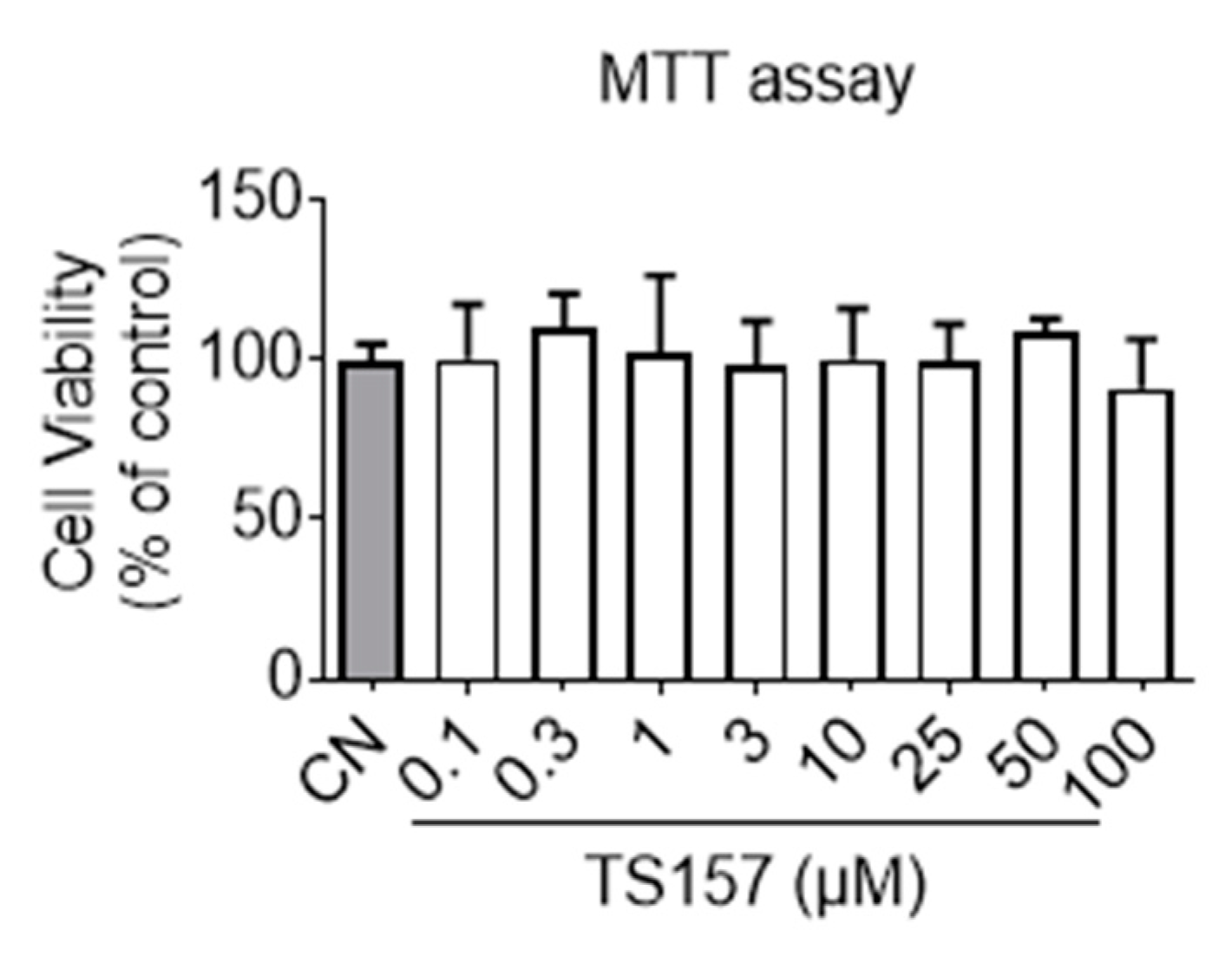
2.1. In Vitro Non-Cytotoxicity and Membrane Permeability of TS-157
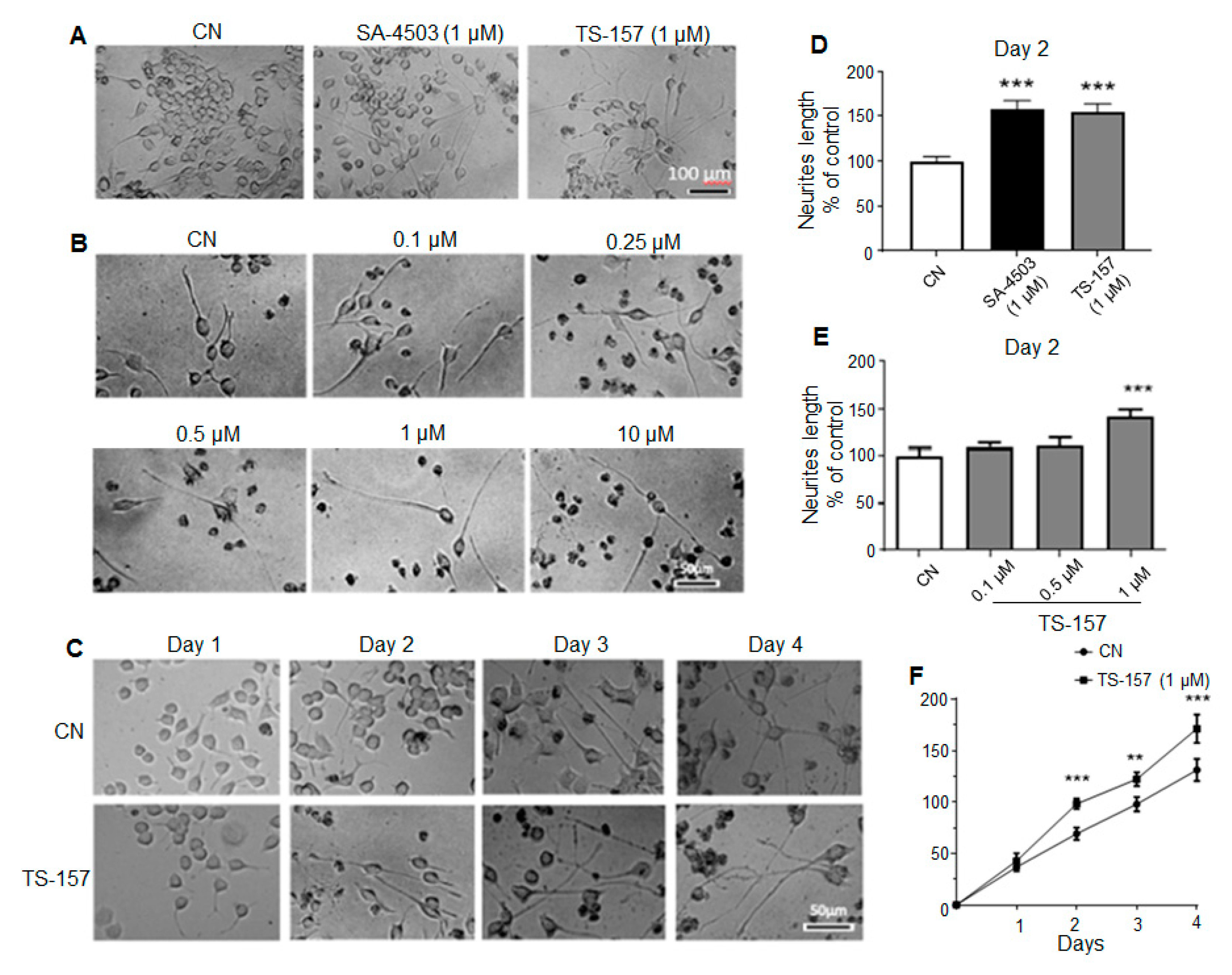
2.2. TS-157 Induces Neurite Outgrowth of N1E-115 Neuronal Cell
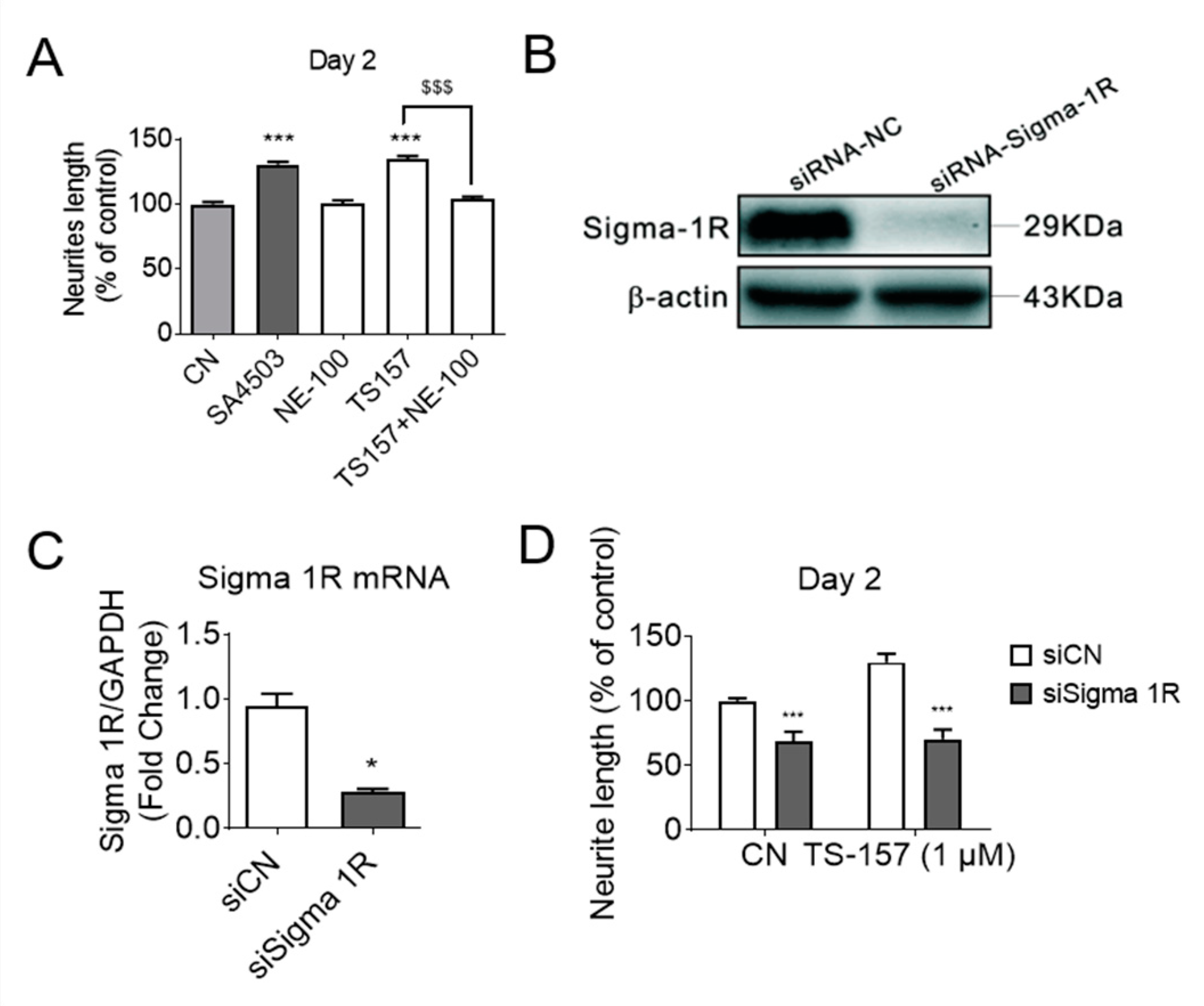
2.3. TS-157 Induces Neurite Outgrowth through σ-1 Receptor
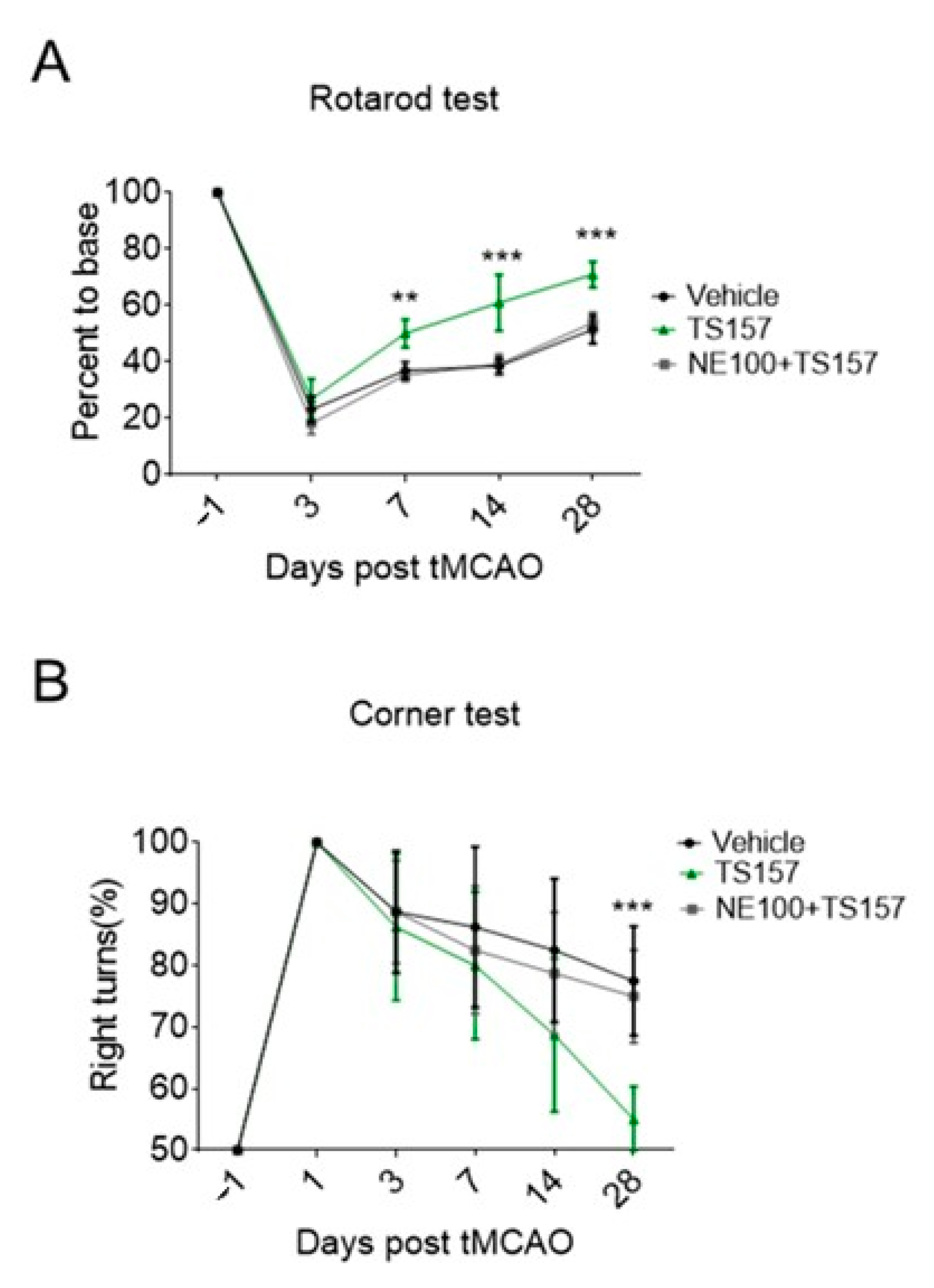
2.4. Motor Function Recovery of Long-Term tMCAO Rats by TS-157 Administration
2.5. No Amelioration for Cerebral Infarct Volume of tMCAO Rats by TS-157
2.6. The σ-1 Antagonist Reverses the Recovery of Motor Function in tMCAO Rats
2.7. ERK Pathway Participates in the Protection of Exercise Capacity of TS-157
3. Discussion
4. Materials and Methods
4.1. Preparation of TS-157
4.2. MTT Assay
4.3. PAMPA Test
4.4. Neurite Outgrowth in N1E-115 Cells
4.5. RNA Isolation and Quantitative Real-Time PCR
4.6. Western Blotting
4.7. The tMCAO in SD Rats
4.8. Rotarod Test
4.9. Corner Test
4.10. Measurement of Infarct Size
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human sigma1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.; Phan, V.; Guillemain, I.; Saunier, M.; Legrand, A.; Anoal, M.; Maurice, T. ς1 receptor-related neuroactive steroids modulate cocaine-induced reward. Neuroscience 2000, 97, 155–170. [Google Scholar] [CrossRef]
- Su, T.P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Hayashi, T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 2010, 332, 388–397. [Google Scholar] [CrossRef]
- Hayashi, T.; Maurice, T.; Su, T.P. Ca(2+) signaling via sigma(1)-receptors: Novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000, 293, 788–798. [Google Scholar]
- Aydar, E.; Palmer, C.P.; Klyachko, V.A.; Jackson, M.B. The Sigma Receptor as a Ligand-Regulated Auxiliary Potassium Channel Subunit. Neuron 2002, 34, 399–410. [Google Scholar] [CrossRef]
- Kourrich, S.; Su, T.P.; Fujimoto, M.; Bonci, A. The sigma-1 receptor: Roles in neuronal plasticity and disease. Trends Neurosci. 2012, 35, 762–771. [Google Scholar] [CrossRef]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farre, D.; Aguinaga, D.; Mallol, J.; Cortes, A.; Casado, V.; Lluis, C.; et al. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Turcotte, M.E.; Halman, S.; Bergeron, R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol. 2007, 578, 143–157. [Google Scholar] [CrossRef]
- Kourrich, S.; Hayashi, T.; Chuang, J.Y.; Tsai, T.S.; Su, Y.P.; Bonci, A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 2013, 152, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Morin-Surun, M.P.; Collin, T.; Denavit-Saubié, M.; Baulieu, E.E.; Monnet, F.P. Intracellular sigma-1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc. Natl. Acad. Sci. USA 1999, 96, 8196–8199. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Hayashi, T.; Harvey, B.K.; Wang, Y.; Wu, W.W.; Shen, R.; Zhang, F.; Becker, Y.K.G.; Hoffer, J.; Su, T.P. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 22468–22473. [Google Scholar] [CrossRef] [PubMed]
- Natsvlishvili, N.; Goguadze, N.; Zhuravliova, E.; Mikeladze, D. Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria. BMC Biochem. 2015, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef]
- Heissa, K.; Raffaeleb, M.; Vanellab, L.; Murabitoc, P.; Prezzaventob, O.; Marrazzob, A.; Aricòb, G.; Castracania, C.C.; Barbagallob, I.; Zappalàa, A.; et al. (+)-Pentazocine attenuates SH-SY5Y cell death, oxidative stress and microglial migration induced by conditioned medium from activated microglia. Neurosci. Lett. 2017, 642, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tavernarakis, N.; Driscoll, M. Necrotic celldeath in C. elegans requires the function of calreticulin and regulators of Ca2+release from the endoplasmic reticulum. Neuron 2001, 31, 957–971. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Chen, I.L.; Korkina, O.; Teng, X.; Abbott, D.W.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-Reperfusion Injury in Stroke. Interv. Neurol. 2013, 1, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.; Back, T.; Lindauer, U.; Busch, C.; Megow, D.; Dreier, J.P.; Dirnagl, U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Fujita, Y.; Shibata, K.; Mori, M.; Yamashita, T. Sigma-1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PLoS ONE 2013, 8, e75760. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Justinova, Z.; Hayashi, E.; Cormaci, G.; Mori, T.; Tsai, S.; Barnes, C.; Goldberg, S.R.; Su, T.P. Regulation of σ-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J. Pharmacol. Exp. Ther. 2010, 332, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Katnik, C.; Garcia, A.S.; Behensky, A.A.; Yasny, I.E.; Shuster, A.M.; Seredenin, S.B.; Petrov, A.V.; Seifu, S.; Mcaleer, J.; Willing, A.E.; et al. Treatment with afobazole at delayed time points following ischemic stroke improves long-term functional and histological outcomes. Neurobiol. Dis. 2014, 62, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.F.; Tuckmantel, W.; Eaton, J.B.; Caldarone, B.; Fedolak, A.; Hanania, T.; Brunner, D.; Lukas, R.J.; Kozikowski, A.P. Identification of novel α4β2-nicotinic acetylcholine receptor (nAChR) agonists based on an isoxazole ether scaffold that demonstrate antidepressant-like activity. J. Med. Chem. 2012, 55, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.F.; Zhang, H.K.; Gunosewoyo, H.; Kozikowski, A.P. From α4β2 nicotinic ligands to the discovery of σ1 receptor ligands: Pharmacophore analysis and rational design. ACS Med. Chem. Lett. 2012, 3, 1054–1058. [Google Scholar] [CrossRef]
- Sun, H.; Shi, M.; Zhang, W.; Zheng, Y.M.; Xu, Y.Z.; Shi, J.J.; Liu, T.; Gunosewoyo, H.; Pang, T.; Gao, Z.B.; et al. Development of novel alkoxyisoxazoles as sigma-1 receptor antagonists with antinociceptive efficacy. J. Med. Chem. 2016, 59, 6329–6343. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.J.; Shi, W.W.; Yang, F.; Tang, J.; Pang, T.; Yu, L.F. Discovery of N-cyclobutylaminoethoxyisoxazole derivatives as novel sigma-1 receptor ligands with neurite outgrowth efficacy in cells. RSC Adv. 2018, 8, 7080–7088. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; Mcconnell, O.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Bezar, I.F.; Petusky, S.; Huang, Y. Comparison of blood–brain barrier permeability assays: In situ brain perfusion, MDR1-MDCKII and PAMPA-BBB. J. Pharm. Sci. 2009, 98, 1980–1991. [Google Scholar] [CrossRef]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood–brain barrier penetration. J. Pharm. Sci. 1999, 88, 815–821. [Google Scholar] [CrossRef]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef]
- Allahtavakoli, M.; Jarrott, B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res. Bull. 2011, 85, 219–224. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Yan, R.; Hu, M. PI3K/Akt and ERK/MAPK signaling promote different aspects of neuron survival and axonal regrowth following rat facial nerve axotomy. Neurochem Res. 2017, 42, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef] [PubMed]
- Nardaia, S.; Lászlóa, M.; Szabób, A.; Alpárc, A.; Hanicsc, J.; Zaholac, P.; Merkelya, B.; Frecskad, E.; Nagya, Z. N,N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, K.; Shamloo, M.; Rickhag, M.; Ladunga, I.; Soriano, L.; Gisselsson, L.; Toresson, H.; Ruslim-Litrus, L.; Oksenberg, D.; Urfer, R.; et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain 2011, 134, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Onoa, Y.; Tanakaa, H.; Takataa, M.; Nagaharaa, Y.; Nodaa, Y.; Tsurumaa, K.; Shimazawaa, M.; Hozumib, I.; Haraa, H. SA4503, a sigma-1 receptor agonist, suppresses motor neuron damage in in vitro and in vivo amyotrophic lateral sclerosis models. Neurosci. Lett. 2014, 559, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.; Zhou, R.; Qi, X.; Wang, J.; Wu, F.; Yang, W.L.; Zhang, W.N.; Sun, T.; Li, Y.X.; Yu, J.Q. Protective effects of aloin on oxygen and glucose deprivation-induced injury in PC12 cells. Brain Res. Bull. 2016, 121, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, Y.; Wang, Y.; Wang, Y.; He, L.; Jiang, Z.; Huang, Z.; Liao, H.; Li, J. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav. Immun. 2015, 50, 298–313. [Google Scholar] [CrossRef] [PubMed]



| Compound | Tested Pe 10−6 cm/s a | Theoretical Pe 10−6 cm/s | LogBB b | CNS MPO c |
|---|---|---|---|---|
| Verapamil | 12.3 ± 0.41 | 16.0 | / | / |
| Atenolol | 0.81 ± 0.22 | 0.8 | / | / |
| TS-157 | 3.15 ± 0.35 | −0.005 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.-J.; Jiang, Q.-H.; Zhang, T.-N.; Sun, H.; Shi, W.-W.; Gunosewoyo, H.; Yang, F.; Tang, J.; Pang, T.; Yu, L.-F. Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia. Molecules 2021, 26, 1212. https://doi.org/10.3390/molecules26051212
Shi J-J, Jiang Q-H, Zhang T-N, Sun H, Shi W-W, Gunosewoyo H, Yang F, Tang J, Pang T, Yu L-F. Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia. Molecules. 2021; 26(5):1212. https://doi.org/10.3390/molecules26051212
Chicago/Turabian StyleShi, Jun-Jie, Qi-Hui Jiang, Tian-Ning Zhang, Hao Sun, Wen-Wen Shi, Hendra Gunosewoyo, Fan Yang, Jie Tang, Tao Pang, and Li-Fang Yu. 2021. "Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia" Molecules 26, no. 5: 1212. https://doi.org/10.3390/molecules26051212
APA StyleShi, J.-J., Jiang, Q.-H., Zhang, T.-N., Sun, H., Shi, W.-W., Gunosewoyo, H., Yang, F., Tang, J., Pang, T., & Yu, L.-F. (2021). Sigma-1 Receptor Agonist TS-157 Improves Motor Functional Recovery by Promoting Neurite Outgrowth and pERK in Rats with Focal Cerebral Ischemia. Molecules, 26(5), 1212. https://doi.org/10.3390/molecules26051212






