Broadband Visible Light-Absorbing [70]Fullerene-BODIPY-Triphenylamine Triad: Synthesis and Application as Heavy Atom-Free Organic Triplet Photosensitizer for Photooxidation
Abstract
1. Introduction
2. Results and Discussion
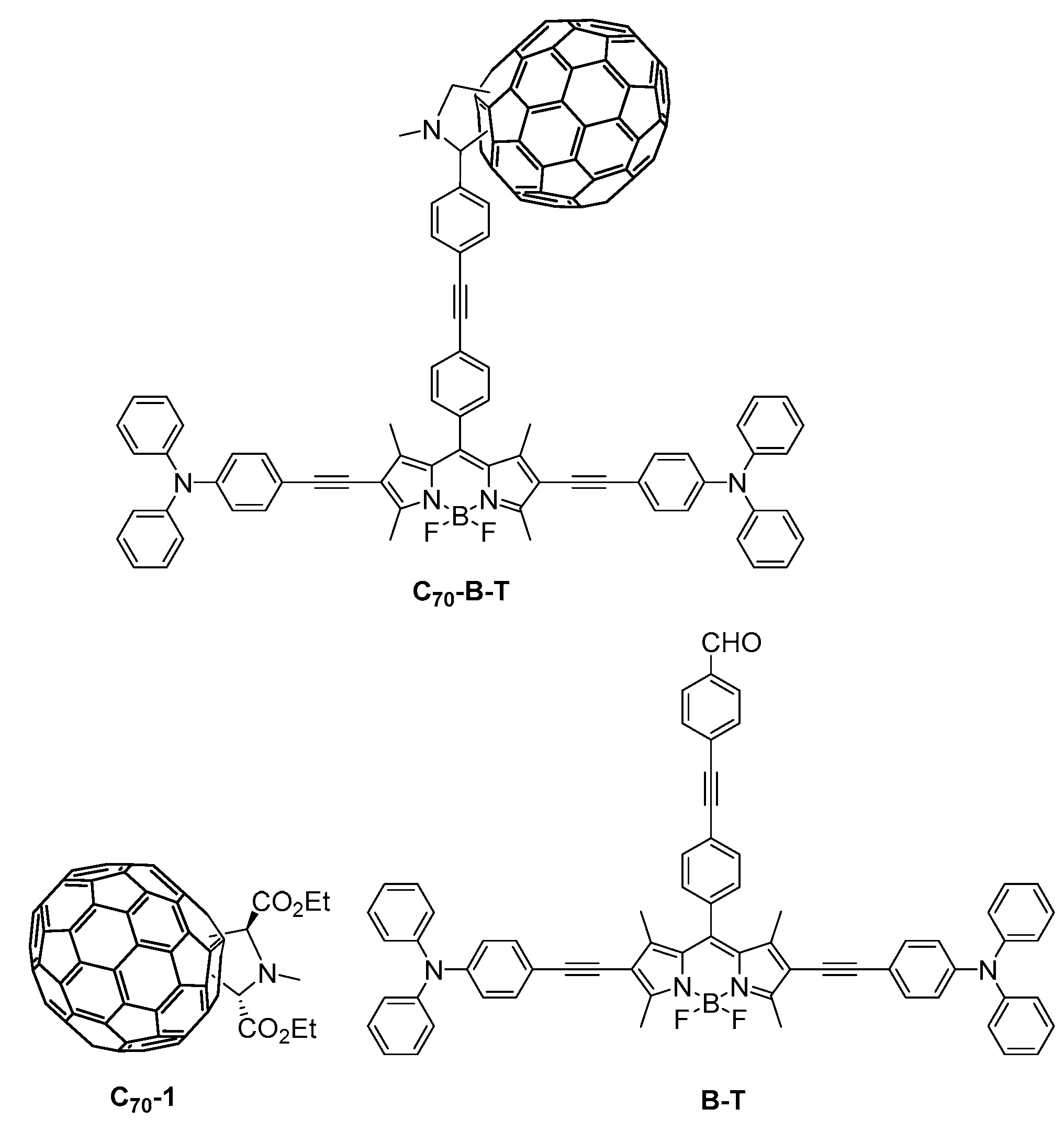
2.1. Synthesis
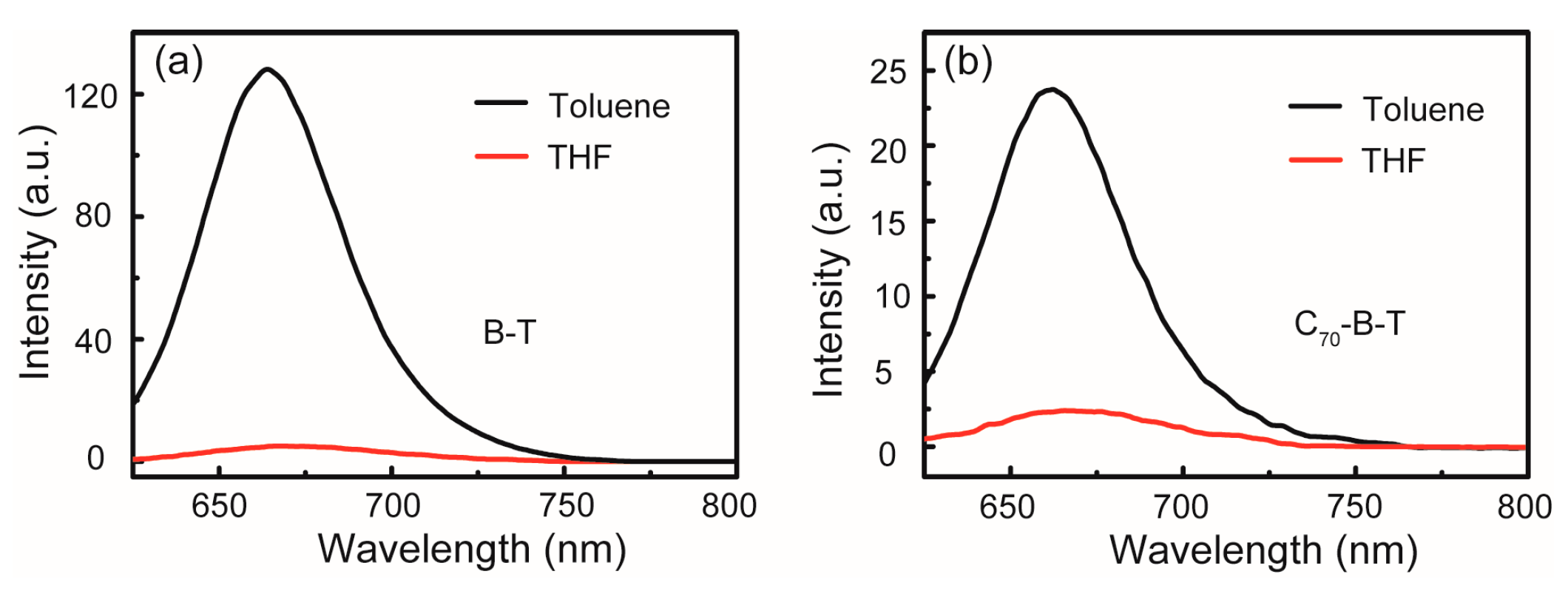
2.2. UV–Vis Absorption and Steady-State Fluorescence
2.3. Time-Resolved Fluorescence Spectroscopy
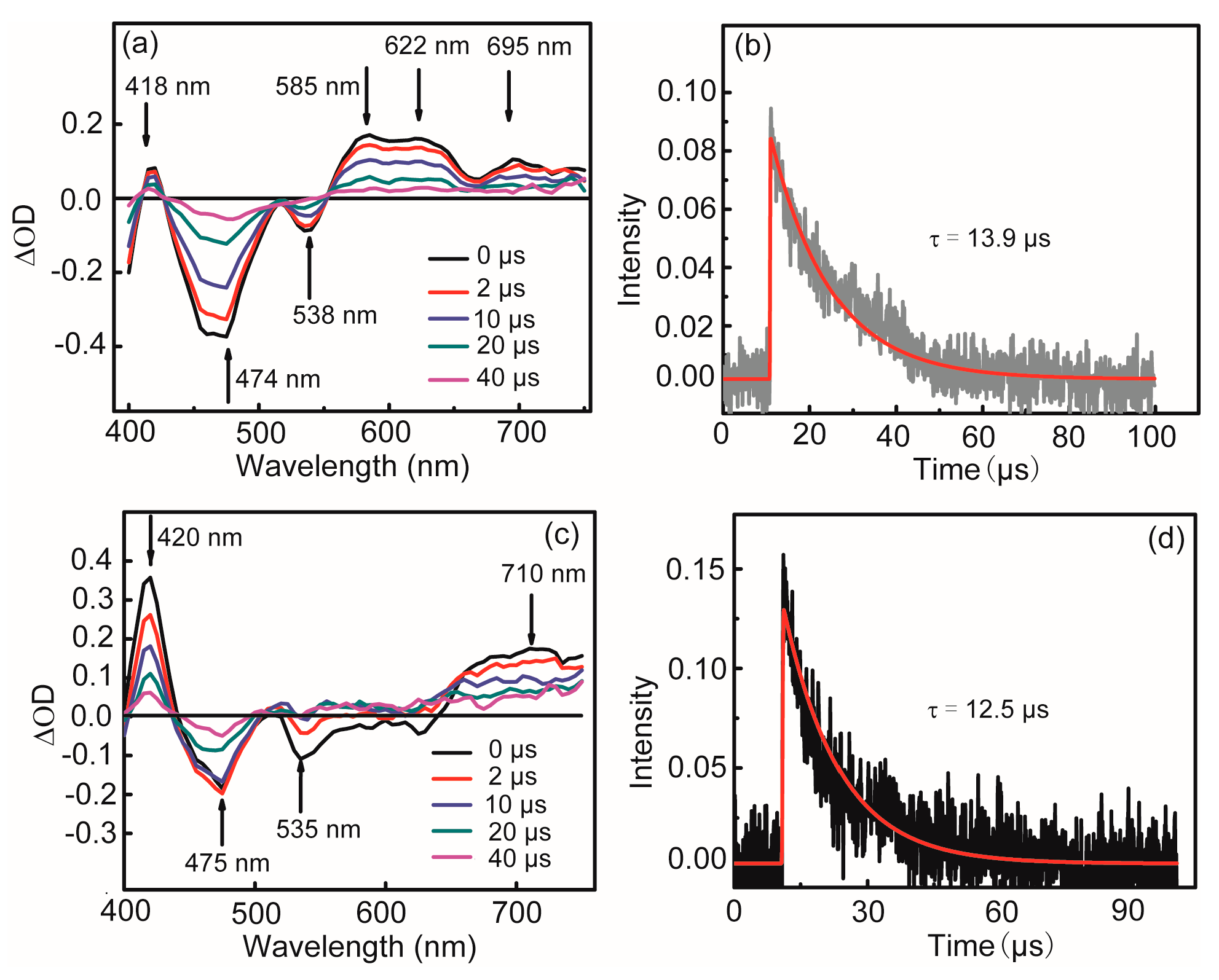
2.4. Nanosecond Time-Resolved Transient Absorption Spectroscopy
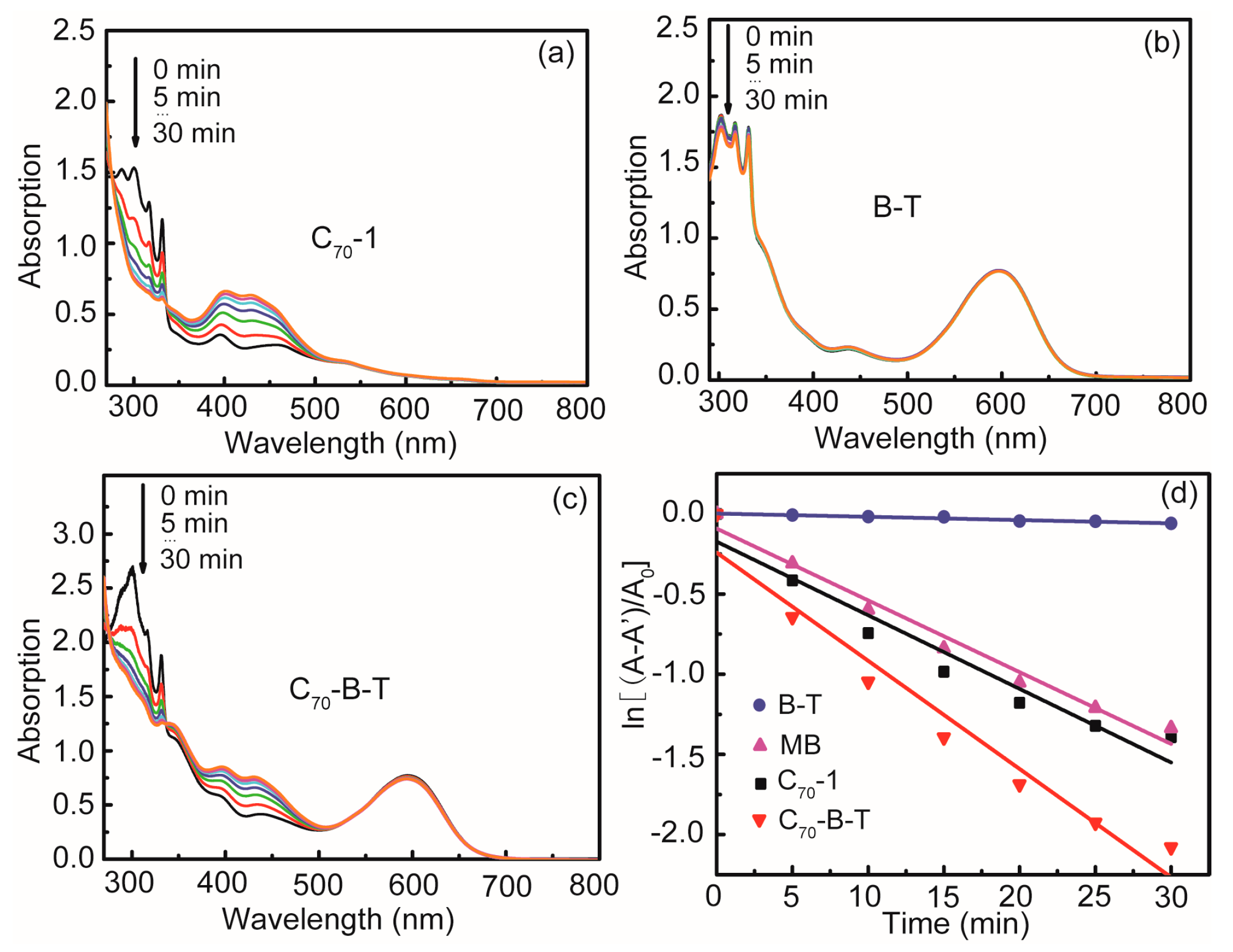
2.5. Photooxidation of 1,5-Dihydroxy Naphthalene Mediated by 1O2
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of C70-1
3.2.2. Synthesis of Compound 2
3.2.3. Synthesis of Compound 3
3.2.4. Synthesis of Compound 5
3.2.5. Synthesis of Compound 6
3.2.6. Synthesis of Compound B-T
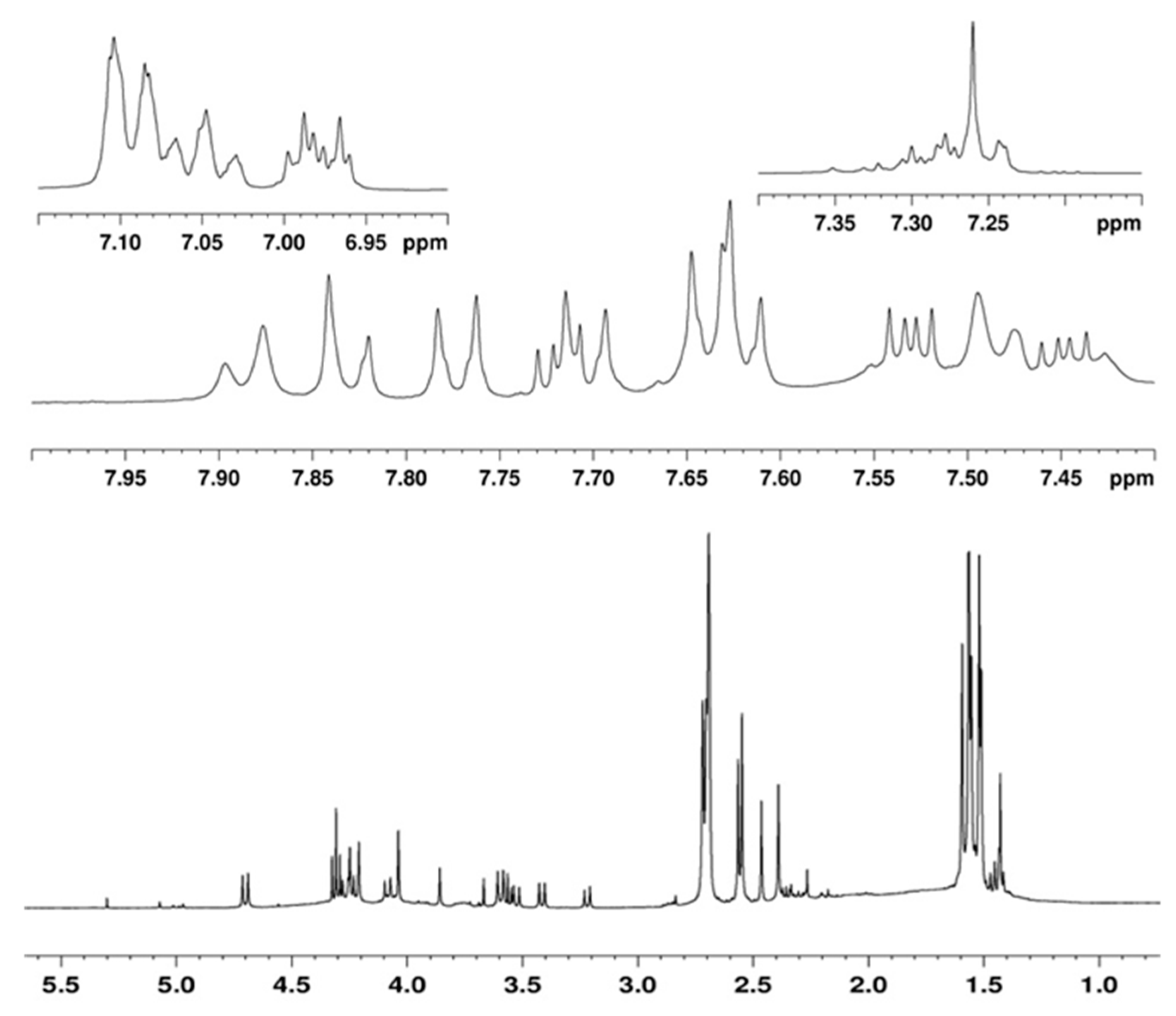
3.2.7. Synthesis of Compound C70-B-T
3.3. Photooxidation Experiment
3.4. Photostability Experiment
3.5. Measurement of Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821–832. [Google Scholar] [CrossRef]
- Zhu, D.-L.; Li, H.-X.; Xu, Z.-M.; Li, H.-Y.; Young, D.J.; Lang, J.-P. Visible light driven, nickel-catalyzed aryl esterification using a triplet photosensitiser thioxanthen-9-one. Org. Chem. Front. 2019, 6, 2353–2359. [Google Scholar] [CrossRef]
- Malimonenko, N.; Butenin, A.; Mester, I.; Kogan, B. Nonlinear photodynamic therapy using saturation of the photosensitizer triplet states. Laser Phys. Lett. 2019, 16, 55602–55604. [Google Scholar] [CrossRef]
- Majumdar, P.; Nomula, R.; Zhao, J. Activatable triplet photosensitizers: Magic bullets for targeted photodynamic therapy. J. Mater. Chem. C 2014, 2, 5982–5997. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-Da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-S.; Ning, Y.; Yin, H.-Y.; Zhang, J.-L. Lutetium(III) porphyrinoids as effective triplet photosensitizers for photon upconversion based on triplet–triplet annihilation (TTA). Inorg. Chem. Front. 2018, 5, 2291–2299. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Z.; Mahboub, M.; Liang, Z.; Jaimes, P.; Xia, P.; Graham, K.R.; Tang, M.L.; Lian, T. Enhanced Near-Infrared-to-Visible Upconversion by Synthetic Control of PbS Nanocrystal Triplet Photosensitizers. J. Am. Chem. Soc. 2019, 141, 9769–9772. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhao, J.Z.; Di Donato, M.; Mazzone, G. Increasing the anti-Stokes shift in TTA upconversion with photosen-sitizers showing red-shifted spin-allowed charge transfer absorption but a non-compromised triplet state energy level. Chem. Commun. 2019, 55, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, C.; Xu, K.; Zhao, J. Application of singlet energy transfer in triplet state formation: Broadband visible light-absorbing triplet photosensitizers, molecular structure design, related photophysics and applications. J. Mater. Chem. C 2015, 3, 8735–8759. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Corroles as triplet photosensitizers. Coord. Chem. Rev. 2019, 379, 121–132. [Google Scholar] [CrossRef]
- Lu, Y.; Conway-Kenny, R.; Wang, J.; Cui, X.; Zhao, J.; Draper, S.M. Exploiting coumarin-6 as ancillary ligands in 1,10-phenanthroline Ir(iii) complexes: Generating triplet photosensitisers with high upconversion capabilities. Dalton Trans. 2018, 47, 8585–8589. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; McCarthy, W.; Conway-Kenny, R.; Twamley, B.; Zhao, J.; Draper, S.M. Novel ruthenium and iridium complexes of N-substituted carbazole as triplet photosensitisers. Chem. Commun. 2017, 54, 1073–1076. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, J.; Wang, J.; Guo, X.; Zhao, D.; Ma, Y. Iridium-Based High-Sensitivity Oxygen Sensors and Photosensitizers with Ultralong Triplet Lifetimes. ACS Appl. Mater. Interfaces 2015, 8, 3591–3600. [Google Scholar] [CrossRef]
- Mai, D.K.; Kang, B.; Vales, T.P.; Badon, I.W.; Cho, S.; Lee, J.; Kim, E.; Kim, H.-J. Synthesis and Photophysical Properties of Tumor-Targeted Water-Soluble BODIPY Photosensitizers for Photodynamic Therapy. Molecules 2020, 25, 3340. [Google Scholar] [CrossRef]
- Hu, W.; Liu, M.; Zhang, X.-F.; Shi, M.; Jia, M.; Hu, X.; Liu, L.; Wang, T. Minimizing the Electron Donor Size of Donor–Acceptor-Type Photosensitizer: Twisted Intramolecular Charge-Transfer-Induced Triplet State and Singlet Oxygen Formation. J. Phys. Chem. C 2020, 124, 23558–23566. [Google Scholar] [CrossRef]
- Dong, Y.; Dick, B.; Zhao, J. Twisted Bodipy Derivative as a Heavy-Atom-Free Triplet Photosensitizer Showing Strong Absorption of Yellow Light, Intersystem Crossing, and a High-Energy Long-Lived Triplet State. Org. Lett. 2020, 22, 5535–5539. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bae, Y.J.; Mauck, C.M.; Mandal, A.; Young, R.M.; Wasielewski, M.R. Singlet Fission in Covalent Terrylene-diimide Dimers: Probing the Nature of the Multiexciton State Using Femtosecond Mid-Infrared Spectroscopy. J. Am. Chem. Soc. 2018, 140, 9184–9192. [Google Scholar] [CrossRef] [PubMed]
- Mauck, C.M.; Hartnett, P.E.; Margulies, E.A.; Ma, L.; Miller, C.E.; Schatz, G.C.; Marks, T.J.; Wasielewski, M.R. Singlet Fission via an Excimer-Like Intermediate in 3,6-Bis(thiophen-2-yl)diketopyrrolopyrrole Derivatives. J. Am. Chem. Soc. 2016, 138, 11749–11761. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Bodipy-Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Tri-plet-State Formation Efficiency and Application in Triplet-Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ge, J.; Escudero, D.; Wang, Z.; Zhao, J.; Jacquemin, D. Molecular Structure–Intersystem Crossing Relationship of Heavy-Atom-Free BODIPY Triplet Photosensitizers. J. Org. Chem. 2015, 80, 5958–5963. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Y.; Hussain, M.; Kundu, S.; Rane, V.; Hayvali, M.; Yildiz, E.A.; Zhao, J.; Yaglioglu, H.G.; Das, R.; et al. Efficient Radical-Enhanced Intersystem Crossing in an NDI-TEMPO Dyad: Photophysics, Electron Spin Polar-ization, and Application in Photodynamic Therapy. Chem. Eur. J. 2018, 24, 18663–18675. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Barbon, A.; Toffoletti, A.; Liu, Y.; An, Y.; Xu, L.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; et al. Radical-Enhanced Intersystem Crossing in New Bodipy Derivatives and Application for Efficient Tri-plet-Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. [Google Scholar] [CrossRef]
- Arbogast, J.W.; Darmanyan, A.P.; Foote, C.S.; Rubin, Y.; Diederich, F.N.; Alvarez, M.M.; Anz, S.J.; Whetten, R.L. Photophysical Properties of C60. J. Phys. Chem. 1991, 95, 11–12. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen spe-cies and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol. 2019, 15, 37–46. [Google Scholar] [CrossRef]
- Siposova, K.; Petrenko, V.I.; Ivankov, O.I.; Musatov, A.; Bulavin, L.A.; Avdeev, M.V.; Kyzyma, O.A. Fullerenes as an Effective Amyloid Fibrils Disaggregating Nanomaterial. ACS Appl. Mater. Interfaces 2020, 12, 32410–32419. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Parveen, A.; Garka, S. Nanoparticle Fullerene (C60) demonstrated stable binding with antibacterial po-tential towards probable targets of drug resistant Salmonella typhi-a computational perspective and in vitro investigation. J. Biomol. Struct. Dyn. 2017, 35, 3449–3468. [Google Scholar] [CrossRef] [PubMed]
- Pochkaeva, E.I.; Podolsky, N.E.; Zakusilo, D.N.; Petrov, A.V.; Charykov, N.A.; Vlasov, T.D.; Penkova, A.V.; Vasina, L.V.; Murin, I.V.; Sharoyko, V.V.; et al. Fullerene derivatives with amino acids, peptides and proteins: From synthesis to biomedical application. Prog. Solid State Chem. 2020, 57, 100255–100284. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2016, 33, 777–794. [Google Scholar] [CrossRef]
- Baker, J.; Fowler, P.W.; Lazzeretti, P.; Malagoli, M.; Zanasi, R. Structure and properties of C70. Chem. Phys. Lett. 1991, 184, 182–186. [Google Scholar] [CrossRef]
- Arbogast, J.W.; Foote, C.S. Photophysical Properties of C70. J. Am. Chem. Soc. 1991, 113, 8886–8889. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, M.; Xu, L.; Shu, C.; Jin, C.; Zheng, J.; Fang, X.; Yang, Y.; Wang, C. Structural Effect and Mechanism of C70-Carboxyfullerenes as Efficient Sensitizers against Cancer Cells. Small 2012, 8, 2070–2077. [Google Scholar] [CrossRef]
- Doi, Y.; Ikeda, A.; Akiyama, M.; Nagano, M.; Shigematsu, T.; Ogawa, T.; Takeya, T.; Nagasaki, T. Intracellular uptake and photodynamic activity of water-soluble [60]- and [70]fullerenes incorporated in liposomes. Chem. Eur. J. 2008, 14, 8892–8897. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Kim, J.-H.; Snow, S.; Kim, J.-H. [C70] Fullerene-sensitized triplet-triplet annihilation upconversion. Chem. Commun. 2013, 49, 10829–10831. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Zhang, J.; Dou, L.; Li, N.; Hu, K.; Gao, T.; Lu, H.; Si, J.; Wang, X.; Yang, W. Rigid axially symmetrical C60-BODIPY triplet photosensitizers: Effect of bridge length on singlet oxygen generation. New J. Chem. 2020, 44, 20419–20427. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Liu, K.-Q.; Wang, X.-F.; Xia, A.-D.; Wang, G.-W. Synthesis and Properties of Axially Symmetrical Rigid Visible Light-Harvesting Systems Containing [60]Fullerene and Perylenebisimide. J. Org. Chem. 2016, 81, 12223–12231. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, W.; Zhao, J.; Huang, D.; Yi, X. Using C60-bodipy dyads that show strong absorption of visible light and long-lived triplet excited states as organic triplet photosensitizers for triplet–triplet annihilation upconversion. J. Mater. Chem. 2012, 22, 20273. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, J.; Sun, J.; Guo, S. Light-Harvesting Fullerene Dyads as Organic Triplet Photosensitizers for Triplet–Triplet Annihilation Upconversions. J. Org. Chem. 2012, 77, 5305–5312. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Zhou, Q.; Zhang, S.; Zhang, B.; Zhou, X.; Liu, S. Solvent effects on triplet–triplet annihilation upconversion kinetics of perylene with a Bodipy-phenyl-C60 photosensitizer. Phys. Chem. Chem. Phys. 2020, 22, 26372–26382. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, M.; Zhou, Q.; Zhou, X.; Liu, S. Application of a bodipy-C70 dyad in triplet-triplet annihilation upconversion of perylene as a metal-free photosensitizer. Org. Biomol. Chem. 2018, 16, 5598–5608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F. BODIPY photosensitizers based on PET and heavy atom effect: A comparative study on the efficient formation of excited triplet state and singlet oxygen in BODIPY dimers and monomers. J. Photochem. Photobiol. A Chem. 2018, 355, 431–443. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, X.; Küçüköz, B.; Li, S.; Zhang, C.; Majumdar, P.; Karatay, A.; Li, X.; Yaglioglu, H.G.; Elmali, A.; et al. Resonance energy transfer-enhanced rhodamine–styryl Bodipy dyad triplet photosensitizers. J. Mater. Chem. C 2014, 2, 3900–3913. [Google Scholar] [CrossRef]
- Wu, W.; Cui, X.; Zhao, J. Hetero Bodipy-dimers as heavy atom-free triplet photosensitizers showing a long-lived triplet excited state for triplet–triplet annihilation upconversion. Chem. Commun. 2013, 49, 9009–9011. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, J.; Wu, W.; Yu, X.; Liu, Y. Accessing the Long-Lived Triplet Excited States in Bodipy-Conjugated 2-(2-Hydroxyphenyl) Benzothiazole/Benzoxazoles and Applications as Organic Triplet Photosensitizers for Photooxidations. J. Org. Chem. 2012, 77, 6166–6178. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Liu, X.; Wang, S.; Wang, Y. BODIPY-Based Fluorescent Surfactant for Cell Membrane Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 3, 593–601. [Google Scholar] [CrossRef]
- Zou, J.; Yin, Z.; Wang, P.; Chen, D.; Shao, J.; Zhang, Q.; Sun, L.; Huang, W.; Dong, X. Photosensitizer synergistic effects: D-A-D structured organic molecule with enhanced fluorescence and singlet oxygen quantum yield for photodynamic therapy. Chem. Sci. 2018, 9, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-Based Molecules, A Platform for Photonic and Solar Cells. Molecules 2020, 26, 153. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, W.-C.; Chen, Y.-C.; Chou, H.-H.; Hsu, C.-Y.; Lin, J.T.; Lin, H.-W. BODIPY dyes with beta-conjugation and their applications for high-efficiency inverted small molecule solar cells. Chem. Commun. 2012, 48, 8913–8915. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Hou, X.-N.; Tian, Y.; Jiang, L.; Deng, S.; Röder, B.; Ermilov, E.A. Photoinduced energy and charge transfer in a bis(triphenylamine)–BODIPY–C60 artificial photosynthetic system. RSC Adv. 2016, 6, 57293–57305. [Google Scholar] [CrossRef]
- Godoy, J.; Vives, G.; Tour, J.M. Synthesis of Highly Fluorescent BODIPY-Based Nanocars. Org. Lett. 2010, 12, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Zhang, X.-R.; Wu, Y.-S.; Zhang, J.-P.; Ai, X.-C.; Wang, Y.; Sun, M.-T. Two-photon photophysical properties of tri-9-anthrylborane. Chem. Phys. Lett. 2007, 436, 280–286. [Google Scholar] [CrossRef]
- Huang, L.; Cui, X.; Therrien, B.; Zhao, J. Energy-funneling-based broadband visible-light-absorbing bodipy-C60 triads and tetrads as dual functional heavy-atom-free organic triplet photosensitizers for photocatalytic organic reactions. Chem. Eur. J. 2013, 19, 17472–17482. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.-Y.; Aboshi, R.; Murata, S. Photooxidation of 1,5-dihydroxynaphthalene with iridium complexes as singlet oxygen sensitizers. Photochem. Photobiol. Sci. 2011, 10, 895–903. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Effects of substituents on the photophysical properties of symmetrical porphyrins. Dye. Pigment. 2013, 96, 440–448. [Google Scholar] [CrossRef]
- Dong, L.; Zheng, Z.; Wang, Y.; Li, X.; Hua, J.; Hu, A. Co-sensitization of N719 with polyphenylenes from the Bergman cyclization of maleimide-based enediynes for dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 11607–11614. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Feng, N. Photoinduced Electron Transfer-based Halogen-free Photosensitizers: Covalent meso -Aryl (Phenyl, Naphthyl, Anthryl, and Pyrenyl) as Electron Donors to Effectively Induce the Formation of the Excited Triplet State and Singlet Oxygen for BODIPY Compounds. Chem. Asian J. 2017, 12, 2447–2456. [Google Scholar] [CrossRef]

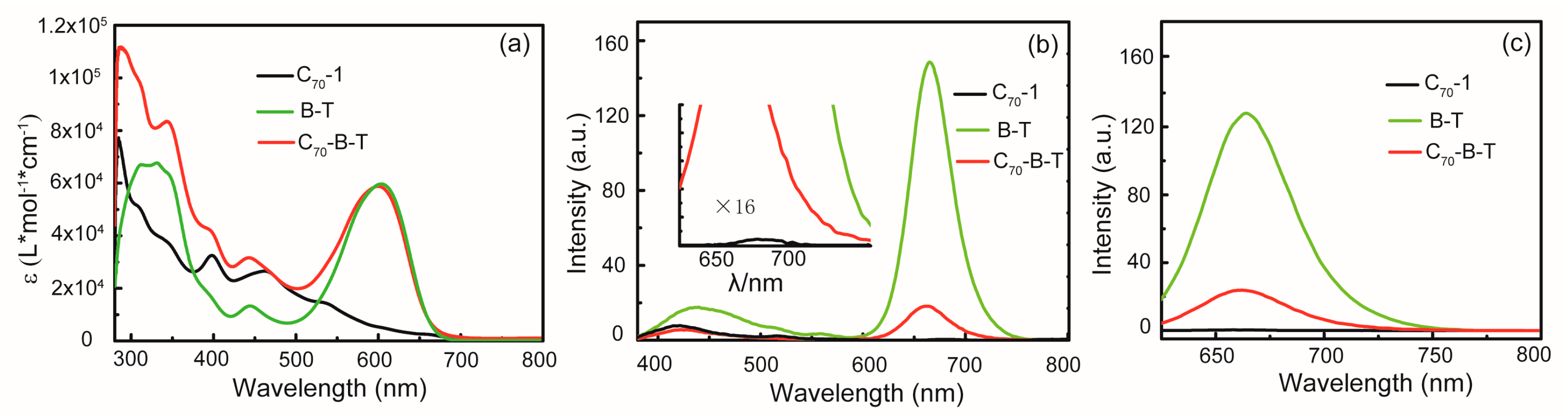

| Compound | Solvent | λabs (nm) | λem (nm) | ΦF b | |
|---|---|---|---|---|---|
| Ex. = 339 nm | Ex. = 605 nm | ||||
| C70-1 | toluene | 284, 307, 398, 462, 537, 666 | 710 | - | - |
| THF | - | - | - | - | |
| B-T | toluene | 330, 443, 603 | 664 | 0.29 | 0.22 |
| THF | - | 668 | - | 0.01 | |
| C70-B-T | toluene | 284, 310, 344, 443, 600 | 664 | 0.03 | 0.04 |
| THF | - | 666 | - | 0.005 | |
| Photosensitizers | kobs b/min−1 | υi c | Φ∆ d |
|---|---|---|---|
| C70-B-T | 67.5 | 6.75 | 0.78 |
| C70-1 | 45.9 | 4.59 | 0.81 e |
| B-T | 2.0 | 0.2 | - |
| MB | 44.8 | 4.48 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-E.; Zhang, J.-H.; Gong, Y.; Dou, L.-F.; Mao, L.-H.; Lu, H.-D.; Wei, C.-X.; Chen, H.; Wang, X.-F.; Yang, W. Broadband Visible Light-Absorbing [70]Fullerene-BODIPY-Triphenylamine Triad: Synthesis and Application as Heavy Atom-Free Organic Triplet Photosensitizer for Photooxidation. Molecules 2021, 26, 1243. https://doi.org/10.3390/molecules26051243
Zhu S-E, Zhang J-H, Gong Y, Dou L-F, Mao L-H, Lu H-D, Wei C-X, Chen H, Wang X-F, Yang W. Broadband Visible Light-Absorbing [70]Fullerene-BODIPY-Triphenylamine Triad: Synthesis and Application as Heavy Atom-Free Organic Triplet Photosensitizer for Photooxidation. Molecules. 2021; 26(5):1243. https://doi.org/10.3390/molecules26051243
Chicago/Turabian StyleZhu, San-E, Jian-Hui Zhang, Yu Gong, Li-Feng Dou, Li-Hua Mao, Hong-Dian Lu, Chun-Xiang Wei, Hong Chen, Xue-Fei Wang, and Wei Yang. 2021. "Broadband Visible Light-Absorbing [70]Fullerene-BODIPY-Triphenylamine Triad: Synthesis and Application as Heavy Atom-Free Organic Triplet Photosensitizer for Photooxidation" Molecules 26, no. 5: 1243. https://doi.org/10.3390/molecules26051243
APA StyleZhu, S.-E., Zhang, J.-H., Gong, Y., Dou, L.-F., Mao, L.-H., Lu, H.-D., Wei, C.-X., Chen, H., Wang, X.-F., & Yang, W. (2021). Broadband Visible Light-Absorbing [70]Fullerene-BODIPY-Triphenylamine Triad: Synthesis and Application as Heavy Atom-Free Organic Triplet Photosensitizer for Photooxidation. Molecules, 26(5), 1243. https://doi.org/10.3390/molecules26051243







