Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer
Abstract
1. Introduction
2. Results
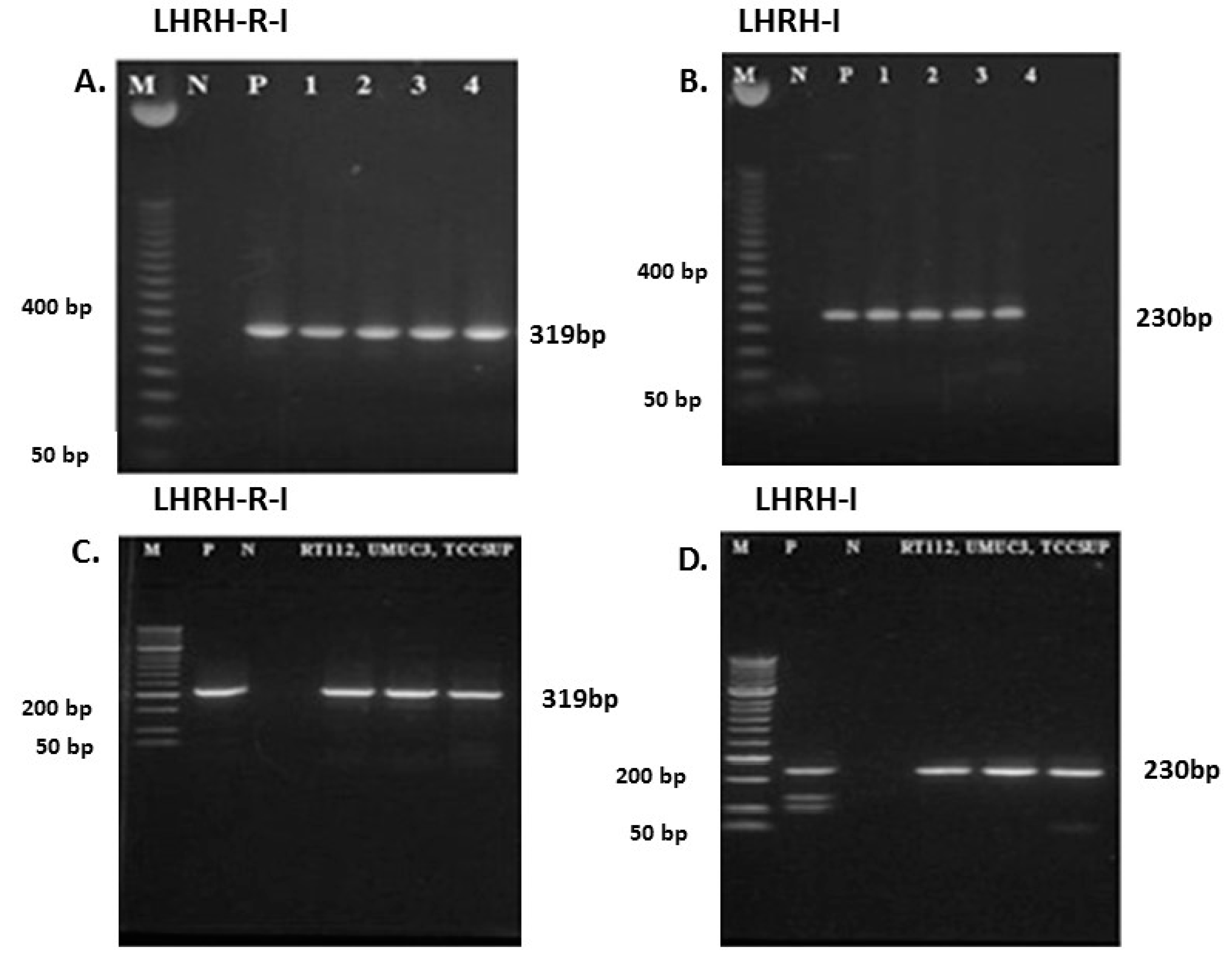
2.1. Expression of mRNA for LHRH-I and LHRH-R-I in Human Bladder Cancer Tissue Samples and in Human Bladder Cancer Cell Lines
2.2. Immunohistochemistry of LHRH Receptor in Human Bladder Cancer Tissue
2.3. Expression of LH-RH Receptors in Human Bladder Cancer Cell Lines In Vitro
2.4. Radioligand Binding Studies
3. Discussion
4. Materials and Methods
4.1. Bladder Cancer Tissue Samples
4.2. Histology
4.3. Cell Culturing
4.4. Isolation of RNA
4.5. RT-PCR
4.6. Immunohistochemistry
4.7. LHRH Receptor Binding Studies
4.8. Western Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hanna, K.S. Updates and novel treatments in urothelial carcinoma. J. Oncol. Pharm. Pr. 2018, 25, 648–656. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Racioppi, M.; D’Agostino, D.; D’Addessi, A.; Marangi, F.; Totaro, A.; Pinto, F.; Sacco, E.; Battaglia, S.; Chiloiro, G.; et al. Advanced bladder cancer: New agents and new approaches. A review. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 9–16. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Miyazawa, K.; Tsukamoto, T.; Kuno, T.; Suzuki, K. Pathobiology and Chemoprevention of Bladder Cancer. J. Oncol. 2011, 2011, 1–23. [Google Scholar] [CrossRef]
- Szepeshazi, K.; Schally, A.V.; Keller, G.; Block, N.L.; Benten, D.; Halmos, G.; Szalontay, L.; Vidaurre, I.; Jaszberenyi, M.; Rick, F.G. Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 (AEZS-108). Oncotarget 2012, 3, 686–699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serrano, C.; Morales, R.; Suárez, C.; Núñez, I.; Valverde, C.; Rodón, J.; Humbert, J.; Padrós, O.; Carles, J. Emerging therapies for urothelial cancer. Cancer Treat. Rev. 2012, 38, 311–317. [Google Scholar] [CrossRef]
- Lei, A.Q.; Cheng, L.; Pan, C.-X. Current treatment of metastatic bladder cancer and future directions. Expert Rev. Anticancer. Ther. 2011, 11, 1851–1862. [Google Scholar] [CrossRef]
- Schally, A.V.; Halmos, G. Targeting to Peptide Receptors. In Drug Delivery in Oncology; Wiley: Weinheim, Germany, 2011; pp. 1219–1261. [Google Scholar]
- Cheng, C.K.; Leung, P.C.K. Molecular Biology of Gonadotropin-Releasing Hormone (GnRH)-I, GnRH-II, and Their Receptors in Humans. Endocr. Rev. 2005, 26, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D. Minireview: GnRH and GnRH Receptor Genes in the Human Genome. Endocrinology 2002, 143, 737–743. [Google Scholar] [CrossRef]
- Engel, J.B.; Schally, A.V. Drug Insight: Clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat. Clin. Pr. Endocrinol. Metab. 2007, 3, 157–167. [Google Scholar] [CrossRef]
- Chi, L.; Zhou, W.; Prikhozhan, A.; Flanagan, C.; Davidson, J.; Golembo, M.; Illing, N.; Millar, R.; Sealfon, S. Cloning and characterization of the human GnRH receptor. Mol. Cell. Endocrinol. 1993, 91, R1–R6. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Reinhart, J.; Catt, K.J. Gonadotropin-Releasing Hormone Receptors: Structure and Signal Transduction Pathways. Endocr. Rev. 1994, 15, 462–499. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Comaru-Schally, A.M.; Nagy, A.; Kovacs, M.; Szepeshazi, K.; Plonowski, A.; Varga, J.L.; Halmos, G. Hypothalamic Hormones and Cancer. Front. Neuroendocr. 2001, 22, 248–291. [Google Scholar] [CrossRef]
- Emons, G.; Schröder, B.; Ortmann, O.; Westphalen, S.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of luteinizing hormone-releasing hormone analogs in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1993, 77, 1458–1464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halmos, G.; Arencibia, J.M.; Schally, A.V.; Davis, R.; Bostwick, D.G. High incidence of receptors for luteinizing hor-mone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J. Urol. 2000, 163, 623–629. [Google Scholar] [CrossRef]
- Schally, A.V.; Rekasi, Z.; Arencibia, J.M. The actions of LH-RH agonists, antagonists, and cytotoxic analogs on the LH-RH receptors on the pituitary and tumors. In Inferility and Reproductive Medicine Clinics of North America; Saunders: Philadelphia, PA, USA, 2001; Volume 2001, pp. 17–44. [Google Scholar]
- Emons, G.; Ortmann, O.; Becker, M.; Irmer, G.; Springer, B.; Laun, R.; Hölzel, F.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines. Cancer Res. 1993, 53, 5439–5446. [Google Scholar] [PubMed]
- Sípos, Éva; Dobos, N.; Rozsa, D.; Fodor, K.; Oláh, G.; Szabo, Z.; Szekvolgyi, L.; Schally, A.V.; Halmos, G. Characterization of luteinizing hormone-releasing hormone receptor type I (LH-RH-I) as a potential molecular target in OCM-1 and OCM-3 human uveal melanoma cell lines. OncoTargets Ther. 2018, 11, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Halmos, G.; Rózsa, B.; Juhász, A.; Treszl, A.; Tóth, G.; Flaskó, T.; Dezsö, B.; Block, N.L.; Schally, A.V. Expression of mRNA for human type-I LHRH receptor transcript forms in human benign prostatic hyperplasia. Int. J. Oncol. 2009, 35, 1053–1059. [Google Scholar] [CrossRef][Green Version]
- Rózsa, B.; Nadji, M.; Schally, A.V.; Dezso, B.; Flaskó, T.; Toth, G.; Mile, M.; Block, N.L.; Halmos, G. Receptors for luteinizing hormone-releasing hormone (LHRH) in benign prostatic hyperplasia (BPH) as potential molecular targets for therapy with LHRH antagonist cetrorelix. Prostate 2010, 71, 445–452. [Google Scholar] [CrossRef]
- Keller, G.; Schally, A.V.; Gaiser, T.; Nagy, A.; Baker, B.; Halmos, G.; Engel, J.B. Receptors for Luteinizing Hormone Releasing Hormone Expressed on Human Renal Cell Carcinomas Can Be Used for Targeted Chemotherapy with Cytotoxic Luteinizing Hormone Releasing Hormone Analogues. Clin. Cancer Res. 2005, 11, 5549–5557. [Google Scholar] [CrossRef][Green Version]
- Bahk, J.Y.; Kim, M.O.; Park, M.S.; Lee, H.Y.; Lee, J.-H.; Chung, B.C.; Min, S.K. Gonadotropin-Releasing Hormone (GnRH) and GnRH Receptor in Bladder Cancer Epithelia and GnRH Effect on Bladder Cancer Cell Proliferation. Urol. Int. 2008, 80, 431–438. [Google Scholar] [CrossRef]
- Imada, S.; Akaza, H.; Otani, M.; Koiso, K. EFFECTS OF ANDROGEN REGULATION SYSTEM ON BLADDER CARCINOGENESIS IN MALE MICE. Jpn. J. Urol. 1995, 86, 1666–1672. [Google Scholar] [CrossRef][Green Version]
- Nagy, A.; Schally, A.V.; Armatis, P.; Szepesházi, K.; Halmos, G.; Kovacs, M.; Zarandi, M.; Groot, K.; Miyazaki, M.; Jungwirth, A.; et al. Cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500–1000 times more potent. Proc. Natl. Acad. Sci. USA 1996, 93, 7269–7273. [Google Scholar] [CrossRef]
- Schally, A.; Nagy, A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors. Eur. J. Endocrinol. 1999, 141, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Nagy, A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol. Metab. 2004, 15, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A. Bladder cancer subtypes defined by genomic alterations. Scand. J. Urol. Nephrol. 2008, 42, 116–130. [Google Scholar] [CrossRef]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J.; Chang, H.Y.; Van De Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef]
- Millar, R.P.; Lu, Z.-L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-Releasing Hormone Receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; McPhaul, M.J. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol. Cell. Endocrinol. 1996, 120, 51–57. [Google Scholar] [CrossRef]
- Boorjian, A.S.; Heemers, H.V.; Frank, I.; Farmer, S.A.; Schmidt, L.J.; Sebo, T.J.; Tindall, D.J. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocr. Relat. Cancer 2009, 16, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.-Å.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef] [PubMed]
- Ponglowhapan, S.; Church, D.; Scaramuzzi, R.; Khalid, M. Luteinizing hormone and follicle-stimulating hormone receptors and their transcribed genes (mRNA) are present in the lower urinary tract of intact male and female dogs. Theriogenology 2007, 67, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Kaufmann, M.; Gorchev, G.; Tsekova, V.; Gründker, C.; Günthert, A.R.; Hanker, L.C.; Velikova, M.; Sindermann, H.; Engel, J.; et al. Dose escalation and pharmacokinetic study of AEZS-108 (AN-152), an LHRH agonist linked to doxorubicin, in women with LHRH receptor-positive tumors. Gynecol. Oncol. 2010, 119, 457–461. [Google Scholar] [CrossRef]
- Engel, J.B.; Tinneberg, H.-R.; Rick, F.G.; Berkes, E.; Schally, A.V. Targeting of Peptide Cytotoxins to LHRH Receptors For Treatment of Cancer. Curr. Drug Targets 2016, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, F.K.; Davis, C.J., Jr.; Sesterhenn, I.A. Histological Typing of Urinary Bladder Tumours; Springer: Berlin/Heidelberg, Germany; World Health Organization: Geneva, Switzerland, 1973; p. 104. [Google Scholar] [CrossRef]
- Eble, J.N.; Epstein, J.; Sesterhenn, I. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, 3rd ed.; WHO Classification of Tumours: Lyon, France, 2004; Volume 7. [Google Scholar]
- Brierley, J.D.; Wittekind, C. UICC TNM Classification of Malignant Tumour; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 272. [Google Scholar]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; Van Rhijn, B.W.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef]



| Positive/Total Number of Cases Examined | Positive (%) | |
|---|---|---|
| LHRH-I mRNA | 19/24 | 79 |
| LHRH-R-I mRNA | 20/24 | 83 |
| Number | Age/Sex | Clinicopathological Findings | Grade | Stage | LHRH-I | LHRH-R-I | IHC Results |
|---|---|---|---|---|---|---|---|
| 1 | 43/female | transitional cell carcinoma | G3 | pT2 | − | + | 1+ |
| 2 | 69/male | transitional cell carcinoma | G1-2 | pT1 | + | − | N/A |
| 3 | 56/male | transitional cell carcinoma | G3 | pT3 | + | + | 1+ |
| 4 | 63/female | transitional cell carcinoma | G1 | N/A | + | − | N/A |
| 5 | 89/female | transitional cell carcinoma | G3 | pT2 | + | + | 1+ |
| 6 | 51/male | transitional cell carcinoma | G1-2 | pT1 | + | + | 2+ |
| 7 | 66/male | transitional cell carcinoma | G2 | pT2, pNx, pMx | + | + | 2+ |
| 8 | 67/male | transitional cell carcinoma | G3 | pT3a, pNx, pMx | + | + | 1+ |
| 9 | 69/male | transitional cell carcinoma | G1 | pT1, pNx, pMx | + | + | 2+ |
| 10 | 50/male | transitional cell carcinoma | G1 | pT1, pNx, pMx | + | + | N/A |
| 11 | 68/male | transitional cell carcinoma | G1 | pT1, pNx, pMx | + | + | N/A |
| 12 | 42/female | transitional cell carcinoma | G2-3 | N/A | − | + | N/A |
| 13 | 79/male | transitional cell carcinoma | G1-2 | pT1 | + | + | 2+ |
| 14 | 84/female | transitional cell carcinoma | G1 | N/A | + | + | N/A |
| 15 | 63/male | transitional cell carcinoma | G2 | N/A | + | + | 2+ |
| 16 | 70/female | transitional cell carcinoma | G3 | pT2 | + | − | N/A |
| 17 | 63/female | transitional cell carcinoma | G2 | pT1 | − | + | N/A |
| 18 | 51/female | transitional cell carcinoma | G1 | N/A | − | + | 3+ |
| 19 | 71/female | transitional cell carcinoma | G3 | pT2 | + | + | 1+ |
| 20 | 86/male | transitional cell carcinoma | G2 | pT1 | + | + | N/A |
| 21 | 57/male | transitional cell carcinoma | G1 | N/A | + | + | N/A |
| 22 | 50/male | transitional cell carcinoma | G3 | pT3 | − | + | 1+ |
| 23 | 84/male | transitional cell carcinoma | G2 | pT1 | + | − | N/A |
| 24 | 74/male | transitional cell carcinoma | G3 | pT2 | + | + | N/A |
| Patient Number | LHRH-R mRNA | Kd (nM) | Bmax (fmol/mg (Protein)) |
|---|---|---|---|
| 3 | + | 5.76 | 274.5 |
| 4 | − | − | − |
| 6 | + | 6.85 | 273.1 |
| 7 | + | 4.94 | 255.0 |
| 8 | + | 6.26 | 685.2 |
| 10 | + | 5.02 | 721.4 |
| 12 | + | 3.61 | 704.5 |
| 14 | + | 4.92 | 578.9 |
| 16 | − | − | − |
| 18 | + | 4.38 | 445.3 |
| 19 | + | 3.83 | 421.6 |
| 22 | + | 4.31 | 371.4 |
| Compound | IC50 Values (nM) |
|---|---|
| [D-Lys6]LHRH | 0.98 |
| Cetrorelix | 0.86 |
| AN-152 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, Z.; Dezső, B.; Fodor, K.; Szegedi, K.; Flaskó, T.; Szabó, E.; Oláh, G.; Sipos, É.; Dobos, N.; Gardi, J.; et al. Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer. Molecules 2021, 26, 1253. https://doi.org/10.3390/molecules26051253
Szabó Z, Dezső B, Fodor K, Szegedi K, Flaskó T, Szabó E, Oláh G, Sipos É, Dobos N, Gardi J, et al. Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer. Molecules. 2021; 26(5):1253. https://doi.org/10.3390/molecules26051253
Chicago/Turabian StyleSzabó, Zsuzsanna, Balázs Dezső, Klára Fodor, Krisztián Szegedi, Tibor Flaskó, Erzsébet Szabó, Gábor Oláh, Éva Sipos, Nikoletta Dobos, János Gardi, and et al. 2021. "Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer" Molecules 26, no. 5: 1253. https://doi.org/10.3390/molecules26051253
APA StyleSzabó, Z., Dezső, B., Fodor, K., Szegedi, K., Flaskó, T., Szabó, E., Oláh, G., Sipos, É., Dobos, N., Gardi, J., Schally, A. V., & Halmos, G. (2021). Expression of Luteinizing Hormone-Releasing Hormone (LHRH) and Type-I LHRH Receptor in Transitional Cell Carcinoma Type of Human Bladder Cancer. Molecules, 26(5), 1253. https://doi.org/10.3390/molecules26051253







