Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. BHMC Selectively Inhibits the Proliferation of MCF-7 Cell and MDA-MB231
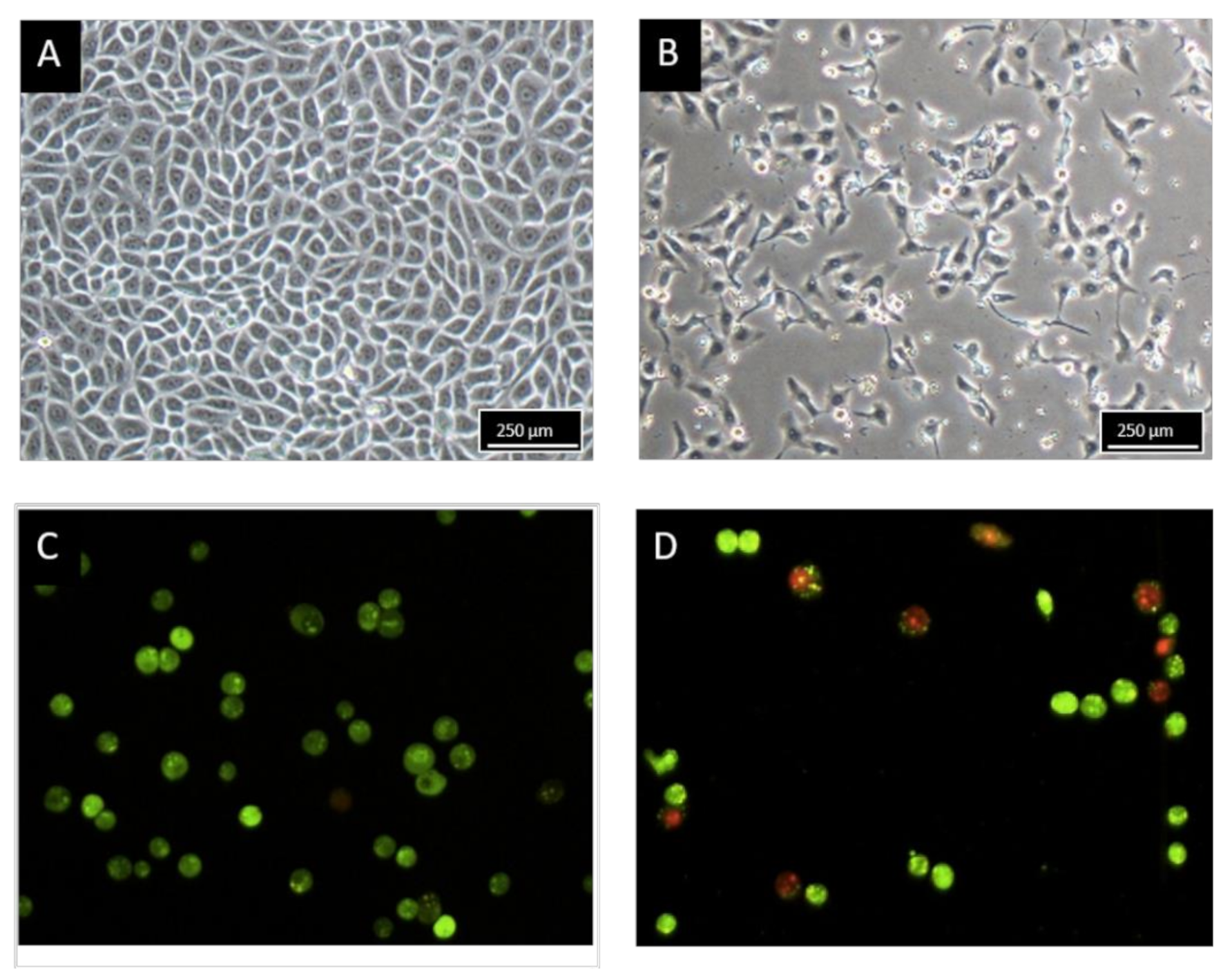
2.2. Morphology Observation of MCF-7 Treated with BHMC
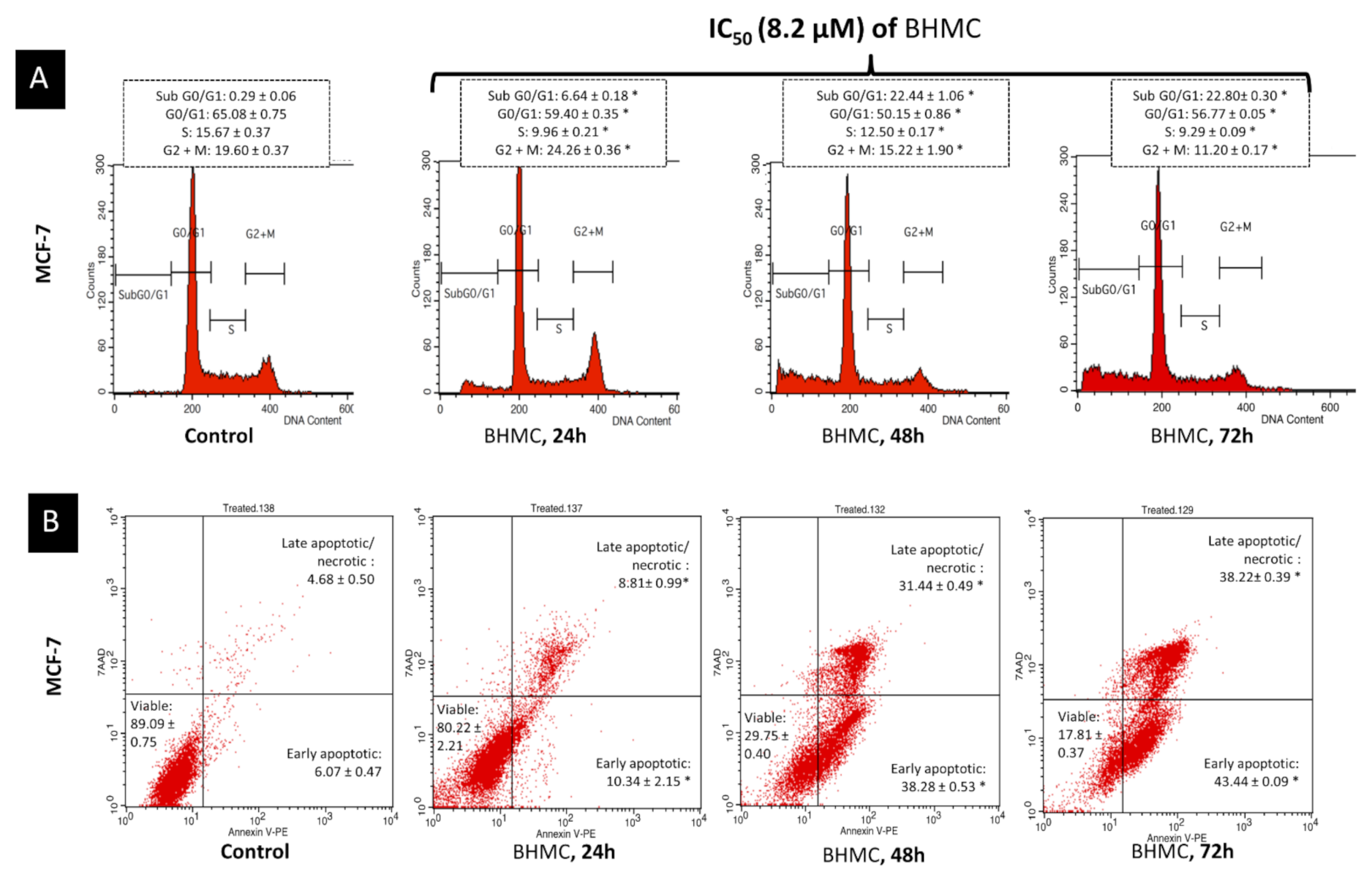
2.3. BHMC Induced G2/M Cell Cycle Arrest Followed by Apoptosis on MCF-7
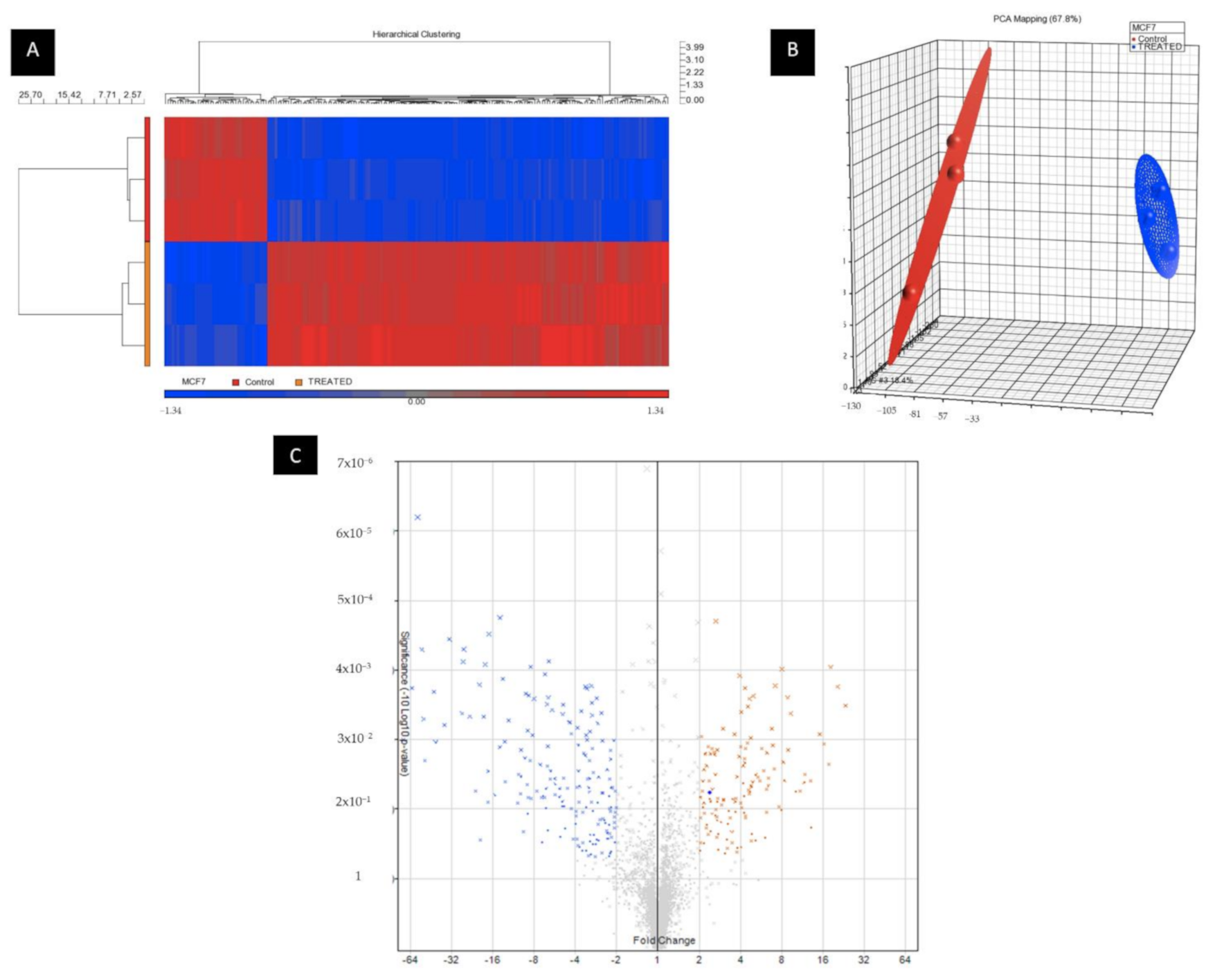
2.4. BHMC Dysregulated miRNA and Gene Expression Profiles of MCF-7 Cells
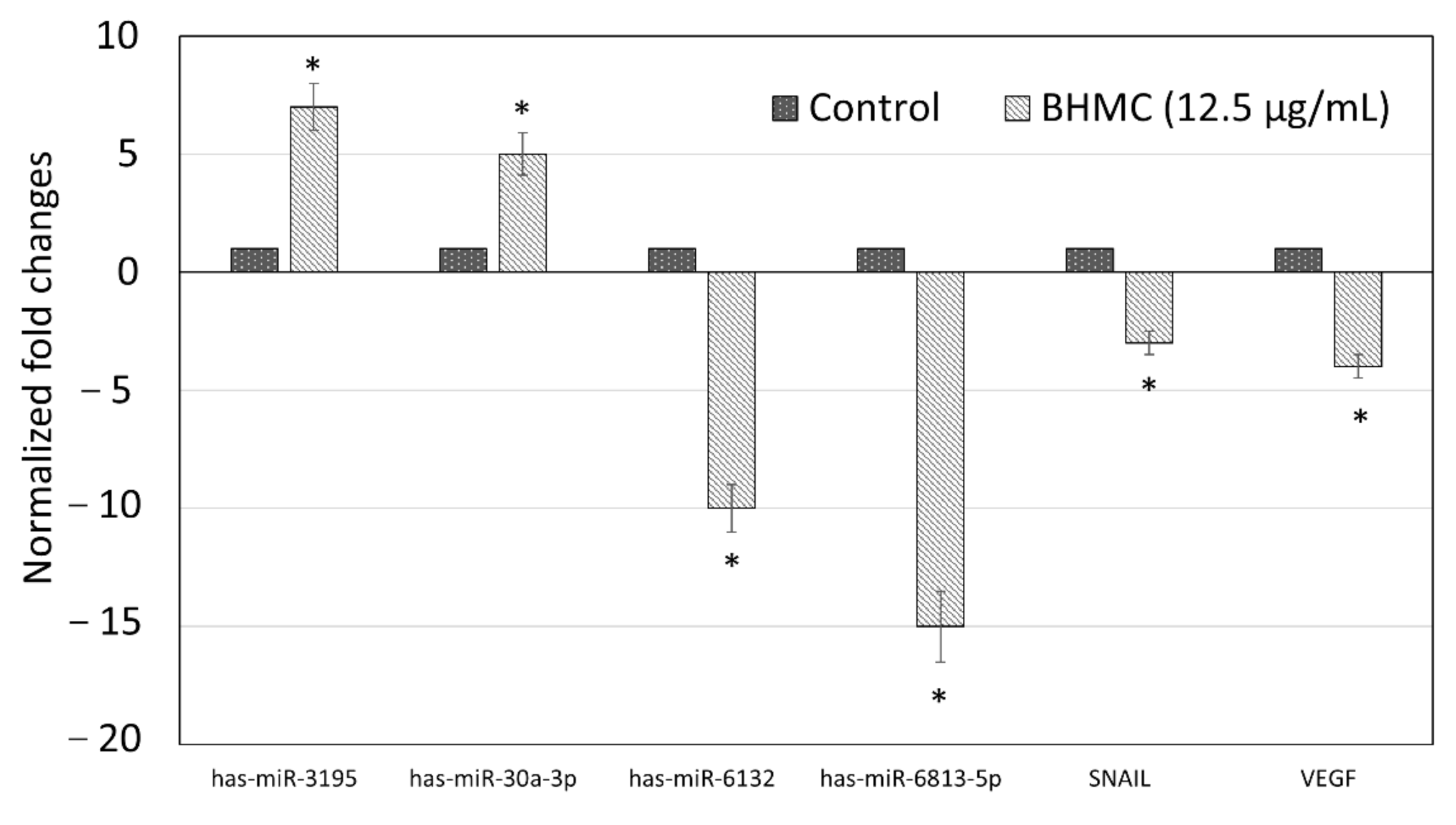
2.5. Validation of Selected Genes and miRNAs by Quantitative Real-Time PCR (qPCR)
3. Discussion
4. Materials and Methods
4.1. Source of Curcumin and Preparation of BHMC
4.2. Breast Cells Culture Conditions
4.3. MTT Cell Viability Assay
4.4. MCF-7 Cell Treatment
4.5. Light and Fluorescent Microscopic Observation
4.6. Flow Cytometry Cell Cycle Analysis
4.7. Flow Cytometry AnnexinV/PI Apoptosis Detection
4.8. Total RNA and miRNA Extraction
4.9. Microarray Analysis of miRNA
4.10. Analysis of Microarrays Data
4.11. Real-Time Quantitative PCR of miRNAs Expression
4.12. Real-Time Quantitative PCR of mRNA Expression
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- World Cancer Research Fund. Breast Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics (accessed on 28 April 2020).
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular classification of breast carcinoma: From traditional, old-fashioned way to a new age, and a new way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.F.; Katki, H.A.; Rosenberg, P.S. Incidence of breast cancer in the United States: Current and future trends. J. Natl. Cancer Inst. 2011, 103, 1397–1402. [Google Scholar] [CrossRef]
- Johnston, S.R.D. New strategies in oestrogen receptor-positive breast cancer. Clin. Cancer Res. 2010, 16, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, F.; He, J.; Du, J.; Zhang, H.; Shi, H.; Chen, Y.; Wei, Y.; Xue, W.; Yan, J.; et al. miR-30a-3p targets MAD2L1 and regulates proliferation of gastric cancer cells. Oncotargets 2019, 12, 11313–11324. [Google Scholar] [CrossRef] [Green Version]
- Razak, N.A.; Akhtar, M.N.; Abu, N.; Ho, W.Y.; Tan, S.W.; Zareen, S.; Taj-ud-din, S.N.; Long, K.; Alitheen, N.B.; Yeap, S.K. The in vivo anti-tumor effect of curcumin derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene) cyclohexanone (BHMC) on 4T1 breast cancer cells. RSC Adv. 2017, 7, 36185–36192. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.-Y.; Moon, A. Natural products for chemoprevention of breast cancer. J. Cancer Prev. 2015, 20, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neidle, S.; Thurston, D.E. Chemical approahes to the discovery and development of cancer therapies. Nat. Rev. Cancer 2005, 5, 285–296. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Schauweker, T.H.; Gregory, R.R.; Varma, M.; Kittrell, F.S.; Medina, D.; Varma, R.S. Nuclear type II sites and malignant cell proliferation: Inhibition by 2,6-Bis-benzylidenecyclohexanones. Cancer Res. 1992, 52, 2482–2488. [Google Scholar]
- Dwivedi, S.; Purohit, P.; Sharma, P. MicroRNAs and diseases: Promising biomarkers for diagnosis and therapeutics. Indian J. Clin. Biochem. 2019, 34, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down- regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef] [Green Version]
- Loh, H.-Y.; Norman, B.P.; Lai, K.-S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The regulatory role of microRNAs in breast cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.-S.; Song, M.-K.; Ryu, J.-C. Integrated analysis of microRNA and mRNA expression profiles highlights alterations in modulation of the apoptosis-related pathway under nonanal exposure. Mol. Cell. Toxicol. 2013, 9, 351–364. [Google Scholar] [CrossRef]
- Othman, N.; Nagoor, N.H. The role of microRNAs in the regulation of apoptosis in lung cancer and its application in cancer treatment. Biomed. Res. Int. 2014, 2014, 318030. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Regalla, K.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-L.; Chang, J.-M.; Chong, I.-W.; Hung, Y.-L.; Chen, Y.-H.; Huang, W.-T.; Kuo, H.-F.; Hsieh, C.-C.; Liu, P.-L. Curcumin inhibits LIN-28a through the activation of miRNA-98 in the lung cancer cell line A549. Molecules 2017, 22, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harun, S.N.A.; Israf, D.A.; Tham, C.L.; Lam, K.W.; Cheema, M.S.; Hashim, N.F.M. The molecular targets and anti-invasive effects of 2,6-bis-(4-hydroxyl-3methoxybenzylidine) cyclohexanone or BHMC in MDA-MB-231 human breast cancer cells. Molecules 2018, 23, 865. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.M.; Yeap, S.K.; Abu, N.; Lim, K.L.; Ky, H.; Zaim, A.; Pauzi, M.; Ho, W.Y.; Tan, S.W.; Kiat, H.; et al. Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Int. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamrus, S.N.H.; Akhtar, M.N.; Yeap, S.K.; Quah, C.K.; Loh, W.-S.; Alitheen, N.B.; Zareen, S.; Tajuddin, S.N.; Hussin, Y.; Shah, S.A.A. Design, synthesis and cytotoxic effects of curcuminoids on HeLa, K562, MCF-7 and MDA-MB-231 cancer cell lines. Chem. Cent. J. 2018, 12, 31. [Google Scholar] [CrossRef]
- Xia, Y.-Q.; Wei, X.-Y.; Li, W.-L.; Kanchana, K.; Xu, C.-C.; Chen, D.-H.; Chou, P.-H.; Jin, R.; Wu, J.-Z.; Liang, G. Curcumin analogue A501 induces G2/M arrest and apoptosis in non-small cell lung cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 6893–6898. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Z.; Cao, Y.; Zhu, C.; Zuo, Y.; Huang, L.; Wen, G.; Shang, N.; Chen, Y.; Yue, X.; et al. MC37, a new mono-carbonyl curcumin analog, induces G2/M cell cycle arrest and mitochondria-mediated apoptosis in human colorectal cancer cells. Eur. J. Pharm. 2017, 795, 139–148. [Google Scholar] [CrossRef]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The curcumin analog EF24 targets NF-kB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS ONE 2013, 8, e71130. [Google Scholar]
- Javad, M.; Esmatabadi, D.; Farhangi, B.; Montazeri, M.; Monfared, H.; Sistani, R.N.; Sadeghizadeh, M. Up-regulation of miR-21 decreases chemotherapeutic effect of dendrosomal curcumin in breast cancer cells. Iran. J. Basic Med. Sci. 2017, 20, 350–359. [Google Scholar]
- Zhou, S.; Zhang, S.; Shen, H.; Chen, W.; Xu, H.; Chen, X.; Sun, D.; Zhong, S.; Zhao, J.; Tang, J. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biol. 2017, 39, 1010428317691680. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, M.; Kemmerling, U.; Aguayo, F.; Bleak, T.C.; Muñoz, J.P.; Calaf, G.M. Curcumin rescues breast cells from epithelial—mesenchymal transition and invasion induced by anti-miR-34a. Int. J. Oncol. 2020, 56, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Gao, Z.; Tian, L.; Liu, M. Expressions of miR-122a and miR-3195 in laryngeal cancer and their effects on the proliferation and apoptosis of laryngeal cancer cell Hep-G2. Adv. Clin. Exp. Med. 2020, 29, 525–534. [Google Scholar] [CrossRef]
- Sohn, E.J.; Won, G.; Lee, J.; Lee, S.; Kim, S. Upregulation of miRNA3195 and miRNA374b mediates the anti-angiogenic properties of melatonin in hypoxic PC-3 prostate cancer cells. J. Cancer 2015, 6, 19–28. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome cancer cell drug resistance using natural products. Evid. Based Complementary Altern. Med. 2015, 2015, 767136. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Gao, Y.; Yu, Q.; Tang, F.; Zhao, P.-W.; Luo, S.-K.; Lin, J.-S.; Mei, H. miR-30a-3p inhibits the proliferation of liver cancer cells by targeting DNMT3a through the PI3K/AKT signaling pathway. Oncol. Lett. 2020, 19, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Cheng, Y.; Lin, L.; Liu, Z.; Du, S.; Ma, L.; Li, J.; Peng, Z.; Yan, J. Global analysis of miRNA signature differentially expressed in insulin-resistant human hepatocellular carcinoma cell line. Int. J. Med. Sci. 2020, 17, 664–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.S.; Liu, X.B.; Wong, B.Y.H.; Ng, R.W.M.; Yuen, A.P.W.; Wei, W.I. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin. Cancer Res. 2008, 14, 2588–2592. [Google Scholar] [CrossRef] [Green Version]
- Foley, N.H.; Bray, I.M.; Tivnan, A.; Bryan, K.; Murphy, D.M.; Buckley, P.G.; Ryan, J.; O’Meara, A.; O’Sullivan, M.; Stallings, R.L. MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol. Cancer 2010, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tamimi, R.M.; Collins, L.C.; Schnitt, S.J.; Gilmore, H.L.; Connolly, J.L.; Colditz, G.A. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: Results from the Nurses’ Health Study. Breast Cancer Res. Treat. 2011, 129, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.N.; Burton, L.J.; Henderson, V.; Randle, D.D.; Morton, D.J.; Smith, B.A.; Taliaferro-Smith, L.; Nagappan, P.; Yates, C.; Zayzafoon, M.; et al. Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS ONE 2014, 9, e104987. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2017, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Pećina-Šlaus, N. Wnt signal transduction pathway and apoptosis: A review. Cancer Cell Int. 2010, 10, 22. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complementary Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W.; Alitheen, N.B.; Long, K.; Yeap, S.K. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci. Rep. 2019, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Abu, N.; Yeap, S.K.; Lim, K.L.; Romli, M.F.; Sharifuddin, S.A.; Long, K.; Alitheen, N.B. Apoptosis and metastasis inhibitory potential of pineapple vinegar against mouse mammary gland cells in vitro and in vivo. Nutr. Metab. 2019, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Hu, Y.; Tong, S.; Williams, B.R.G.; Smyth, G.K.; Gantier, M.P. The use of miRNA microarrays for the analysis of cancer samples with global miRNA decrease. RNA 2013, 19, 876–888. [Google Scholar] [CrossRef] [Green Version]
- Causin, R.L.; Pessôa-Pereira, D.; Souza, K.C.B.; Evangelista, A.F.; Reis, R.M.V.; Fregnani, J.H.T.G.; Maria, C.M.M. Identification and performance evaluation of housekeeping genes for microRNA expression normalization by reverse transcription-quantitative PCR using liquid-based cervical cytology samples. Oncol. Lett. 2019, 18, 4753–4761. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2014, 3, 71–85. [Google Scholar]
- Xiao, B.; Shi, X.; Bai, J. miR-30a regulates the proliferation and invasion of breast cancer cells by targeting Snail. Oncol. Lett. 2019, 17, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Weber, R.; Bertoni, A.P.S.; Bessestil, L.W.; Brasil BM de, A.A.; Brum, I.i.S.; Furlanetto, T.W. Validation of reference genes for normalization gene expression in reverse transcription quantitative PCR in human normal thyroid and goiter tissue. Biomed. Res. Int. 2014, 2014, 198582. [Google Scholar] [CrossRef]

| Cell Lines | Treatment | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| MCF-7 | BHMC (µM) | 23.50 ± 2.41 | 12.50 ± 1.87 | 10.98 ± 1.33 |
| Curcumin (µM) | 55.72 ± 2.77 | 45.15 ± 2.12 | 39.79 ± 1.51 | |
| MDA-MB-231 | BHMC (µM) | 29.35 ± 3.15 | 20.05 ± 1.94 | 19.69 ± 3.14 |
| Curcumin (µM) | 32.00 ± 2.81 | 26.00 ± 2.33 | 23.00 ± 2.15 | |
| MCF-10A | BHMC (µM) | 180.00 ± 3.11 | 108.00 ± 2.15 | 98.40 ± 3.22 |
| Curcumin (µM) | 188.00 ± 4.52 | 112.00 ± 4.57 | 100.00 ± 4.31 | |
| SI MCF10A/MCF7 | BHMC (µM) | 7.66 | 8.64 | 8.96 |
| Curcumin (µM) | 3.37 | 2.48 | 2.51 | |
| SI MCF10A/MDA-MB-231 | BHMC (µM) | 6.14 | 5.38 | 5.00 |
| Curcumin (µM) | 5.88 | 4.31 | 4.35 |
| Transcript ID | Upregulated Fold Change | Transcript ID | Downregulated Fold Change |
|---|---|---|---|
| hsa-miR-184 | 17.58 | hsa-miR-6779-5p | −33.07 |
| hsa-miR-3195 | 16.21 | hsa-miR-1587 | −35.89 |
| hsa-miR-149-5p | 13.06 | hsa-miR-4725-3p | −41.51 |
| hsa-miR-30a-3p | 12.9 | hsa-miR-6132 | −61.96 |
| hsa-miR-532-3p | 11.73 | hsa-miR-6813-5p | −71.82 |
| MicroRNA Targets | Primer Sequence (5′–3′) |
|---|---|
| has-miR-3195 | F: GCGCCGGGCCC |
| R: CAGGTCCAGTTTTTTTTTTTTTTTAAC | |
| has-miR-30a-3p | F: GCTTTCAGTCGGATGTTTG |
| R: GGTCCAGTTTTTTTTTTTTTTTGCT | |
| has-miR-6132 | F: GCAGGGCTGGGAT |
| R: GGTCCAGTTTTTTTTTTTTTTTGCA | |
| has-miR-6813-5p | F: AGGGGCTGGGGTTTC |
| R: CCAGTTTTTTTTTTTTTTTAGAACCTG |
| Gene | Primer Sequence (5′–3′) |
|---|---|
| SNAIL | F: GCCGACTTTTGTGGTCTTCC |
| R: GGTACAAGTATGCCTCTGCCA | |
| VEGF | F: GCTGTGGACTTGAGTTGGG |
| R: GCTGGGTTTGTCGGTGTT | |
| ACTB | F: AGAGCTACGAGCTGCCTGAC |
| R: AGCACTGTGTTGGCGTACAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeap, S.K.; Mohd Ali, N.; Akhtar, M.N.; Razak, N.A.; Chong, Z.X.; Ho, W.Y.; Boo, L.; Zareen, S.; Kurniawan, T.A.; Avtar, R.; et al. Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells. Molecules 2021, 26, 1277. https://doi.org/10.3390/molecules26051277
Yeap SK, Mohd Ali N, Akhtar MN, Razak NA, Chong ZX, Ho WY, Boo L, Zareen S, Kurniawan TA, Avtar R, et al. Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells. Molecules. 2021; 26(5):1277. https://doi.org/10.3390/molecules26051277
Chicago/Turabian StyleYeap, Swee Keong, Norlaily Mohd Ali, Muhammad Nadeem Akhtar, Nursyamirah Abd Razak, Zhi Xiong Chong, Wan Yong Ho, Lily Boo, Seema Zareen, Tonni Agustiono Kurniawan, Ram Avtar, and et al. 2021. "Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells" Molecules 26, no. 5: 1277. https://doi.org/10.3390/molecules26051277
APA StyleYeap, S. K., Mohd Ali, N., Akhtar, M. N., Razak, N. A., Chong, Z. X., Ho, W. Y., Boo, L., Zareen, S., Kurniawan, T. A., Avtar, R., Ng, S. Y. L., Ong, A. H. K., & Alitheen, N. B. (2021). Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells. Molecules, 26(5), 1277. https://doi.org/10.3390/molecules26051277







