Abstract
Cytotoxic flavonoids of Murraya tetramera were investigated in this study. A novel flavonoid and twelve known flavonoids, including seven flavones (1–7), three flavanones (8–10), and three chalcones (11–13) were isolated from the leaves and twigs of Murraya tetramera. Chemical structures were elucidated by NMR combined with MS spectral analysis, and the new compound (6) was confirmed as 3′,5′-dihydroxy-5,6,7,4′-tetramethoxyflavone. Furthermore, all the isolated flavonoids were evaluated for their cytotoxicities against murine melanoma cells (B16), and human breast cancer cells (MDA-MB-231) by CCK-8 assay. Among them, compounds 7, 13, and 5 exhibited potent cytotoxic activities against B16 cell lines (IC50 = 3.87, 7.00 and 8.66 μg/mL, respectively). Compounds 5, 13, and 12 displayed potent cytotoxicities against MDA-MB-231 cell lines (IC50 = 3.80, 5.95 and 7.89 μg/mL, respectively). According to the correlation of the structure and activity analysis, 5-hydroxyl and 8-methoxyl substituents of the flavone, 8-methoxyl substituent of the flavanone, and 3′,5′-methoxyl substituents of the chalcone could be critical factors of the high cytotoxicity. The results indicated that the active flavonoids have potential to be developed as leading compounds for treating cancers.
1. Introduction
Cancer is a leading cause of death among most countries in the 21st century, and the incidence and mortality are rapidly growing worldwide [1]. Melanoma of the skin among males and breast cancer among females are two of most prevalent cancers in 2019 [2]. Melanoma arises from epidermal melanocytes and induces 80% of the dermatological cancer-related deaths [3]. Breast cancer is the second major cause of cancer deaths in female worldwide [4]. Serious side effects and drug resistance caused by conventional cancer treatment of chemotherapy and radiotherapy remain the major problems during the treatment [5,6]. Accordingly, plant secondary metabolites have been attracting more attention in drug development owing to multiple factors, and the various compounds discovered in plants made them as a rich source of cancer drug candidates [7,8,9,10]. Thousands of flavonoids have been isolated from stems, flowers, fruits, roots, and barks of the plants, moreover, many effective cytotoxic flavonoids from various plants were considered as potential leading compounds for the development of anticancer drugs [8,11,12,13].
The genus Murraya (family Rutaceae) is a common plant source of polymethoxylated and polyhydroxylated flavonoids [14]. Murraya tetramera Huang (M. tetramera) is a small tree that is widely distributed in Guangxi and Yunnan provinces of China. The folk medicine has been applied for treating coughs, bronchitis, rheumatism, asthma, and traumatic injury, etc. [15,16]. M. tetramera contains various flavonoids, coumarins, alkaloids, and sesquiterpenes [17,18,19,20]. Some of the isolated compounds have exhibited significant cytotoxic effects [20,21,22].
To explore potent cytotoxic flavonoids from M. tetramera as potential leading compounds for treating cancers and make comprehensive utilization of its natural resources, a phytochemical investigation was carried out, and the cytotoxicities were evaluated against murine melanoma cells (B16) and human breast cancer cells (MDA-MB-231) by CCK-8 assay.
2. Results and Discussions
2.1. Flavonoids Isolated from M. tetramera
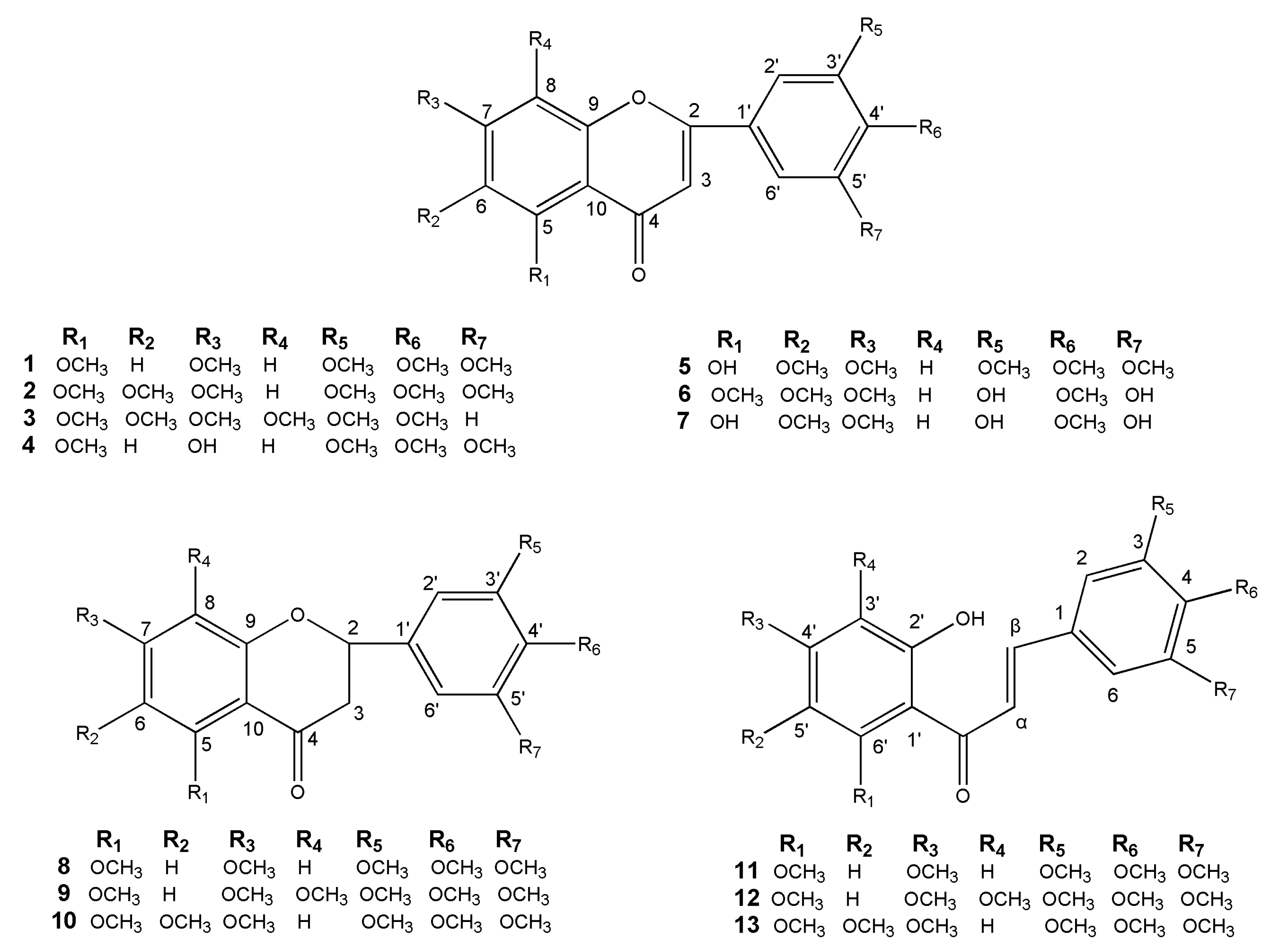
A novel flavonoid and twelve known flavonoids, including seven flavones (1–7), three flavanones (8–10) and three chalcones (11–13) were isolated from the leaves and twigs of M. tetramera. The new one was identified as 3′,5′-dihydroxy-5,6,7,4′-tetramethoxyflavone (6) and the others were 3′,4′,5,5′,7-pentamethoxyflavone (1) [23], 5,6,7,3′,4′,5′-hexamethoxyflavone (2) [24,25], nobiletin (3) [26], 7-hydroxy-3′,4′,5,5′-tetra methoxyflavone (4) [27], 5-hydroxy-6,7,3′,4′,5′-pentamethoxyflavone (5) [28], 5,3′,5′-trihydroxy-6,7,4′-trimethoxyflavone (7) [29], 5,7,3′,4′,5′-pentamethoxyflavanone (8) [30], 3′,4′,5′,5,7,8-hexamethoxy flavanone (9) [31,32], 5,6,7,3′,4′,5′-hexamethoxyflavanone (10) [25], 2′-hydroxy-3,4,5,4′,6′-pentamethoxychalcone (11) [23,33], 2′-hydroxy-3,4,5,3′,4′,6′-hexamethoxychalcone (12) [33], and 2′-hydroxy-3,4,5,4′,5′,6′-hexamethoxychalcone (13) [34]. Their structures were shown in Figure 1. The 1H and 13C-NMR data of the twelve known flavonoids were listed in the Supplementary Materials.
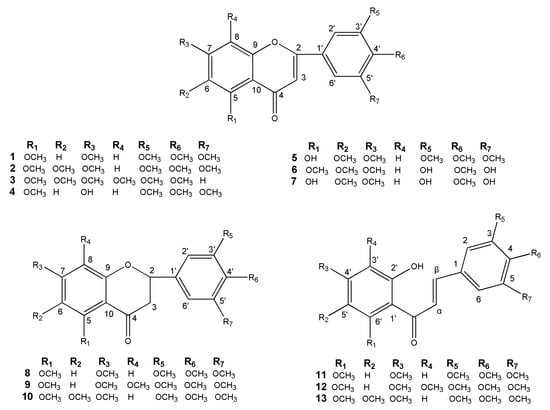
Figure 1.
Chemical structures of flavonoids 1–13.
2.2. Structure Elucidation of the New Flavone
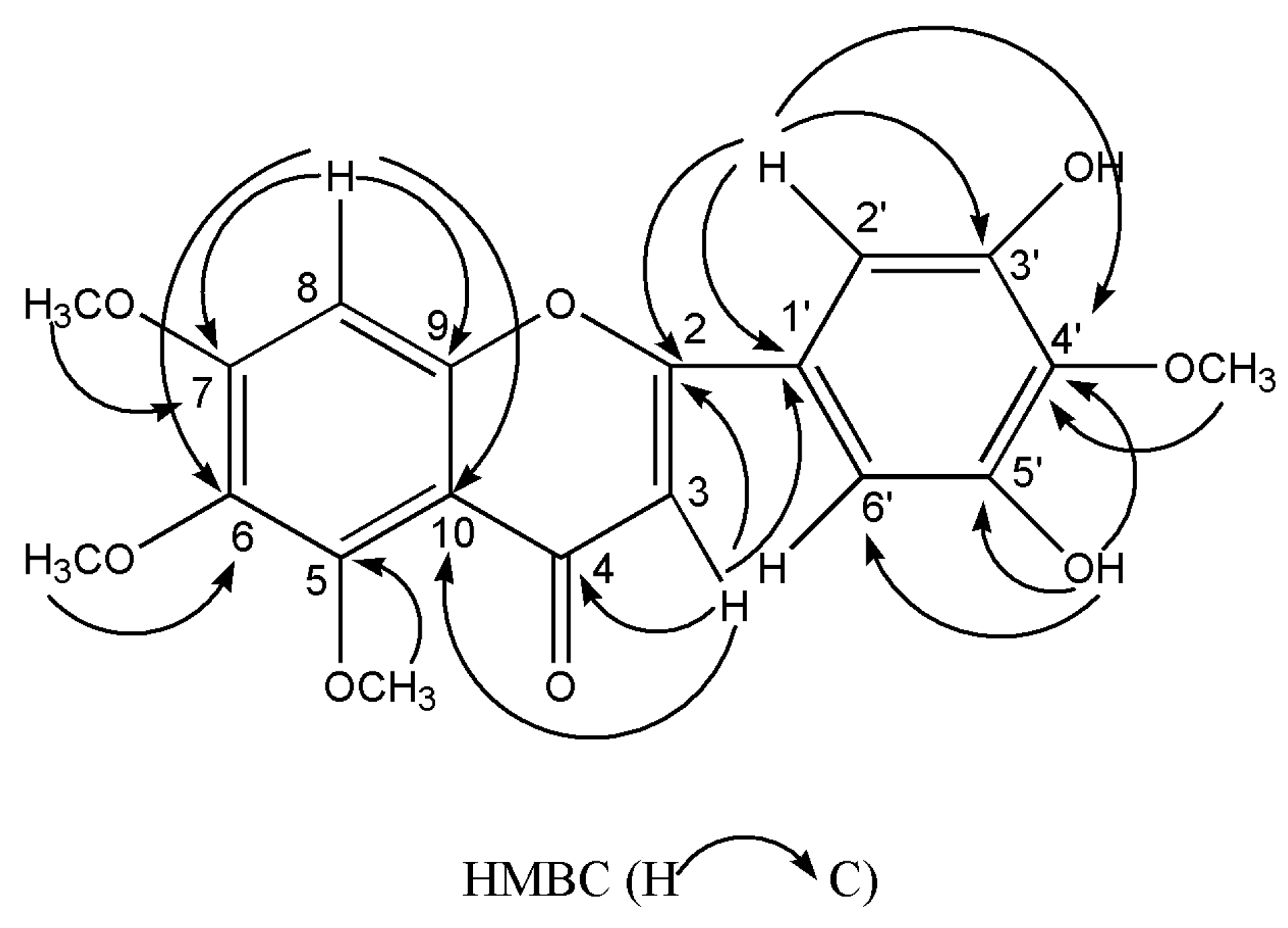
Compound 6 was collected as yellow needles. The molecular formula of C19H18O8 was deduced from the peak at m/z 375.1075 [M+H]+ (calculated for C19H19O8, 375.1074) in the HR-ESI-MS and the 19 carbon resonances in the 13C-NMR data. The 13C-NMR exhibited the typical flavone signals at δC 176.0 (C-4), δC 161.0 (C-2) and δC 107.2 (C-3). The 1H-NMR displayed a typical flavone H-3 signal at δH 6.45 (1H, s), a characteristic flavone signal of H-2′ and 6′ at δH 7.00 (2H, s), and an aromatic proton at δH 7.10 (1H, s). Moreover, the 1H-NMR exhibited the existence of two hydroxy protons at δH 9.51 (2H, s), and four methoxyl peaks at δH 3.96, 3.80, 3.77 and 3.76 (each 3H, s). The HMBC displayed correlations arising from H-8 to C-10/C-6/C-9/C-7, from H-2′,6′ to C-1′/C-4′/C-3′,5′/C-2, from H-3 to C-10/C-1′/C-2/C-4, from 3′,5′-OH to C-2′,6′/, C-4′/C-3′,5′, from 7-OCH3, 5-OCH3, 6-OCH3 and 4′-OCH3 to C-7, C-5, C-6 and C-4′, respectively. The key HMBC correlations were indicated in Figure 2. Accordingly, compound 6 was deduced as 3′,5′-dihydroxy-5,6,7,4′-tetramethoxyflavone. The 1H and 13C-NMR data were listed in Table 1. All spectra are available in the Supplementary Materials.
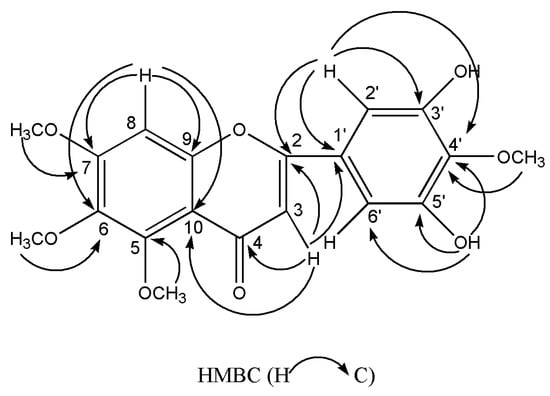
Figure 2.
Key HMBC correlations of compound 6.

Table 1.
1H and 13C-NMR data of flavone 6 in DMSO-d6.
2.3. Cytotoxicities of Isolated Flavonoids
Flavonoids 1–13 were evaluated for their cytotoxicities against B16 and MDA-MB-231 cell lines by CCK-8 assay and the results were displayed in Table 2. Among them, compounds 7, 13, and 5 exhibited potent cytotoxic activities against B16 cell lines (IC50 = 3.87, 7.00, and 8.66 μg/mL, respectively). Compounds 5, 13, and 12 displayed potent cytotoxicities against MDA-MB-231 cell lines (IC50 = 3.80, 5.95 and 7.89 μg/mL, respectively). However, flavonoids 1, 6 and 11 showed weak anticancer efficacy against the two tested tumor cell lines (IC50 > 100 μg/mL).

Table 2.
Cytotoxicities of flavonoids 1–13 from Murraya tetramera.
The diverse cytotoxicities might be attributed to the different substituents of the flavonoids. Among flavones 1–7, flavones 5, 7, and 3 exhibited higher cytotoxicities against B16 cells (IC50 = 8.66, 3.87, and 11.18 µg/mL) and MDA-MB-231 cells (IC50 = 3.80, 14.93, and 23.46 µg/mL) than others. Thus, 5-hydroxyl and 8-methoxyl substituents of the flavone were essential for high cytotoxicity, which corresponds to the state of literature [8,35]. In addition, compared with flavone 6, flavone 2 showed a higher cytotoxicity against B16 and MDA-MB-231 cells with IC50 values of 14.74 and 34.19 µg/mL. Therefore, if the methoxy substituents in position 3′ and 4′ of flavone was substituted by hydroxyl substituents, as found in flavone 6, the cytotoxicity was significantly reduced. Among the flavanones 8–10, flavanone 9 exhibited the highest cytotoxicity against B16 and MDA-MB-231 cells (IC50 = 12.76 and 16.02 µg/mL). Thus, 8-methoxyl substituent of the flavanone could be a critical factor of the cytotoxic activity, which corresponds to the state of literature [35]. Among chalcones 11–13, chalcones 12 and 13 exhibited higher cytotoxicities against B16 cells (IC50 = 11.53 and 7.00 µg/mL) and MDA-MB-231 cells (IC50 = 7.89 and 5.95 µg/mL) compared with chalcone 11. Hence, 3′ and 5′-methoxyl substituents of the chalcone could be a major factor of the cytotoxic activity. Overall, according to the correlation of the structure and activity analysis, the position of methoxy and hydroxyl substituents in the flavonoids may be the major factors of the anticancer efficacy. Further investigation is essential to clarify the structure-active relationships.
3. Materials and Methods
3.1. General Information
The NMR spectrometer (Bruker Avance III, Bruker, Karlsruhe, Germany) was used to record the NMR spectra at 500 MHz (1H) and at 125 MHz (13C). The mass spectrometer (Bruker Q-TOF, Bruker, Karlsruhe, Germany) was used to measure the HR-ESI-MS. Preparative HPLC was carried out using a Rainbow Kromasil-C18 column (10 × 250 mm, 10 µm) on a Waters Delta Prep 4000 instrument with a dual λ absorbance detector (Waters 2487, Waters, Milford, USA). MCI GEL CHP20P of 75–150 μm (Kaiteki Company, Tokyo, Japan) was selected for column chromatography. Silica gel G plates were used for TLC analysis (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). The deuterated DMSO-d6 and CDCl3 were supplied by Cambridge Isotope Labo-ratories, Inc. (Andover, USA). DMEM, RPMI 1640 and fetal bovine serum were supplied by Gibco Inc. (New York, USA). Penicillin and streptomycin were provided by Solarbio science & technology Co., Ltd. (Beijing, China). CCK-8 reagent was obtained from Beyotime Biotechnology (Shanghai, China). All the analytical solvents of analytical grade were supplied by Beijing Chemical Plant (Beijing, China).
3.2. Plant Material
The leaves and twigs of M. tetramera were harvested at Xishuangbanna, Yunnan Province, China in May 2014 and were identified by Dr. Liu, Q.R. (College of Life Sciences, Beijing Normal University, Beijing, China). The certificate specimen (BNU-CMH-Dushushan-2014-05-025-001) was stored at the Herbarium of Faculty of Geographical Science, Beijing Normal University.
3.3. Extraction and Isolation
The methanol extract of leaves and twigs of M. tetramera was obtained from our previous study and 90 fractions were received from the methanol extract by eluting with a stepwise gradient of PE/EtOAc and CHCl3/CH3OH [36]. Fr. 55–57 (4.27 g), Fr. 59–60 (3.77 g), Fr. 66–67 (1.96 g), Fr. 68–69 (2.26 g) and Fr. 75 (1.51 g) were separated by MCI column chromatography with a mobile phase of EtOH-H2O (3:7, 5:5, 7:3 and EtOH), and then further purified by preparative HPLC using a stepwise gradient of MeOH-H2O (2:8→MeOH) to obtain flavone 1 (20 mg, 0.0008% yield), flavone 2 (150 mg, 0.006% yield), flavone 3 (60 mg, 0.0024% yield), flavone 4 (9.5 mg, 0.0004% yield), flavone 5 (2.1 mg, 0.00008% yield), flavone 6 (2.1 mg, 0.00008% yield), flavone 7 (2.8 mg, 0.0001% yield), flavanone 8 (20 mg, 0.0008% yield), flavanone 9 (200 mg, 0.008% yield), flavanone 10 (15 mg, 0.0006% yield), chalcone 11 (6.2 mg, 0.0002% yield), chalcone 12 (50 mg, 0.002% yield) and chalcone 13 (45 mg, 0.0018% yield), respectively. The compounds were stored at 4 °C in a refrigerator for subsequent experiments.
3.4. Cytotoxicity Assay
The cytotoxicities of flavonoids 1–13 were determined by the standard CCK-8 assay [20,37]. B16 (Number: GDC0039) were originally provided by China Center for Type Culture Collection (Wuhan, China) and MDA-MB-231 (Number: CL0208) were obtained from the Fenghui Biotechnology Co., Ltd. (Changsha, China). Doxorubicin hydrochloride (DOX), the positive control, was purchased from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). B16 cells were cultured in RPMI 1640 medium and MDA-MB-231 cells were cultured in DMEM medium. The medium supplemented with 10% fetal bovine serum (Gibco Inc.), 100 U/mL penicillin and 0.1 mg/mL streptomycin. The tested cell lines were incubated at 37 °C, 5% CO2 and 90% humidity in the CO2 incubator (Binder, Tuttlingen, Germany). Firstly, 100 μL of the cell suspension was seeded into each well of 96-well plates (6 × 103 per well), and then incubated for 12–24 h to allow cellular attachment. After removing the medium, fresh medium containing seven concentrations of test compounds was added into cultured cells of 100 μL per well and incubated for 48 h. Secondly, 10 μL CCK-8 reagent was added into each well and placed in a CO2 incubator for 1 h. Finally, the absorbance was recorded using a microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm. The 50% inhibitory concentration (IC50) values were calculated using Probit analysis (SPSS V20.0).
4. Conclusions
A novel flavonoid and twelve known flavonoids, including seven flavones (1–7), three flavanones (8–10), and three chalcones (11–13) were isolated from the leaves and twigs of M. tetramera. The novel one (compound 6) was identified as 3′,5′-dihydroxy-5,6,7,4′-tetramethoxyflavone. Results of cytotoxicity assay indicated that flavones 5 and 7 with 5-hydroxyl substituent, flavones 3 and flavanone 9 with 8-methoxyl substituent, chalcone 12 with 3′-methoxyl substituent and chalcone 13 with 5′-methoxyl substituent exhibited significant cytotoxic activities against B16 and MDA-MB-231 cell lines. According to the correlation of the structure and activity analysis, the position of methoxy and hydroxyl substituents in the flavonoids were the major factors of the high anticancer efficacy. The results indicated that the active flavonoids have potential to be developed as leading compounds for treating cancers.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of compound 6, Figure S2: 13C-NMR spectrum of compound 6, Figure S3: HMBC spectrum of compound 6, Figure S4: HR-ESI-MS spectrum of compound 6, Table S1: 1H-NMR data of the twelve known flavonoids, Table S2: 13C-NMR data of the twelve known flavonoids.
Author Contributions
Conceptualization, C.-X.Y., K.Z., W.-J.Z. and X.-X.Y.; methodology, C.-X.Y., K.Z., X.L. and J.L.; investigation, C.-X.Y., K.Z., X.L. and J.L.; data curation, C.-X.Y. and K.Z.; writing—original draft preparation, C.-X.Y.; writing—review and editing, C.-X.Y., K.Z., W.-J.Z. and X.-X.Y.; funding acquisition, C.-X.Y. and W.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Tianjin, grant number 18JCQNJC83700 and the Research Project of Tianjin Municipal Education Commission, grant number 2018KJ191.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting this study is available in the manuscript and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. Ca-Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Zhang, X.L.; Lu, Y.; Zhao, L.Y.; Guo, Y.X.; Guo, S.S.; Kang, Q.Z.; Liu, J.J.; Dai, L.P.; Zhang, L.G.; et al. Protein 4.1R affects photodynamic therapy for B16 melanoma by regulating the transport of 5-aminolevulinic acid. Exp. Cell Res. 2021, 399, 112465. [Google Scholar] [CrossRef]
- Han, B.; Sha, L.J.; Yu, X.M.; Yang, M.; Cao, Y.; Zhao, J. Identification of dual therapeutic targets assisted by in situ automatous DNA assembly for combined therapy in breast cancer. Biosens. Bioelectron. 2021, 176, 112913. [Google Scholar] [CrossRef] [PubMed]
- Vo, P.H.T.; Nguyen, T.D.T.; Tran, H.T.; Nguyen, Y.N.; Doan, M.T.; Nguyen, P.H.; Lien, G.T.K.; To, D.C.; Tran, M.H. Cytotoxic components from the leaves of Erythrophleum fordii induce human acute leukemia cell apoptosis through caspase 3 activation and PARP cleavage. Bioorg. Med. Chem. Lett. 2021, 31, 127673. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Controlled Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Pan, L.; Chai, H.; Kinghorn, A.D. The continuing search for antitumor agents from higher plants. Phytochem. Lett. 2010, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.; Tayarani-Najaran, Z. Potent cytotoxic natural flavonoids: The limits of perspective. Curr. Pharm. Des. 2018, 24, 5555–5579. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.R.A.; Ma, T.M.T. In vitro cytotoxic screening of 300 selected Chinese medicinal herbs against human gastric adenocarcinoma SGC-7901 cells. Afr. J. Pharm. Pharmacol. 2012, 6, 592–600. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Passreiter, C.M.; Suckow-Schnitker, A.; Kulawik, A.; Addae-Kyereme, J.; Wright, C.W.; Wätjen, W. Prenylated flavanone derivatives isolated from Erythrina addisoniae are potent inducers of apoptotic cell death. Phytochemistry 2015, 117, 237–244. [Google Scholar] [CrossRef]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2010, 49, 276–282. [Google Scholar] [CrossRef]
- Liang, H.Z.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Polymethoxylated flavonoids from Murraya paniculata (L.) Jack. Biochem. Syst. Ecol. 2020, 93, 104162. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1997; p. 145. [Google Scholar]
- Lv, H.N.; Wen, R.; Zhou, Y.; Zeng, K.W.; Li, J.; Guo, X.Y.; Tu, P.F.; Jiang, Y. Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J. Nat. Prod. 2015, 78, 2432–2439. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, H.N.; Wang, W.G.; Tu, P.F.; Jiang, Y. Flavonoids and anthraquinones from Murraya tetramera C. C. Huang (Rutaceae). Biochem. Syst. Ecol. 2014, 57, 78–80. [Google Scholar] [CrossRef]
- Lv, H.N.; Zhou, Y.; Wen, R.; Shi, M.L.; Zeng, K.W.; Xia, F.; Tu, P.F.; Jiang, Y. Murradiate and murradiol, two structurally unique heterodimers of carbazole-monoterpene and carbazole-phenylethanol from Murraya tetramera. Phytochem. Lett. 2016, 15, 113–115. [Google Scholar] [CrossRef]
- Lyu, H.N.; Zhou, Y.; Wen, R.; Tu, P.F.; Jiang, Y. Nitric oxide inhibitory carbazole alkaloids from the folk medicine Murraya tetramera C.C. Huang. Chem. Biodivers. 2020, 17, e2000490. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic compounds isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Wu, M.Z.; Chen, H.P.; Liu, Y.P. Compounds isolated from Murraya tetramera Huang and their cytotoxic activity. Nat. Prod. Res. Dev. 2019, 31, 627–632. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Chen, H.P.; Chen, L.; Liu, Y.P.; Li, Y. Carbazole alkaloids from Murraya tetramera Huang and their cytotoxic activity. Nat. Prod. Res. Dev. 2019, 31, 269–272, 305. [Google Scholar] [CrossRef]
- Mateeva, N.N.; Kode, R.N.; Redda, K.K. Synthesis of novel flavonoid derivatives as potential HIV-integrase inhibitors. J. Heterocycl. Chem. 2002, 39, 1251–1258. [Google Scholar] [CrossRef]
- Kong, C.H.; Liang, W.J.; Hu, F.; Xu, X.H.; Wang, P.; Jiang, Y.; Xing, B.S. Allelochemicals and their transformations in the Ageratum conyzoides intercropped citrus orchard soils. Plant Soil 2004, 264, 149–157. [Google Scholar] [CrossRef]
- Passador, E.A.P.; Da Silva, M.F.D.G.F.; Fo, E.R.; Fernandes, J.B.; Vieira, P.C.; Pirani, J.R. A pyrano chalcone and a flavanone from Neoraputia magnifica. Phytochemistry 1997, 45, 1533–1537. [Google Scholar] [CrossRef]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Bioph. Res. Co. 2005, 337, 1330–1336. [Google Scholar] [CrossRef]
- Facundo, V.A.; Morais, S.M.; Braz Filho, R. Chemical constituents of Ottionia corcovadensis Miq. from Amazon Forest: 1H and 13C chemical shift assignments. Quim. Nova 2004, 27, 79–83. [Google Scholar] [CrossRef]
- Rwangabo, P.C.; Claeys, M.; Pieters, L.; Corthout, J.; Vandenberghe, D.A.; Vlietinck, A.J. Umuhengerin, a new antimicrobially active flavonoid from Lantana trifolia. J. Nat. Prod. 1988, 51, 966–968. [Google Scholar] [CrossRef]
- Kinoshita, T.; Firman, K. Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry 1996, 42, 1207–1210. [Google Scholar] [CrossRef]
- Da Silva, B.F.; Rodrigues-Fo, E. Production of a benzylated flavonoid from 5,7,3′,4′,5′-pentamethoxyflavanone by Penicillium griseoroseum. J. Mol. Catal. B Enzym. 2010, 67, 184–188. [Google Scholar] [CrossRef]
- Ferracin, R.J.; Da Silva, M.; Fernandes, J.B.; Vieira, P.C. Flavonoids from the fruits of Murraya paniculata. Phytochemistry 1998, 47, 393–396. [Google Scholar] [CrossRef]
- Sherie, E.A.; Gupta, R.K.; Krishnamurti, M. Synthesis of chalcones, flavanones isolated from Popowia cauliflora and their analogues. Agric. Biol. Chem. 1981, 45, 531–533. [Google Scholar] [CrossRef][Green Version]
- Yao, H.; Jin, Y.R.; Shan, J.; Wang, X.Z.; Zhang, M.C.; Li, X.W. Two new natural methoxyflavonoids from leaves of Murraya paniculata(L.) Jack. Chem. Res. Chin. Univ. 2013, 29, 884–887. [Google Scholar] [CrossRef]
- Gupta, R.K.; Krishnamurti, M.; Parthasarathi, J. Synthesis of some recently isolated chalcones, their analogues and corresponding flavanones. Agric. Biol. Chem. 1979, 43, 2603–2605. [Google Scholar] [CrossRef]
- Cao, X.D.; Ding, Z.S.; Jiang, F.S.; Ding, X.H.; Chen, J.Z.; Chen, S.H.; Lv, G.Y. Antitumor constituents from the leaves of Carya cathayensis. Nat. Prod. Res. 2012, 26, 2089–2094. [Google Scholar] [CrossRef]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Liang, J.Y.; Lei, N.; Du, S.S.; Deng, Z.W. Chemical constituents of Murraya tetramera Huang and their repellent activity against Tribolium castaneum. Molecules 2017, 22, 1379. [Google Scholar] [CrossRef]
- Wu, S.B.; Ji, Y.P.; Zhu, J.J.; Zhao, Y.; Xia, G.; Hu, Y.H.; Hu, J.F. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids 2009, 74, 761–765. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).