Development, Validation, and Comparison of Two Mass Spectrometry Methods (LC-MS/HRMS and LC-MS/MS) for the Quantification of Rituximab in Human Plasma
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Proteotypic Peptides
2.2. Liquid Chromatography and Mass Spectrometry
2.3. Result of Validation
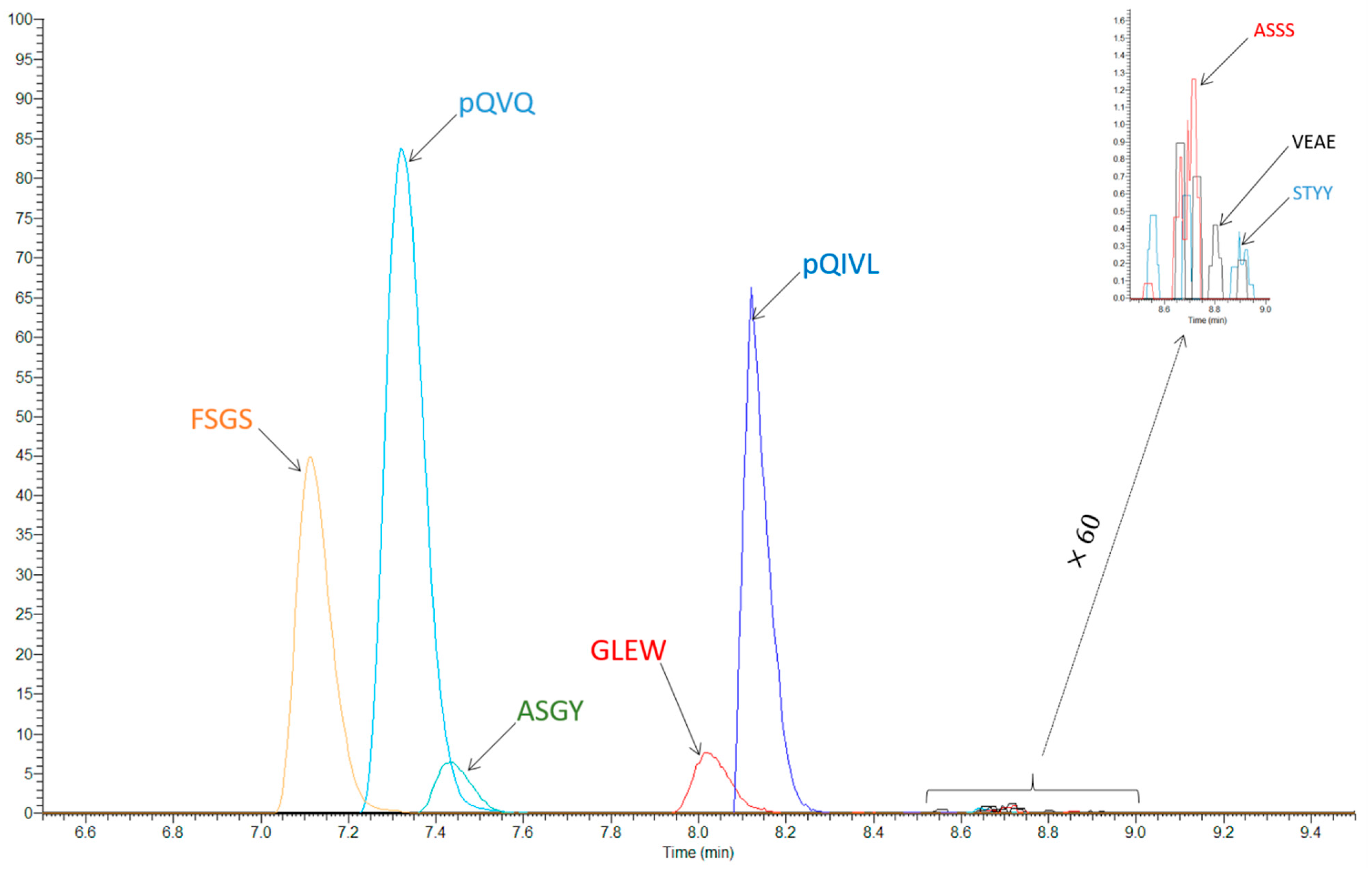
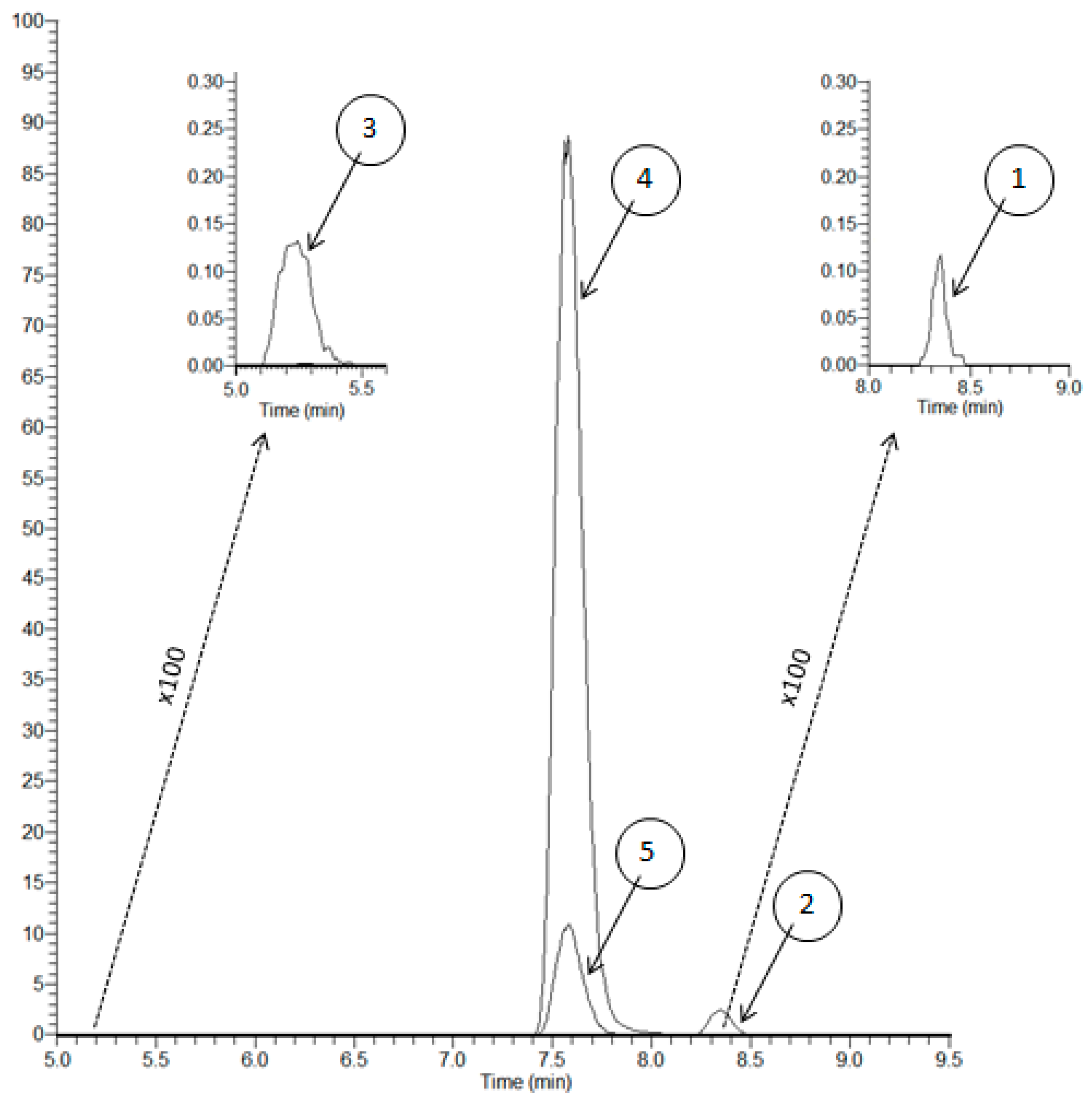
2.3.1. Selectivity
2.3.2. Calibration, Accuracy, Precision, LLOQ, and Dilution
2.3.3. Matrix Effects, Carryover, and Sample Stability
2.4. Application
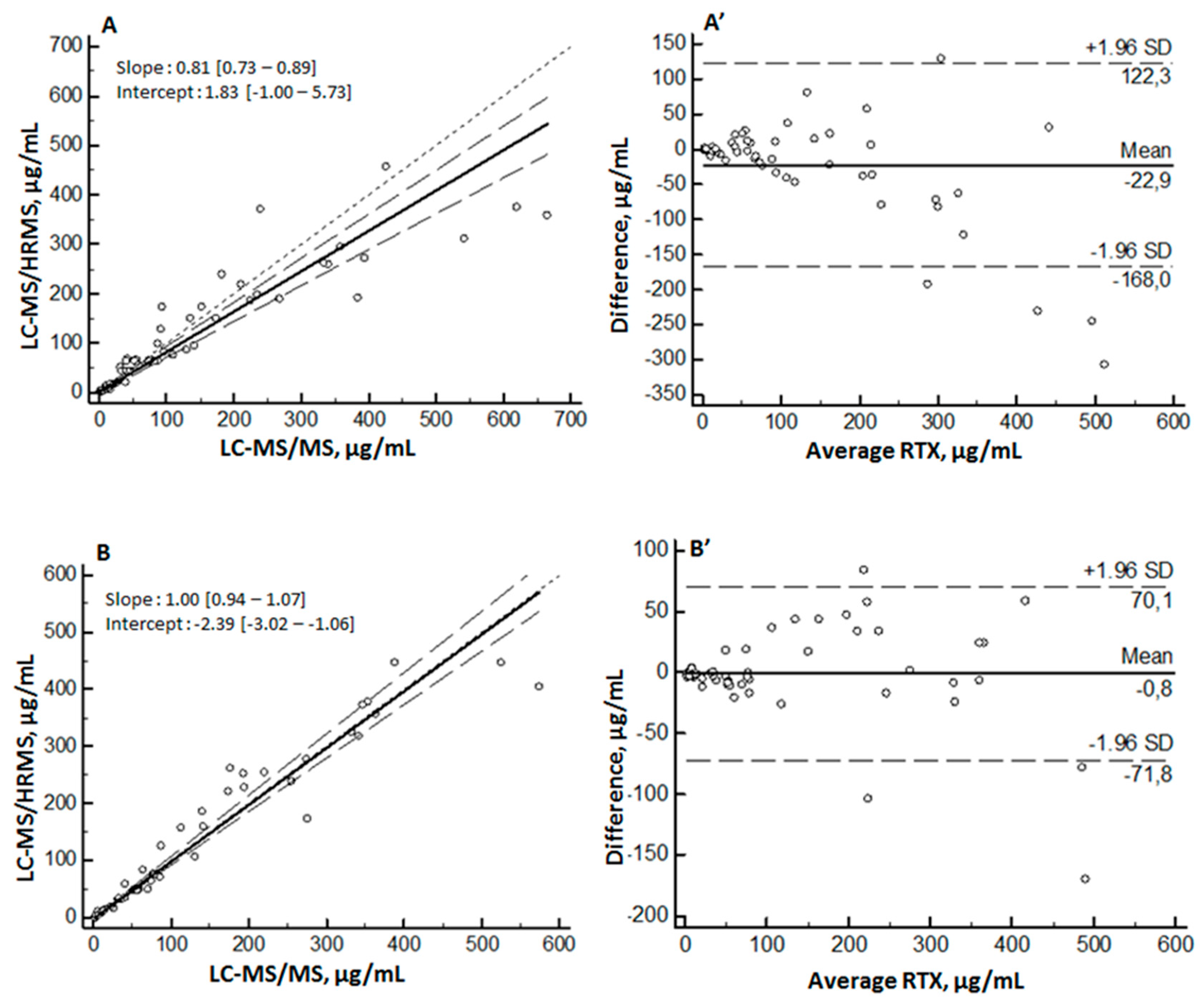
2.5. Comparison with Previously Published MS Methods
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Chromatographic and Mass Spectrometric Conditions and Instrumentation
3.2.1. LC-MS/HRMS
3.2.2. LC-MS/MS
3.3. Selection of Peptides for Quantification
3.4. Sample Preparation
3.5. Method Validation
3.6. Application and Method Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| IgG | immunoglobulin G |
| CR3 | complement receptor 3 |
| Fcγ | constant fragment gamma |
| HC | heavy chain |
| LC | light chain |
| QVQ | peptide QVQLQQPGAELVKPGASVK |
| pQVQ | peptide QVQLQQPGAELVKPGASVK with pyroglutamination of N-terminal glutamine |
| ASGY | peptide ASGYTFTSYNMHWVK |
| pQIVL | peptide QIVLSQSPAILSASPGEK with pyroglutamination of N-terminal glutamine |
| FSGS | peptide FSGSGSGTSYSLTISR |
| GLEW | peptide GLEWIGAIYPGNGDTSYNQK |
| ASSS | peptide ASSSVSYIHWFQQKPGSSPKPWIYATSNLASGVPVR |
| VEAE | peptide VEAEDAATYYCQQWTSNPPTFGGGTK |
| STYY | peptide STYYGGDWYFNVWGAGTTVTVSAASTK |
| LLOQ | lower limit of quantification |
| PRM | parallel reaction monitoring |
| MRM | multiple-reaction monitoring |
| SD | standard deviation |
| QCs | quality controls |
| nSMOL | nano-surface and molecular-orientation limited |
| FA | formic acid |
References
- Kazkaz, H.; Isenberg, D. Anti B Cell Therapy (Rituximab) in the Treatment of Autoimmune Diseases. Curr. Opin. Pharmacol 2004, 4, 398–402. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Karras, A.; Khouatra, C.; Aumaître, O.; Cohen, P.; Maurier, F.; Decaux, O.; Ninet, J.; Gobert, P.; et al. Rituximab versus Azathioprine for Maintenance in ANCA-Associated Vasculitis. N Engl. J. Med. 2014, 371, 1771–1780. [Google Scholar] [CrossRef]
- Cartron, G.; Trappe, R.U.; Solal-Céligny, P.; Hallek, M. Interindividual Variability of Response to Rituximab: From Biological Origins to Individualized Therapies. Clin. Cancer Res. 2011, 17, 19–30. [Google Scholar] [CrossRef]
- Harrold, J.M.; Straubinger, R.M.; Mager, D.E. Combinatorial Chemotherapeutic Efficacy in Non-Hodgkin Lymphoma Can Be Predicted by a Signaling Model of CD20 Pharmacodynamics. Cancer Res. 2012, 72, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Paci, A.; Desnoyer, A.; Delahousse, J.; Blondel, L.; Maritaz, C.; Chaput, N.; Mir, O.; Broutin, S. Pharmacokinetic/Pharmacodynamic Relationship of Therapeutic Monoclonal Antibodies Used in Oncology: Part 1, Monoclonal Antibodies, Antibody-Drug Conjugates and Bispecific T-Cell Engagers. Eur. J. Cancer 2020, 128, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Croca, S.; Gerona, D.; De La Torre, I.; Isenberg, D.; McDonald, V.; Leandro, M.; Cambridge, G. Serum Rituximab Levels and Efficiency of B Cell Depletion: Differences between Patients with Rheumatoid Arthritis and Systemic Lupus Erythematosus. Rheumatology (Oxford) 2013, 52, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Rekeland, I.G.; Fluge, Ø.; Alme, K.; Risa, K.; Sørland, K.; Mella, O.; de Vries, A.; Schjøtt, J. Rituximab Serum Concentrations and Anti-Rituximab Antibodies During B-Cell Depletion Therapy for Myalgic Encephalopathy/Chronic Fatigue Syndrome. Clin. Ther. 2019, 41, 806–814. [Google Scholar] [CrossRef]
- Iacona, I.; Lazzarino, M.; Avanzini, M.A.; Rupolo, M.; Arcaini, L.; Astori, C.; Lunghi, F.; Orlandi, E.; Morra, E.; Zagonel, V.; et al. Rituximab (IDEC-C2B8): Validation of a Sensitive Enzyme-Linked Immunoassay Applied to a Clinical Pharmacokinetic Study. Ther. Drug Monit. 2000, 22, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Lalmanach, G.; Godat, E.; Maurel, M.C.; Canepa, S.; Belghazi, M.; Paintaud, G.; Degenne, D.; Chatelut, E.; Cartron, G.; et al. Evaluation of a Peptide ELISA for the Detection of Rituximab in Serum. J. Immunol. Methods 2007, 325, 127–139. [Google Scholar] [CrossRef]
- Hampson, G.; Ward, T.H.; Cummings, J.; Bayne, M.; Tutt, A.L.; Cragg, M.S.; Dive, C.; Illidge, T.M. Validation of an ELISA for the Determination of Rituximab Pharmacokinetics in Clinical Trials Subjects. J. Immunol. Methods 2010, 360, 30–38. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, X.; Weaver, R.J.; Calliste, L.; Xia, C.; He, Y.J.; Chen, L. Validation of a GyrolabTM Assay for Quantification of Rituximab in Human Serum. J. Pharmacol. Toxicol. Methods 2012, 65, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R.; Cornec, D.; Dasari, S.; Ladwig, P.M.; Hummel, A.M.; Cheu, M.; Murray, D.L.; Willrich, M.A.; Snyder, M.R.; Hoffman, G.S.; et al. Using Mass Spectrometry to Quantify Rituximab and Perform Individualized Immunoglobulin Phenotyping in ANCA-Associated Vasculitis. Anal. Chem. 2016, 88, 6317–6325. [Google Scholar] [CrossRef]
- Mekhssian, K.; Mess, J.-N.; Garofolo, F. Application of High-Resolution MS in the Quantification of a Therapeutic Monoclonal Antibody in Human Plasma. Bioanalysis 2014, 6, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Truffot, A.; Jourdil, J.-F.; Seitz-Polski, B.; Malvezzi, P.; Brglez, V.; Stanke-Labesque, F.; Gautier-Veyret, E. Simultaneous Quantification of Rituximab and Eculizumab in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry and Comparison with Rituximab ELISA Kits. Clin. Biochem. 2020. [Google Scholar] [CrossRef]
- Iwamoto, N.; Takanashi, M.; Hamada, A.; Shimada, T. Validated LC/MS Bioanalysis of Rituximab CDR Peptides Using Nano-Surface and Molecular-Orientation Limited (NSMOL) Proteolysis. Biol. Pharm. Bull. 2016, 39, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Willeman, T.; Jourdil, J.-F.; Gautier-Veyret, E.; Bonaz, B.; Stanke-Labesque, F. A Multiplex Liquid Chromatography Tandem Mass Spectrometry Method for the Quantification of Seven Therapeutic Monoclonal Antibodies: Application for Adalimumab Therapeutic Drug Monitoring in Patients with Crohn’s Disease. Anal. Chim. Acta 2019, 1067, 63–70. [Google Scholar] [CrossRef]
- LISA TRACKER–Theradiag. Available online: https://www.theradiag.com/lisa-tracker/ (accessed on 1 February 2021).
- Du, J.; Wang, H.; Zhong, C.; Peng, B.; Zhang, M.; Li, B.; Huo, S.; Guo, Y.; Ding, J. Structural Basis for Recognition of CD20 by Therapeutic Antibody Rituximab. J. Biol. Chem. 2007, 282, 15073–15080. [Google Scholar] [CrossRef]
- Ryan, A.M.; Sokolowski, S.A.; Ng, C.-K.; Shirai, N.; Collinge, M.; Shen, A.C.; Arrington, J.; Radi, Z.; Cummings, T.R.; Ploch, S.A.; et al. Comparative Nonclinical Assessments of the Proposed Biosimilar PF-05280586 and Rituximab (MabThera®). Toxicol. Pathol. 2014, 42, 1069–1081. [Google Scholar] [CrossRef]
- Visser, J.; Feuerstein, I.; Stangler, T.; Schmiederer, T.; Fritsch, C.; Schiestl, M. Physicochemical and Functional Comparability between the Proposed Biosimilar Rituximab GP2013 and Originator Rituximab. BioDrugs 2013, 27, 495–507. [Google Scholar] [CrossRef]
- Seo, N.; Huang, Z.; Kuhns, S.; Sweet, H.; Cao, S.; Wikström, M.; Liu, J. Analytical and Functional Similarity of Biosimilar ABP 798 with Rituximab Reference Product. Biologicals 2020, 68, 79–91. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Donners, A.A.M.; Hack, C.E.; Huitema, A.D.R.; van Maarseveen, E.M. Six-Step Workflow for the Quantification of Therapeutic Monoclonal Antibodies in Biological Matrices with Liquid Chromatography Mass Spectrometry-A Tutorial. Anal. Chim. Acta 2019, 1080, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Siepen, J.A.; Keevil, E.-J.; Knight, D.; Hubbard, S.J. Prediction of Missed Cleavage Sites in Tryptic Peptides Aids Protein Identification in Proteomics. J. Proteome Res. 2007, 6, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Chelius, D.; Jing, K.; Lueras, A.; Rehder, D.S.; Dillon, T.M.; Vizel, A.; Rajan, R.S.; Li, T.; Treuheit, M.J.; Bondarenko, P.V. Formation of Pyroglutamic Acid from N-Terminal Glutamic Acid in Immunoglobulin Gamma Antibodies. Anal. Chem. 2006, 78, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Goetze, A.M.; Bass, R.B.; Flynn, G.C. N-Terminal Glutamate to Pyroglutamate Conversion in Vivo for Human IgG2 Antibodies. J. Biol. Chem. 2011, 286, 11211–11217. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Angeles, A.; Hamuro, L.L.; Arnold, M.E.; Shen, J.X. A Novel and Cost Effective Method of Removing Excess Albumin from Plasma/Serum Samples and Its Impacts on LC-MS/MS Bioanalysis of Therapeutic Proteins. Anal. Chem. 2014, 86, 8336–8343. [Google Scholar] [CrossRef]
- Passing, H.; Bablok, W. Comparison of Several Regression Procedures for Method Comparison Studies and Determination of Sample Sizes. Application of Linear Regression Procedures for Method Comparison Studies in Clinical Chemistry, Part II. J. Clin. Chem. Clin. Biochem. 1984, 22, 431–445. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.J. Regression Analysis. Lancet 1986, 1, 908–909. [Google Scholar] [CrossRef]





| Compound | Peptide | Precursor Ion | Product Ion | |||
|---|---|---|---|---|---|---|
| (m/z) | Charge | Ion | (m/z) | Charge | ||
| RTX | FSGS | 803.8890 | +2 | y8 | 926.4942 | +1 |
| y10 | 1084.5629 | +1 | ||||
| pQVQ | 980.5467 | +2 | y13 | 1252.7260 | +1 | |
| y6 | 558.3246 | +1 | ||||
| pQIVL | 904.4936 | +2 | y11 | 1069.5888 | +1 | |
| y7 | 675.3308 | +1 | ||||
| SIL-RTX | FSGS | 808.8931 | +2 | y8 y10 | 936.5024 1094.5716 | +1 +1 |
| pQVQ | 988.5609 | +2 | y13 y6 | 1268.7544 566.3360 | +1 +1 | |
| pQIVL | 908.5007 | +2 | y11 y7 | 1077.6030 683.3450 | +1 +1 | |
| Compound | Peptide | Q1 1 m/z (charge) | Q3 2 | CE 3 (eV) | CVt 4 (V) | |
|---|---|---|---|---|---|---|
| Ion | m/z (charge) | |||||
| RTX | pQVQ | 980.5 (+2) | y6 | 558.3 (+1) | 35 | 80 |
| y10 | 1027.6 (+1) | |||||
| y12 | 1155.7 (+1) | |||||
| y13 | 1252.7 (+1) | |||||
| pQIVL | 904.5 (+2) | y4 | 430.2 (+1) | 20 | 90 | |
| y7 | 675.3 (+1) | |||||
| y9 | 788.4 (+1) | |||||
| y11 | 1069.6 (+1) | 30 | ||||
| y12 | 1156.6 (+1) | |||||
| SIL-RTX | pQVQ | 988.6 (+2) | y6 | 566.3 (+1) | 35 | 80 |
| y10 | 1043.6 (+1) | |||||
| y12 | 1171.7 (+1) | |||||
| y13 | 1268.7 (+1) | |||||
| pQIVL | 908.5 (+2) | y4 | 438.2 (+1) | 20 | 90 | |
| y7 | 683.3 (+1) | |||||
| y9 | 796.4 (+1) | |||||
| y11 | 1077.6 (+1) | 30 | ||||
| y12 | 1164.6 (+1) | |||||
| FSGS | pQVQ | |||||
|---|---|---|---|---|---|---|
| Spiked (µg/mL) | Found (µg/mL) (mean ± s.d.) | Precision (%) | Accuracy (%) | Found (µg/mL) (mean ± s.d.) | Precision (%) | Accuracy (%) |
| 10 | 9 ± 1 | 12.6 | 91.8 | 9 ± 2 | 16.6 | 88.4 |
| 25 | 26 ± 3 | 9.7 | 103.1 | 26 ± 3 | 11.8 | 102.8 |
| 50 | 51 ± 3 | 5.4 | 101.7 | 51 ± 5 | 9.1 | 102.0 |
| 100 | 98 ± 3 | 3.3 | 98.3 | 100 ± 6 | 6.0 | 100.0 |
| 150 | 150 ± 7 | 4.9 | 99.8 | 148 ± 8 | 5.6 | 98.6 |
| 200 | 200 ± 5 | 2.4 | 99.8 | 204 ± 5 | 2.6 | 102.1 |
| pQVQ | |||
|---|---|---|---|
| Spiked (µg/mL) | Found (µg/mL) (mean ± s.d.) | Precision (%) | Accuracy (%) |
| 5.0 | 5 ± 1 | 11.3 | 108.2 |
| 10.0 | 10 ± 1 | 6.2 | 103.1 |
| 20 | 20 ± 2 | 7.7 | 97.8 |
| 50 | 47 ± 4 | 9.2 | 94.1 |
| 100 | 96 ± 7 | 7.2 | 96.0 |
| 250 | 247 ± 16 | 6.7 | 98.6 |
| 500 | 510 ± 20 | 3.8 | 102.0 |
| Concentration (µg/mL) | Precision (%) | Accuracy (%) | |||
|---|---|---|---|---|---|
| Peptide | Spiked | Found (mean ± s.d.) | Within-Day | Between-Day | |
| FSGS | 20 | 21 ± 2 | 8.4 | 7.3 | 106 |
| 80 | 86 ± 7 | 5.3 | 6.9 | 107 | |
| 160 | 170 ± 13 | 7.3 | 0.5 | 106 | |
| pQVQ | 20 | 22 ± 3 | 11.3 | 7.8 | 109 |
| 80 | 84 ± 9 | 8.5 | 7.6 | 105 | |
| 160 | 168 ± 15 | 8.7 | 3.1 | 105 | |
| Concentration (µg/mL) | Precision (%) | Accuracy (%) | |||
|---|---|---|---|---|---|
| Peptide | Spiked | Found (mean ± s.d.) | Within-Day | Between-Day | |
| pQVQ | 15 | 16 ± 2 | 8.4 | 5.2 | 104 |
| 75 | 76 ± 7 | 6.4 | 8.3 | 101 | |
| 300 | 291 ± 22 | 5.3 | 5.5 | 97 | |
| Method | Peptide | Ion Suppression | Min; Max |
|---|---|---|---|
| LC-MS/HRMS | FSGS | −67% | −54%; −78% |
| pQVQ | −11% | 15%; −35% | |
| pQIVL | −93% | −88%; −95% | |
| LC-MS/MS | pQVQ | −17% | 0%; −34% |
| pQIVL | −22% | 1%; −44% |
| Temperature | Concentration (µg/mL) | Precision (%) | Accuracy (%) | |
|---|---|---|---|---|
| Spiked | Found (mean ± s.d.) | |||
| +4 °C | 15 | 16 ± 1 | 7.9 | 107.5 |
| 75 | 75 ± 2 | 3.2 | 101.0 | |
| 300 | 297 ± 6 | 2.0 | 98.8 | |
| −20 °C | 15 | 14 ± 2 | 13.2 | 95.8 |
| 75 | 72 ± 3 | 4.3 | 95.3 | |
| 300 | 319 ± 21 | 6.7 | 106.2 | |
| Patient | FSGS | pQVQ | ||||
|---|---|---|---|---|---|---|
| Found (µg/mL) (mean ± s.d.) | Repro. (%) | Diff. (%) | Found (µg/mL) (mean ± s.d.) | Repro. (%) | Diff. (%) | |
| P1 | 100 ± 2 | 1.5 | 3.0 | 102 ± 7 | 6.5 | 9.5 |
| P3 | 242 ± 25 | 10.3 | −14.1 | 232 ± 15 | 6.4 | −11.0 |
| P5 | 244 ± 19 | 7.7 | −12.2 | 229 ± 24 | 10.5 | −6.8 |
| P7 | 305 ± 8 | 2.7 | 4.6 | 286 ± 33 | 11.5 | 8.0 |
| P9 | <2 | - | - | <2 | - | - |
| P13 | 174 ± 13 | 7.7 | −12.2 | 180 ± 7 | 4.1 | −1.1 |
| P15 | 139 ± 2 | 1.2 | −1.8 | 147 ± 14 | 9.3 | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millet, A.; Khoudour, N.; Lebert, D.; Machon, C.; Terrier, B.; Blanchet, B.; Guitton, J. Development, Validation, and Comparison of Two Mass Spectrometry Methods (LC-MS/HRMS and LC-MS/MS) for the Quantification of Rituximab in Human Plasma. Molecules 2021, 26, 1383. https://doi.org/10.3390/molecules26051383
Millet A, Khoudour N, Lebert D, Machon C, Terrier B, Blanchet B, Guitton J. Development, Validation, and Comparison of Two Mass Spectrometry Methods (LC-MS/HRMS and LC-MS/MS) for the Quantification of Rituximab in Human Plasma. Molecules. 2021; 26(5):1383. https://doi.org/10.3390/molecules26051383
Chicago/Turabian StyleMillet, Aurélien, Nihel Khoudour, Dorothée Lebert, Christelle Machon, Benjamin Terrier, Benoit Blanchet, and Jérôme Guitton. 2021. "Development, Validation, and Comparison of Two Mass Spectrometry Methods (LC-MS/HRMS and LC-MS/MS) for the Quantification of Rituximab in Human Plasma" Molecules 26, no. 5: 1383. https://doi.org/10.3390/molecules26051383
APA StyleMillet, A., Khoudour, N., Lebert, D., Machon, C., Terrier, B., Blanchet, B., & Guitton, J. (2021). Development, Validation, and Comparison of Two Mass Spectrometry Methods (LC-MS/HRMS and LC-MS/MS) for the Quantification of Rituximab in Human Plasma. Molecules, 26(5), 1383. https://doi.org/10.3390/molecules26051383







