Physicochemical, Thermal and Rheological Properties of Pectin Extracted from Sugar Beet Pulp Using Subcritical Water Extraction Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of SBP
2.2. Modeling and Optimization of Operational Parameters
2.3. Characterization of Pectin
2.3.1. FTIR Characterization
2.3.2. Chemical Analysis
2.4. Functional Properties of Pectin
2.4.1. Color Attributes
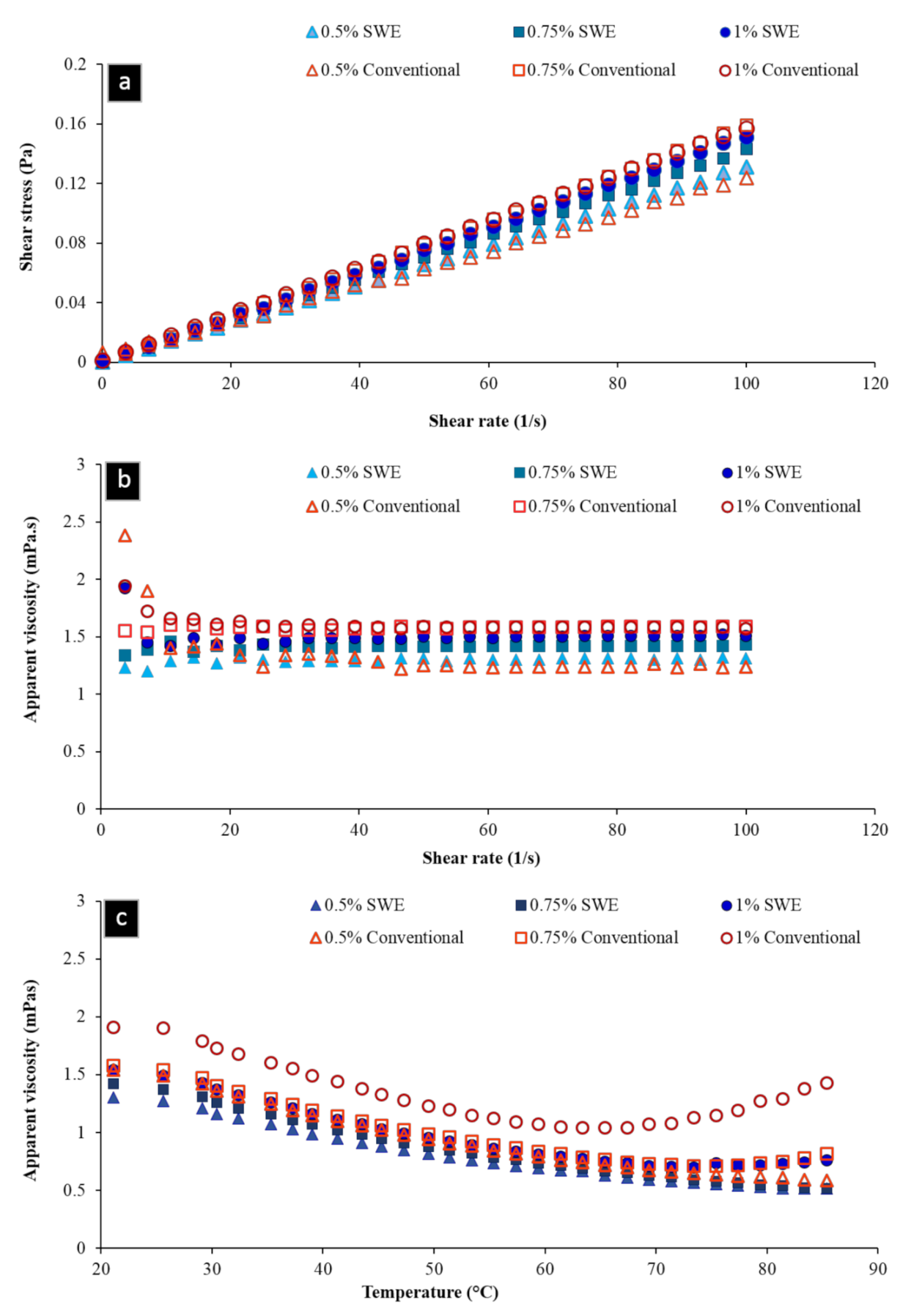
2.4.2. Rheological Measurements
2.4.3. Pasting Properties
2.4.4. Thermal Properties
3. Materials and Methods
3.1. Materials
3.2. Chemical Analysis of SBP
3.3. Preparation and Extraction
3.3.1. Preparation of SBP
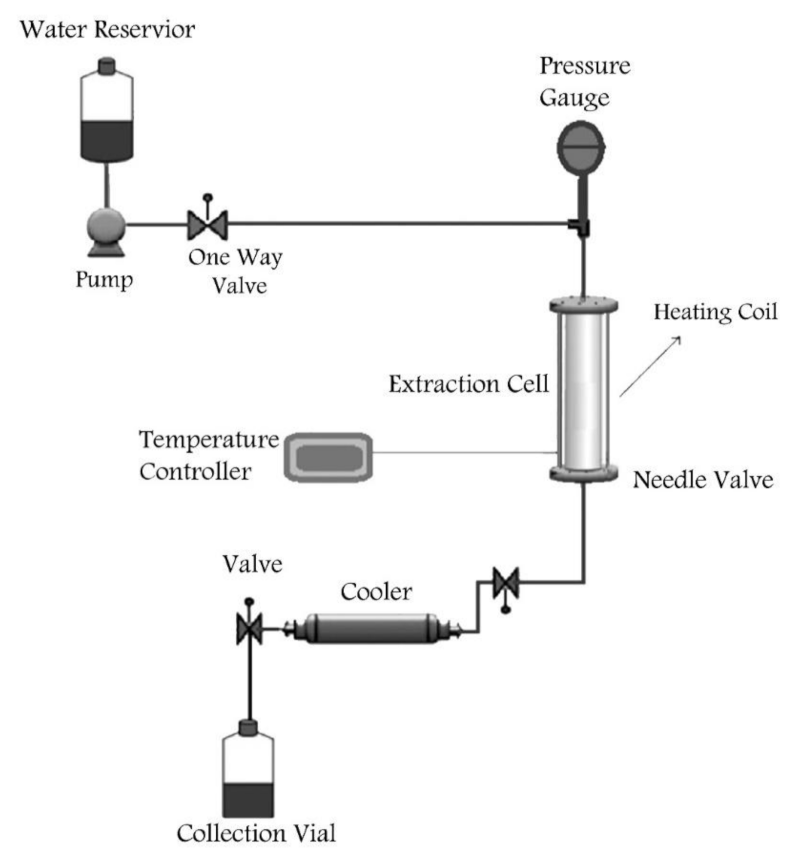
3.3.2. Subcritical Water Extraction of Pectin
3.3.3. Conventional Extraction of Pectin
3.3.4. Experimental Design
3.3.5. Purification of Pectic Substances
3.4. Physico-Chemical Analysis of Pectin
3.4.1. FTIR Characterization of Pectin
3.4.2. Determination of Galacturonic Acid
3.4.3. Degree of Methylation (DM) and Degree of Acetylation (DA)
3.4.4. Determination of Ferulic Acid Content
3.4.5. Molecular Weight (MW)
3.5. Functional Properties of Pectin
3.5.1. Color Measurement
3.5.2. Rheological Measurements
3.5.3. Pasting Property Measurement and Thermal Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Asadi, M. Beet-Sugar Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780471763475. [Google Scholar]
- Kühnel, S.; Schols, H.A.; Gruppen, H. Aiming for the complete utilization of sugar-beet pulp: Examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Wang, Y.; Wang, L.J.; Li, D.; Adhikari, B. Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohydr. Polym. 2013, 95, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Yapo, B.M.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Ravash, N.; Peighambardoust, S.H.; Soltanzadeh, M.; Pateiro, M.; Lorenzo, J.M. Impact of high-pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality aspects and safety of pulsed electric field (PEF) processing on dairy products: A comprehensive review. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martinez-Jimenez, F.; Rezende, C.A.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Subcritical water hydrolysis of sugarcane bagasse: An approach on solid residues characterization. J. Supercrit. Fluids 2016, 108, 69–78. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Ballesteros, I.; Oliva, J.M.; Cabañas, A.; Ballesteros, M. Hydrothermal pretreatment conditions to enhance ethanol production from poplar biomass. In Biotechnology for Fuels and Chemicals; Davison, B.H., Wyman, C.E., Finkelstein, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 87–100. ISBN 978-1-4612-7497-1. [Google Scholar]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of Dried Apricots Pretreated by Ultrasound-Assisted Osmotic Dehydration and Application of Active Coatings. Food Technol. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Fasihnia, S.H.; Khosrowshahi, N.K.; Gullón, B.; Lorenzo, J.M. Optimization of the Amount of ZnO, CuO, and Ag Nanoparticles on Antibacterial Properties of Low-Density Polyethylene (LDPE) Films Using the Response Surface Method. Food Anal. Methods 2020. [Google Scholar] [CrossRef]
- Olmos, J.C.; Hansen, M.E.Z. Enzymatic depolymerization of sugar beet pulp: Production and characterization of pectin and pectic-oligosaccharides as a potential source for functional carbohydrates. Chem. Eng. J. 2012, 192, 29–36. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L. jun Effect of particle size of sugar beet pulp on the extraction and property of pectin. J. Food Eng. 2018, 218, 44–49. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.Q.; Jia, X.; Wei, Z.; Liu, Z.Y. Box-Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydr. Polym. 2012, 88, 342–346. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Wathelet, B.; Paquot, M. New method for a two-step hydrolysis and chromatographic analysis of pectin neutral sugar chains. J. Agric. Food Chem. 2004, 52, 4652–4659. [Google Scholar] [CrossRef]
- Jung, J.; Wicker, L. Laccase mediated conjugation of sugar beet pectin and the effect on emulsion stability. Food Hydrocoll. 2012, 28, 168–173. [Google Scholar] [CrossRef]
- Li, W.; Fan, Z.; Wu, Y.; Jiang, Z.; Shi, R. Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste with subcritical water. J. Sci. Food Agric. 2019, 99, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Brunner, G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocoll. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Liew, S.Q.; Teoh, W.H.; Tan, C.K.; Yusoff, R.; Ngoh, G.C. Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int. J. Biol. Macromol. 2018, 116, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Klinchongkon, K.; Khuwijitjaru, P.; Wiboonsirikul, J.; Adachi, S. Extraction of Oligosaccharides from Passion Fruit Peel by Subcritical Water Treatment. J. Food Process Eng. 2017, 40, e12269. [Google Scholar] [CrossRef]
- Idrovo Encalada, A.M.; Pérez, C.D.; Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N.; Encalada, A.M.I.; Pérez, C.D.; Gerschenson, L.N.; Rojas, A.M.; Fissore, E.N. Gelling pectins from carrot leftovers extracted by industrial-enzymes with ultrasound pretreatment. LWT 2019, 111, 640–646. [Google Scholar] [CrossRef]
- Rombouts, F.M.; Thibault, J.F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and rheological properties of pectins extracted from industrial sugar beet by-products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; Lim, J.; Ko, S.; Lee, K.G.; Lee, S.H.; Lee, S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- Dartois, A.; Singh, J.; Kaur, L.; Singh, H. Influence of Guar Gum on the in Vitro Starch Digestibility—Rheological and Microstructural Characteristics. Food Biophys. 2010, 5, 149–160. [Google Scholar] [CrossRef]
- Gularte, M.A.; Rosell, C.M. Physicochemical properties and enzymatic hydrolysis of different starches in the presence of hydrocolloids. Carbohydr. Polym. 2011, 85, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Kulicke, W.-M.; Eidam, D.; Kath, F.; Kix, M.; Kull, A.H. Hydrocolloids and Rheology: Regulation of Visco-elastic Characteristics of Waxy Rice Starch in Mixtures with Galactomannans. Starch Stärke 1996, 48, 105–114. [Google Scholar] [CrossRef]
- Rosell, C.M.; Yokoyama, W.; Shoemaker, C. Rheology of different hydrocolloids–rice starch blends. Effect of successive heating–cooling cycles. Carbohydr. Polym. 2011, 84, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Abioye, V.F.; Ade-Omowaye, B.I.O.; Babarinde, G.O.; Adesigbin, M.K. Chemical, physico-chemical and sensory properties of soy-plantain flour. Afr. J. Food Sci. 2011, 5, 176–180. [Google Scholar]
- Biliaderis, C.G.; Maurice, T.J.; Vose, J.R. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980, 45, 1669–1674. [Google Scholar] [CrossRef]
- Tester, R.F.; Sommerville, M.D. The effects of non-starch polysaccharides on the extent of gelatinisation, swelling and α-amylase hydrolysis of maize and wheat starches. Food Hydrocoll. 2003, 17, 41–54. [Google Scholar] [CrossRef]
- Krüger, A.; Ferrero, C.; Zaritzky, N.E. Modelling corn starch swelling in batch systems: Effect of sucrose and hydrocolloids. J. Food Eng. 2003, 58, 125–133. [Google Scholar] [CrossRef]
- AACC Approved Methods of the American Association of Cereal Chemist; AACC: Eagan, MN, USA, 2006.
- Jafarzadeh-Moghaddam, M.; Shaddel, R.; Peighambardoust, S.H. Sugar beet pectin extracted by ultrasound or conventional heating: A comparison. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Z.; Fu, X.; Luo, F.; Peng, Z. Effect of enzymatic pretreatment on the synthesis and properties of phosphorylated amphoteric starch. Carbohydr. Polym. 2012, 88, 917–925. [Google Scholar] [CrossRef]
- Aydar, A. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processess; IntechOpen: London, UK, 2018; pp. 157–169. ISBN 978-953-51-3878-5. [Google Scholar]
- Boyacı, İ.H. A new approach for determination of enzyme kinetic constants using response surface methodology. Biochem. Eng. J. 2005, 25, 55–62. [Google Scholar] [CrossRef]
- Garleb, K.A.; Bourquin, L.D.; Fahey, G.C., Jr. Galacturonate in Pectic Substances from Fruits and Vegetables: Comparison of Anion Exchange HPLC with Pulsed Amperometric Detection to Standard Colorimetric Procedure. J. Food Sci. 1991, 56, 423–426. [Google Scholar] [CrossRef]
- Levigne, S.; Thomas, M.; Ralet, M.C.; Quemener, B.; Thibault, J.F. Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocoll. 2002, 16, 547–550. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Schols, H.A.; Pilnik, W. Determination of the degree of methylation and acetylation of pectins by h.p.l.c. Top. Catal. 1986, 1, 65–70. [Google Scholar] [CrossRef]


| Conventional Extraction | SWE | ||||
|---|---|---|---|---|---|
| Term | Coefficient 1 | Yield | Term | Coefficient 1 | Yield |
| Intercept | β0 | 16.80 | Intercept | β0 | 19.37 |
| X1 | β1 | 1.08 ** | X1 | β1 | 1.79 *** |
| X2 | β2 | 0.62 | X2 | β2 | 1.00 *** |
| X3 | β3 | −2.74 *** | X3 | β3 | −0.31 * |
| X12 | β11 | −0.11 | X12 | β11 | −1.43 *** |
| X22 | Β22 | −0.54 | X22 | Β22 | −0.52 ** |
| X32 | Β33 | 0.15 | X32 | Β33 | −0.10 |
| X1 (Temperature) × X2 (Time) | β12 | 0.26 | X1 (Temperature) × X2 (Time) | β12 | −1.78 *** |
| X1 (Temperature) × X3 (pH) | β13 | −0.058 | X1 (Temperature) × X3 (L/S ratio) | β13 | 0.32 |
| X2 (Time) × X3 (pH) | Β23 | −0.79 | X2 (Time) × X3 (L/S ratio) | Β23 | 0.40 * |
| R2 | 0.9158 | R2 | 0.98 | ||
| Adj.R2 | 0.8401 | Adj.R2 | 0.96 | ||
| F | 12.09 | F | 68.93 | ||
| Model | 0.0003 *** | Model | <0.0001 *** | ||
| Lack of fit | p = 0.6 | Lack of fit | p = 0.1 | ||
| Std. Dev | 1.11 | Std. Dev | 0.44 | ||
| CV | 6.76 | CV | 2.44 | ||
| CE Pectin | SWE Pectin | |
|---|---|---|
| Constituents | ||
| GalA (% w/w) | 68.15 ± 1.63 | 73.00 ± 1.98 |
| DM (% mole) | 57.02 ± 1.52 | 84.19 ± 2.07 |
| DA (% mole) | 19.55 ± 1.30 | 25.96 ± 2.04 |
| FA (% w/w) | 0.81 ± 0.05 | 1.87 ± 0.29 |
| MW (kDa *) | 102.27 ± 0.1 | 23.51 ± 0.4 |
| Color Parameters | ||
| L* | 46.0 ± 1.0 | 44.9 ± 1.0 |
| a* | 2.1 ± 1.0 | 2.2 ± 0.3 |
| b* | 21.1 ± 1.0 | 29.4 ± 0.3 |
| ΔE | 42.5 | 46.3 |
| Brabender Viscosity Parameters * | DSC Gelatinization Parameters ** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PT (°C) | PV (BU) | HPV (BU) | BD (BU) | FV(BU) | SB (BU) | TO (°C) | TP (°C) | TC (°C) | TC − TO | ΔH (J/g) | |
| Starch *** | 72.4 ± 0.3 a | 377 ± 1.9 a | 210 ± 3.0 b | 167 ± 0.4 a | 483 ± 2 a | 273 ± 2.0 a | 65.7 ± 0.2 b | 71.3 ± 0.3 b | 75.8 ± 0.4 a | 10.1 | 0.80 ± 0.10 c |
| Starch + CEP (1%) ‡ | 71.6 ± 0.1 b | 342 ± 1.0 c | 210 ± 0.5 b | 132 ± 0.3 b | 437 ± 1 b | 227 ± 2.0 b | 67.8 ± 0.1 a | 72.0 ± 0.5 a | 75.6 ± 0.5 a | 7.8 | 2.20 ± 0.01 a |
| Starch + SWE Pectin 1% | 70.3 ± 0.2 c | 355 ± 2.6 b | 254 ± 2.7 a | 101 ± 0.1 c | 427 ± 4 c | 173 ± 2.5 c | 65.8 ± 0.4 b | 70.4 ± 0.1 c | 74.2 ± 0.5 b | 8.4 | 1.61 ± 0.03 b |
| Run | SWE Pectin | CE Pectin | Response (Yield, %) | |||||
|---|---|---|---|---|---|---|---|---|
| Independent Variable | Response (Yield, %) | Independent Variable | ||||||
| X1 | X2 | X3 | X1 | X2 | X3 | |||
| Temperature (°C) | Time (min) | L/S Ratio (w/w %) | Temperature (°C) | Time (h) | pH | |||
| 1 | 130 (1) | 20 (−1) | 50 (1) | 19.3 | 80 (0) | 3 (0) | 1.25 (0) | 16,93 |
| 2 | 110 (−1) | 40 (1) | 30 (−1) | 18.81 | 70 (−1) | 4 (1) | 1 (−1) | 18.76 |
| 3 | 103 (−1.68) | 30 (0) | 40 (0) | 11.75 | 80 (0) | 3 (0) | 1.25 (0) | 16.19 |
| 4 | 120 (0) | 30 (0) | 57 (1.68) | 18.83 | 90 (1) | 4 (1) | 1 (−1) | 20.75 |
| 5 | 130 (1) | 40 (1) | 30 (−1) | 17.7 | 80 (0) | 3 (0) | 1.25 (0) | 15.18 |
| 6 | 120 (0) | 30 (0) | 23 (−1.68) | 19.2 | 97 (1.68) | 3 (0) | 1.25 (0) | 19 |
| 7 | 120 (0) | 30 (0) | 40 (0) | 19.18 | 90 (1) | 2 (−1) | 1 (−1) | 18.21 |
| 8 | 110 (−1) | 20 (−1) | 30 (−1) | 14.2 | 70 (−1) | 2 (−1) | 1.5 (1) | 13.66 |
| 9 | 120 (0) | 30 (0) | 40 (0) | 19.18 | 80 (0) | 3 (0) | 1.25 (0) | 18.32 |
| 10 | 120 (0) | 13 (−1.68) | 40 (0) | 15.75 | 70 (−1) | 2 (−1) | 1 (−1) | 17 |
| 11 | 120 (0) | 30 (0) | 40 (0) | 19.75 | 63 (−1.68) | 3 (0) | 1.25 (0) | 14.26 |
| 12 | 110 (−1) | 40 (1) | 50 (1) | 17.9 | 90 (1) | 4 (1) | 1.5 (1) | 14.48 |
| 13 | 120 (0) | 30 (0) | 40 (0) | 19.75 | 80 (0) | 1.3 (−1.68) | 1.25 (0) | 13.52 |
| 14 | 120 (0) | 47 (1.68) | 40 (0) | 19.9 | 70 (−1) | 4 (1) | 1.5 (1) | 12 |
| 15 | 110 (−1) | 20 (−1) | 50 (1) | 12.04 | 90 (1) | 2 (−1) | 1.5 (1) | 14.84 |
| 16 | 120 (0) | 30 (0) | 40 (0) | 19.18 | 80 (0) | 4.7 (1.68) | 1.25 (0) | 17.24 |
| 17 | 120 (0) | 30 (0) | 40 (0) | 19.2 | 80 (0) | 3 (0) | 1.7 (1.8) | 11.9 |
| 18 | 130 (1) | 20 (−1) | 30 (−1) | 20.55 | 80 (0) | 3 (0) | 0.85 (−1.6) | 22.46 |
| 19 | 137 (1.68) | 30 (0) | 40 (0) | 18.61 | 80 (0) | 3 (0) | 1.25 (0) | 16 |
| 20 | 130 (1) | 40 (1) | 50 (1) | 18.4 | 80 (0) | 3 (0) | 1.25 (0) | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peighambardoust, S.H.; Jafarzadeh-Moghaddam, M.; Pateiro, M.; Lorenzo, J.M.; Domínguez, R. Physicochemical, Thermal and Rheological Properties of Pectin Extracted from Sugar Beet Pulp Using Subcritical Water Extraction Process. Molecules 2021, 26, 1413. https://doi.org/10.3390/molecules26051413
Peighambardoust SH, Jafarzadeh-Moghaddam M, Pateiro M, Lorenzo JM, Domínguez R. Physicochemical, Thermal and Rheological Properties of Pectin Extracted from Sugar Beet Pulp Using Subcritical Water Extraction Process. Molecules. 2021; 26(5):1413. https://doi.org/10.3390/molecules26051413
Chicago/Turabian StylePeighambardoust, Seyed Hadi, Maryam Jafarzadeh-Moghaddam, Mirian Pateiro, José M. Lorenzo, and Rubén Domínguez. 2021. "Physicochemical, Thermal and Rheological Properties of Pectin Extracted from Sugar Beet Pulp Using Subcritical Water Extraction Process" Molecules 26, no. 5: 1413. https://doi.org/10.3390/molecules26051413








