Surface Characterization, Biocompatibility and Antifungal Efficacy of a Denture-Lining Material Containing Cnidium officinale Extracts
Abstract
1. Introduction
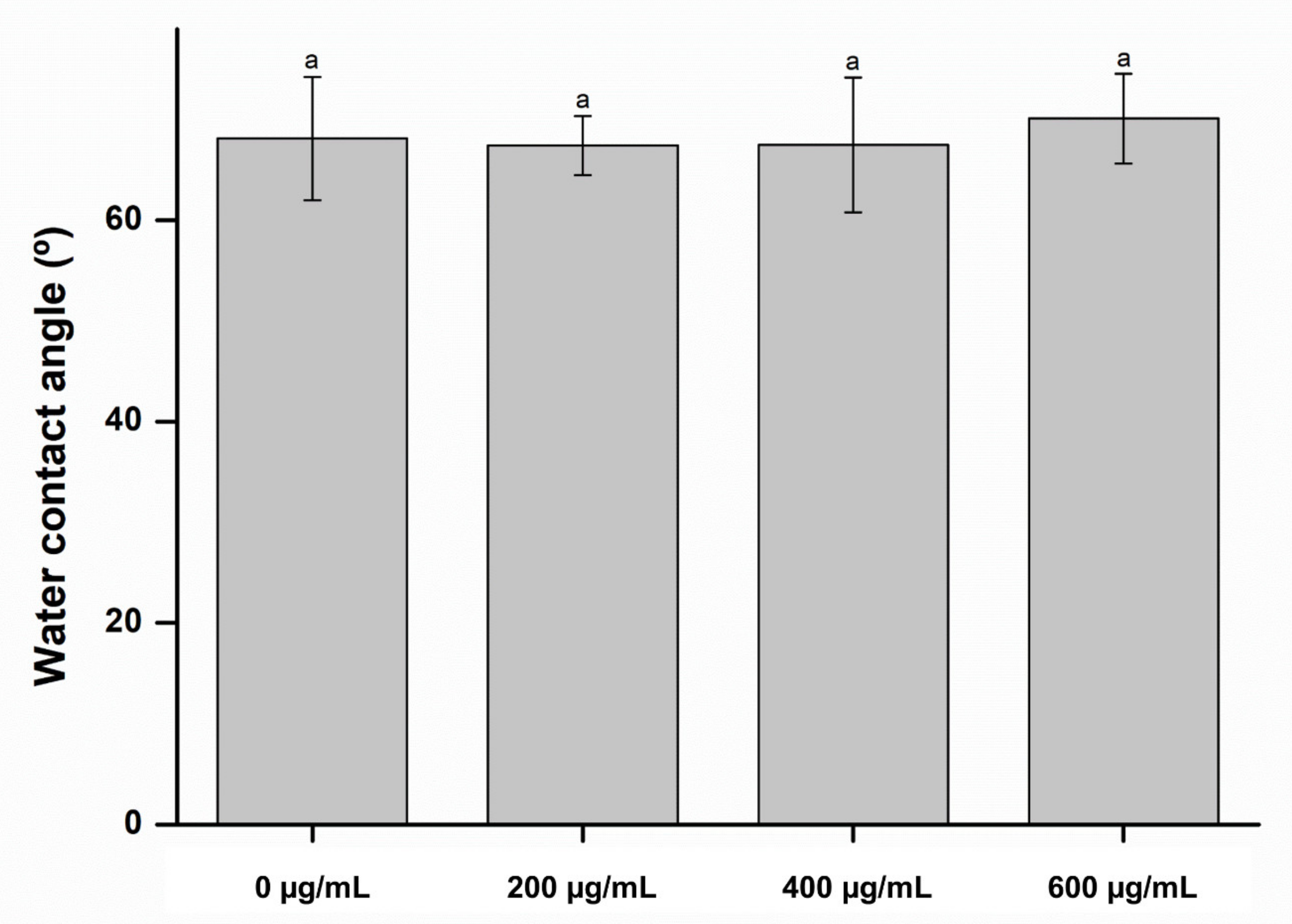
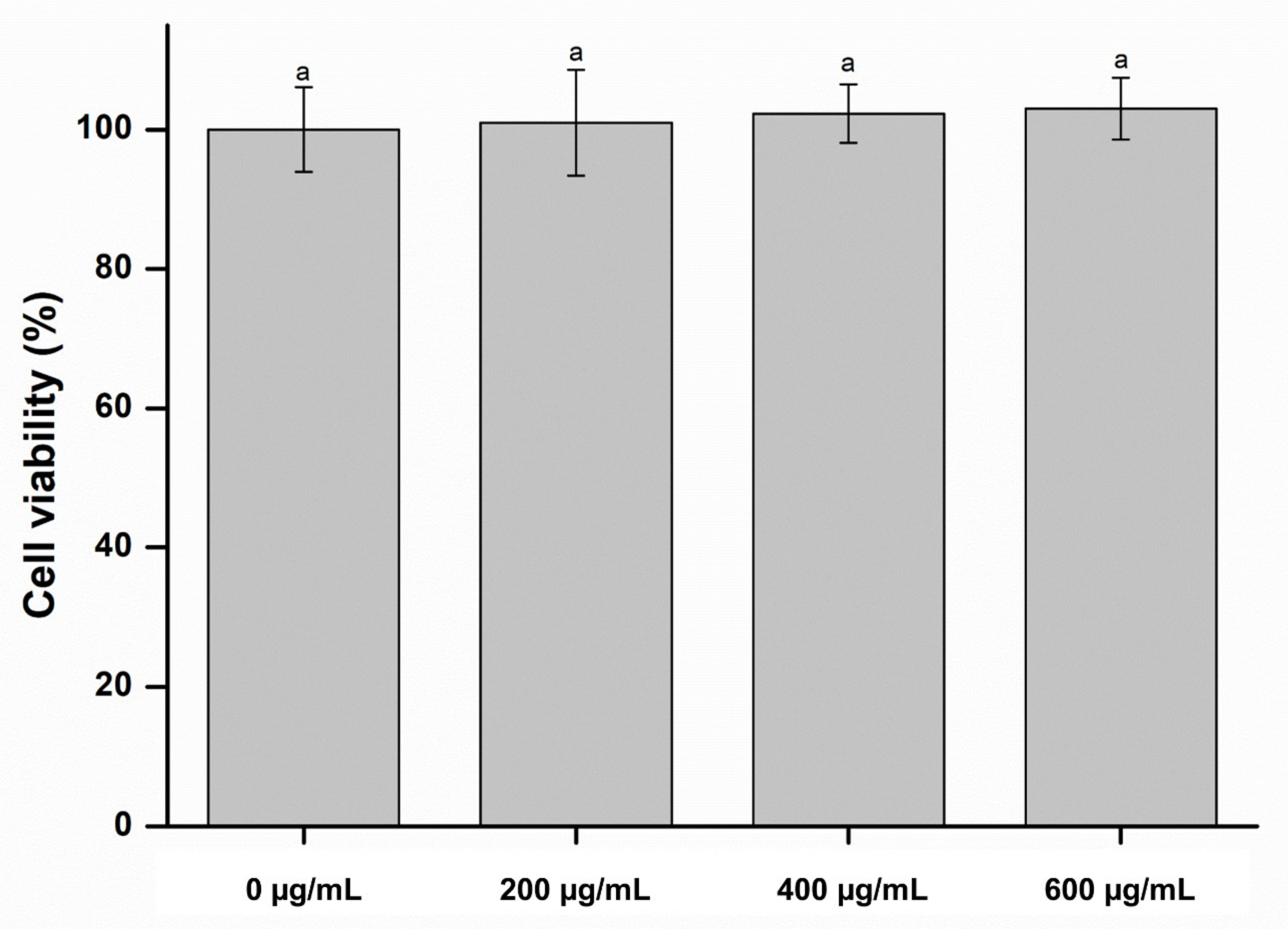
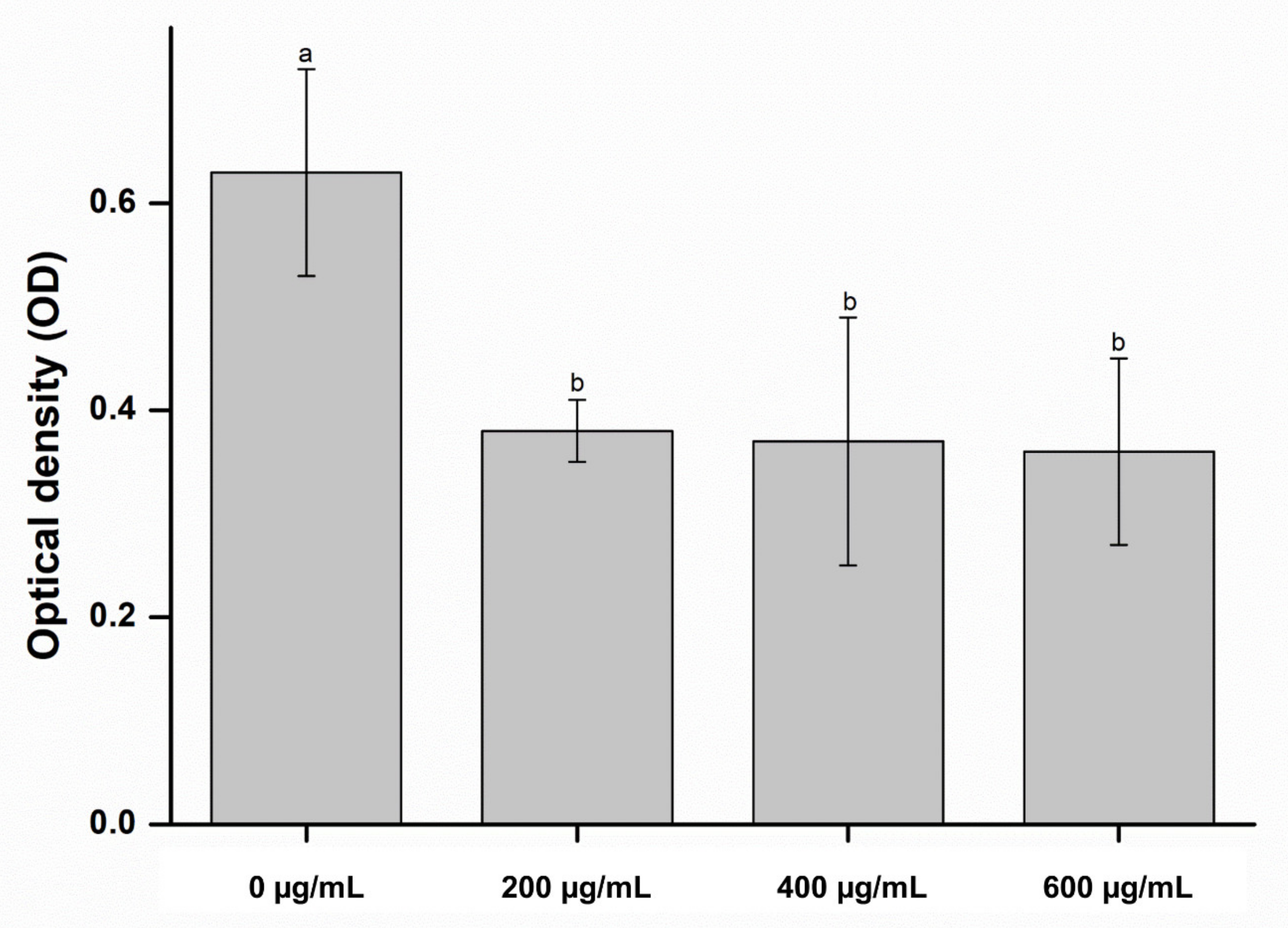
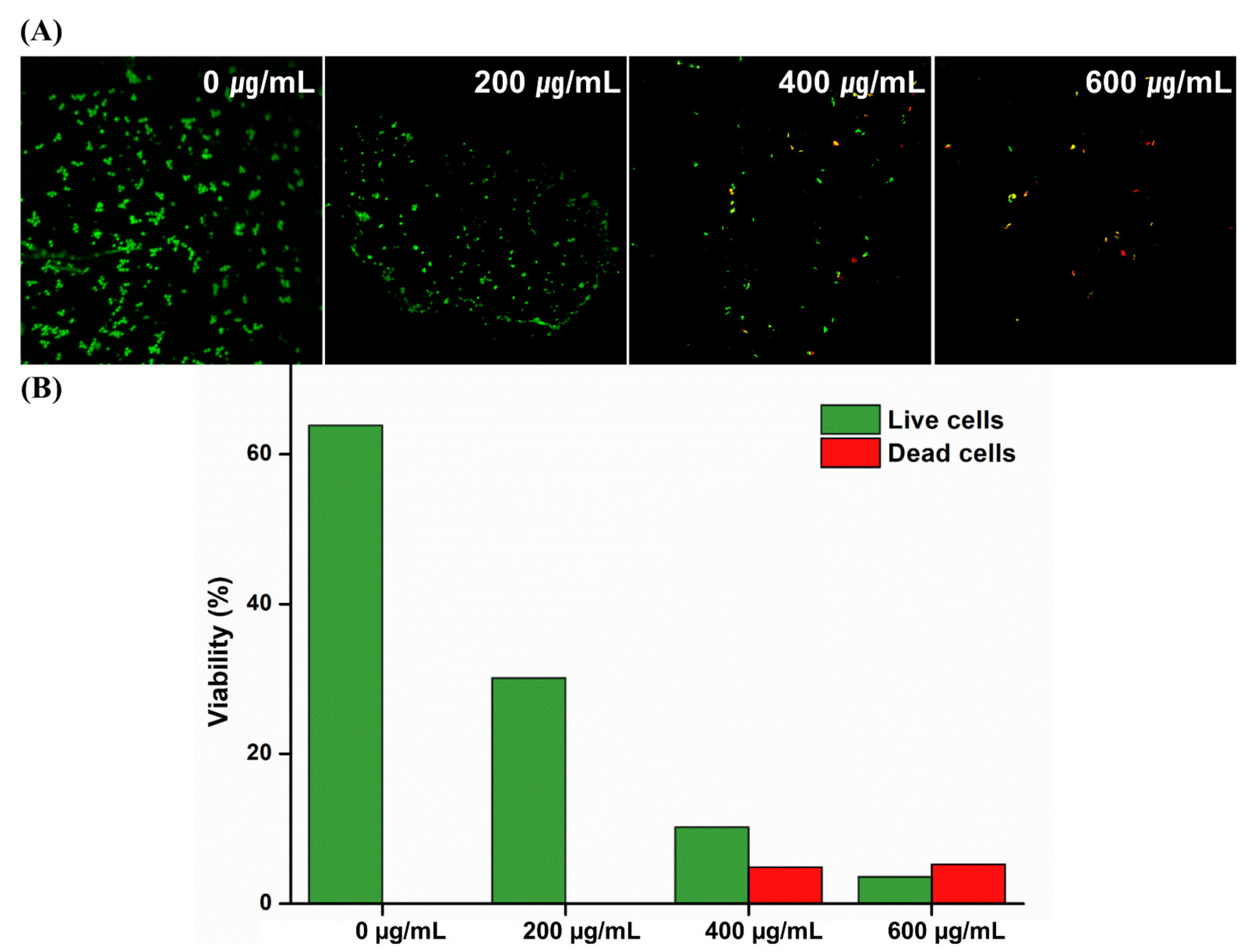
2. Results and Discussion
3. Materials and Methods
3.1. Extraction
3.2. Preparation of the Denture-lining Material Containing Cnidium Officinale
3.3. Measurement of the Color Change
3.4. Measurement of the Wettability
3.5. Evaluation of Biological Properties
3.6. Preparation of C. Albicans Strains
3.7. Evaluation of Optical Density (OD)
3.8. Evaluation of Microbial Viability
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, S.A.; Ghotaslou, R.; Akbarzadeh, A.; Azima, N.; Aeinfar, A.; Khorramdel, A. Evaluation of antibacterial and antifungal properties of a tissue conditioner used in complete dentures after incorporation of ZnO‒Ag nanoparticles. J. Dent. Res. Dent. Clin. Dent. Prospects. 2019, 13, 11–18. [Google Scholar] [CrossRef]
- Naito, Y.; Yumoto, H.; Kumar Hs, K.; Matsuo, T.; Hirota, K.; Miyake, Y.; Nagao, K.; Tomotake, Y.; Jimbo, R.; Ichikawa, T. Antifungal and Mechanical Properties of Tissue Conditioner Containing Plant-Derived Component: An In Vitro Study. J. Prosthodont. 2018, 27, 665–669. [Google Scholar] [CrossRef]
- Kim, H.J.; Son, J.S.; Kwon, T.Y. Antifungal Effect of a Dental Tissue Conditioner Containing Nystatin-Loaded Alginate Microparticles. J. Nanosci. Nanotechnol. 2018, 18, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Landayan, J.I.; Manaloto, A.C.; Lee, J.Y.; Shin, S.W. Effect of aging on tear strength and cytotoxicity of soft denture lining materials; in vitro. J. Adv. Prosthodont. 2014, 6, 115–120. [Google Scholar] [CrossRef]
- De Foggi, C.C.; Ayres, M.S.B.; Feltrin, G.P.; Jorge, J.H.; Machado, A.L. Effect of surface characteristics of soft liners and tissue conditioners and saliva on the adhesion and biofilm formation. Am. J. Dent. 2018, 31, 45–52. [Google Scholar]
- Chaves, C.A.; Vergani, C.E.; Thomas, D.; Young, A.; Costa, C.A.; Salih, V.M.; Machado, A.L. Biological effects of soft denture reline materials on L929 cells in vitro. J. Tissue Eng. 2014, 5, 2041731414540911. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, M.J.; Oh, S.H.; Kwon, J.S. Novel Dental Poly (Methyl Methacrylate) Containing Phytoncide for Antifungal Effect and Inhibition of Oral Multispecies Biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, Y.K.; Lim, B.S.; Kim, C.W. Changes in properties of short-term-use soft liners after thermocycling. J. Oral Rehabil. 2004, 31, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.G.; Sugio, C.Y.C.; de Campos Chaves, G.; Procópio, A.L.F.; Urban, V.M.; Neppelenbroek, K.H. Determining acceptable limits for water sorption and solubility of interim denture resilient liners. J. Prosthet. Dent. 2019, 121, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; El-Fiqi, A.; Jo, J.K.; Kim, D.A.; Kim, S.C.; Jun, S.K.; Kim, H.W.; Lee, H.H. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent. Mater. 2016, 32, 1564–1574. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, Y.R.; Lee, M.J.; Kang, M.K. Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kang, M.K. Analysis of the Antimicrobial, Cytotoxic, and Antioxidant Activities of Cnidium officinale Extracts. Plants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Youn, U.J.; Kim, S.; Woo, M.H.; Min, B.S. Anti-inflammatory activity of compounds from the rhizome of Cnidium officinale. Arch. Pharm. Res. 2018, 41, 977–985. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, Y.R.; Kim, C.S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Cnidium officinale extract and butylidenephthalide inhibits retinal neovascularization in vitro and in vivo. BMC Complement Altern. Med. 2016, 16, 231. [Google Scholar] [CrossRef]
- Bae, K.E.; Choi, Y.W.; Kim, S.T.; Kim, Y.K. Components of rhizome extract of Cnidium officinale Makino and their in vitro biological effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Fouda, S.M.; Näpänkangas, R.; Raustia, A. Flexural and Surface Properties of PMMA Denture Base Material Modified with Thymoquinone as an Antifungal Agent. J. Prosthodont. 2020, 29, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, M.J.; Oh, S.H.; Kim, K.M. Changes in the physical properties and color stability of aesthetic restorative materials caused by various beverages. Dent. Mater. J. 2019, 38, 33–40. [Google Scholar] [CrossRef]
- De Andrade Lima Chaves, C.; de Souza Costa, C.A.; Vergani, C.E.; Chaves de Souza, P.P.; Machado, A.L. Effects of soft denture liners on L929 fibroblasts, HaCaT keratinocytes, and RAW 264.7 macrophages. Biomed. Res. Int. 2014, 2014, 840613. [Google Scholar]
- Lee, M.J.; Kim, M.J.; Kwon, J.S.; Lee, S.B.; Kim, K.M. Cytotoxicity of Light-Cured Dental Materials according to Different Sample Preparation Methods. Materials 2017, 10, 288. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef]
- Kwon, J.S.; Lee, M.J.; Kim, J.Y.; Kim, D.; Ryu, J.H.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Novel anti-biofouling light-curable fluoride varnish containing 2-methacryloyloxyethyl phosphorylcholine to prevent enamel demineralization. Sci. Rep. 2019, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kwon, J.S.; Jiang, H.B.; Choi, E.H.; Park, G.; Kim, K.M. The antibacterial effect of non-thermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Sci. Rep. 2019, 9, 1938. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Ren, X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B 2019, 196, 111509. [Google Scholar] [CrossRef]
- Lee, M.J.; Mangal, U.; Kim, S.J.; Yoon, Y.P.; Ahn, E.S.; Jang, E.S.; Kwon, J.S.; Choi, S.H. Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation. Polymers 2020, 12, 1716. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Shim, Y.-S.; An, S.-Y.; Kang, M.-K. Surface Characterization, Biocompatibility and Antifungal Efficacy of a Denture-Lining Material Containing Cnidium officinale Extracts. Molecules 2021, 26, 1440. https://doi.org/10.3390/molecules26051440
Lee M-J, Shim Y-S, An S-Y, Kang M-K. Surface Characterization, Biocompatibility and Antifungal Efficacy of a Denture-Lining Material Containing Cnidium officinale Extracts. Molecules. 2021; 26(5):1440. https://doi.org/10.3390/molecules26051440
Chicago/Turabian StyleLee, Myung-Jin, Youn-Soo Shim, So-Youn An, and Min-Kyung Kang. 2021. "Surface Characterization, Biocompatibility and Antifungal Efficacy of a Denture-Lining Material Containing Cnidium officinale Extracts" Molecules 26, no. 5: 1440. https://doi.org/10.3390/molecules26051440
APA StyleLee, M.-J., Shim, Y.-S., An, S.-Y., & Kang, M.-K. (2021). Surface Characterization, Biocompatibility and Antifungal Efficacy of a Denture-Lining Material Containing Cnidium officinale Extracts. Molecules, 26(5), 1440. https://doi.org/10.3390/molecules26051440






