Metal-Organic Frameworks as Versatile Heterogeneous Solid Catalysts for Henry Reactions
Abstract
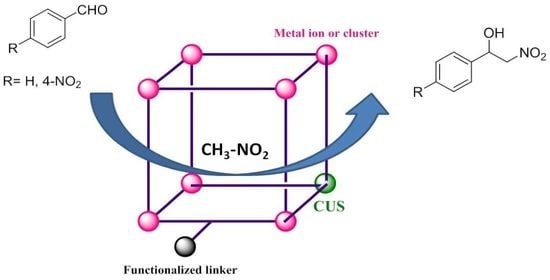
:1. Introduction
2. Metal Nodes as Active Sites
2.1. Co Metal Nodes as Active Sites
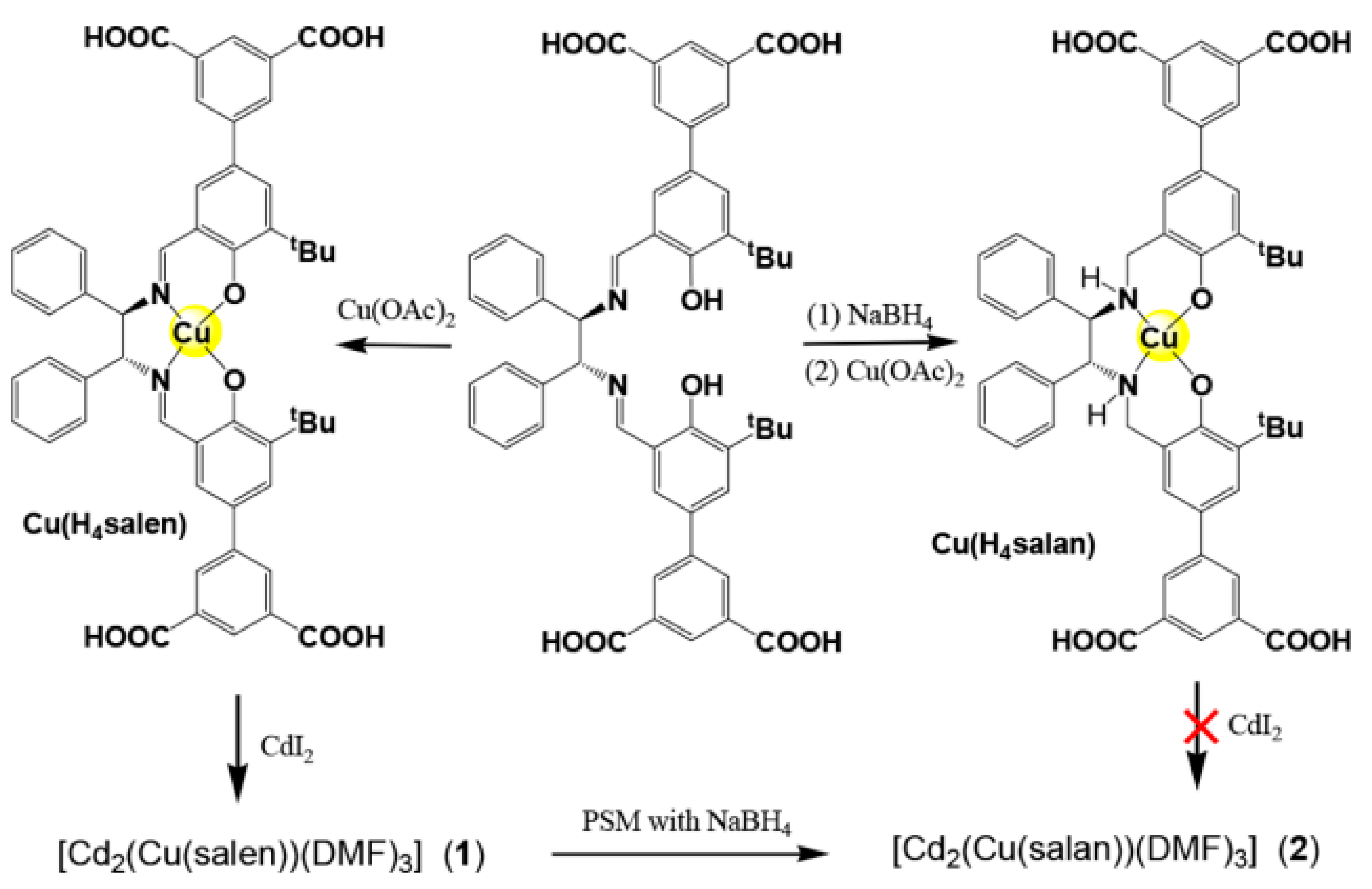
2.2. Cd Metal Nodes as Active Sites
2.3. Zn Metal Nodes as Active Sites
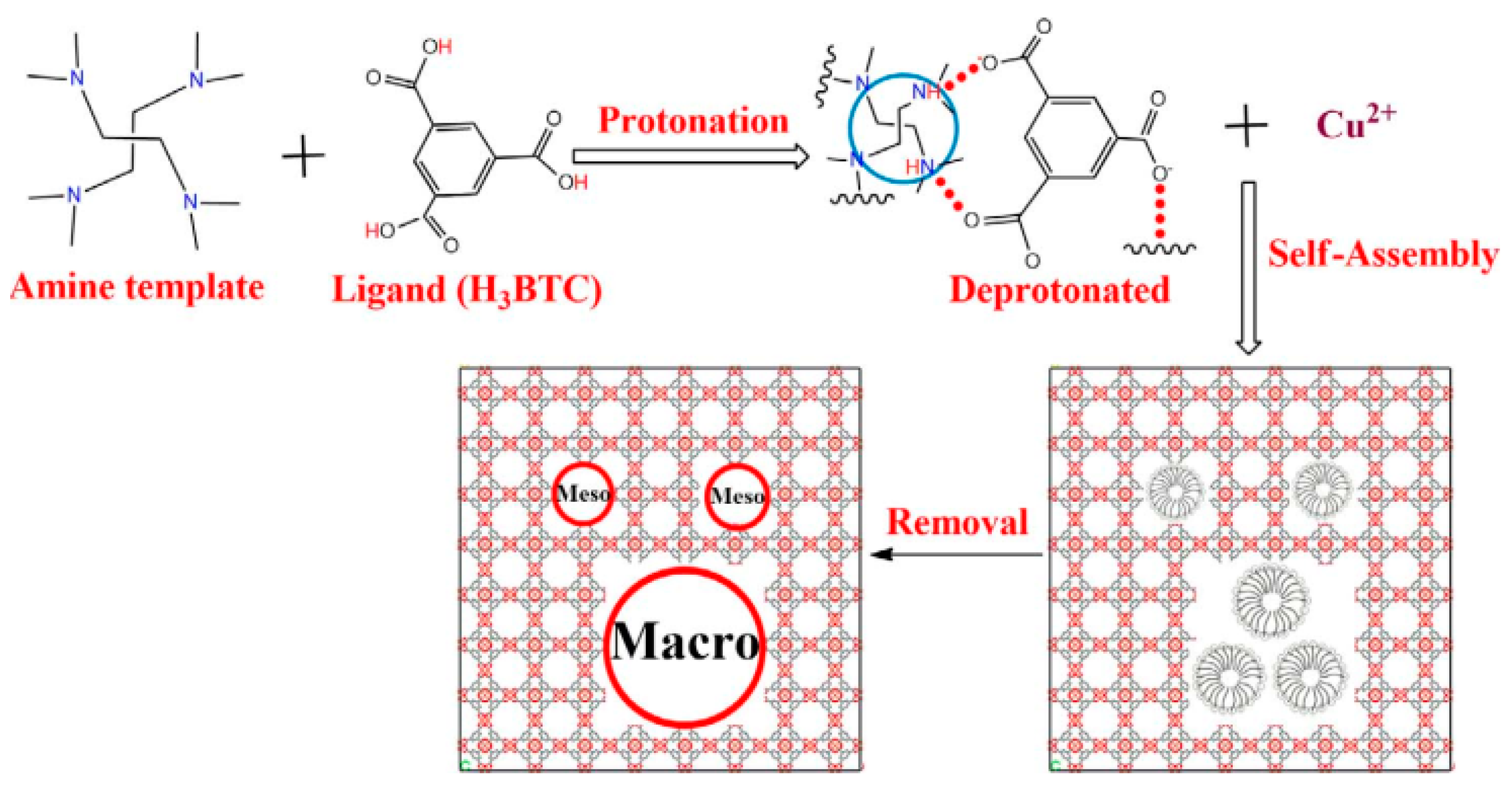
2.4. Cu Metal Nodes as Active Sites
2.5. Sm Metal Nodes as Active Sites
3. MOFs as Organocatalysts for the Henry Reaction
3.1. MOFs with Primary Amine Sites
3.2. MOFs with Secondary Amine Sites
3.3. MOFs with Amide Sites
3.4. MOFs with Urea Sites
4. Conclusions and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Henry, L. Formation synthétique d’alcools nitrés. Bull. Soc. Chim. Fr 1895, 120, 1265–1268. [Google Scholar]
- Cirujano, F.G.; Martin, N.; Wee, L.H. Design of hierarchical architectures in Metal-Organic-Frameworks for catalysis and adsorption. Chem. Mater. 2020, 32, 10268–10295. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Metal-organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles. Chem. Soc. Rev. 2014, 43, 5750–5765. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Mixed-metal or mixed-linker metal organic frameworks as heterogeneous catalysts. Catal. Sci. Technol. 2016, 6, 5238–5261. [Google Scholar] [CrossRef]
- Martin, N.; Cirujano, F.G. Synthesis of high added value molecules with MOF catalysts. Org. Biomol. Chem. 2020, 18, 8058–8073. [Google Scholar] [CrossRef]
- Cirujano, F.G. Engineered MOFs and Enzymes for the Synthesis of Active Pharmaceutical Ingredients. ChemCatChem 2019, 11, 5671–5685. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Opanasenko, M.; Čejka, J.; Garcia, H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 2013, 3, 2509–2540. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Yang, C.X.; Chang, N.; Yan, X.P. Metal-organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal-organic frameworks as heterogeneous catalysts for oxidation reactions. Catal. Sci. Technol. 2011, 1, 856–867. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-Organic Frameworks as Catalysts for Oxidation Reactions. Chem. Eur. J. 2016, 22, 8012–8024. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Herance, R.; Garcia, H. Metal organic frameworks as solid promoters for aerobic autoxidations. Catal. Today 2018, 306, 2–8. [Google Scholar] [CrossRef]
- i Xamena, F.X.; Abad, A.; Corma, A.; Garcia, H. MOFs as catalysts: Activity, reusability and shape-selectivity of a Pd-containing MOF. J. Catal. 2007, 250, 294–298. [Google Scholar]
- Dhakshinamoorthy, A.; Opanasenko, M.; Cejka, J.; Garcia, H. Metal Organic Frameworks as Solid Catalysts in Condensation Reactions of Carbonyl Groups. Adv. Synth. Catal. 2013, 355, 247–268. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-organic frameworks catalyzed C-C and C-Heteroatom coupling reactions. Chem. Soc. Rev. 2015, 44, 1922–1947. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Formation of C-C and C-Heteroatom Bonds by C-H Activation by Metal Organic Frameworks as Catalysts or Supports. ACS Catal. 2019, 9, 1081–1102. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.; Jin, Y.; Fu, Y.; Zhong, Y.; Zhu, W. Facile synthesis of MIL-100 (Fe) under HF-free conditions and its application in the acetalization of aldehydes with diols. Chem. Eng. J. 2015, 259, 183–190. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal organic frameworks as solid acid catalysts for acetalization of aldehydes with methanol. Adv. Synth. Catal. 2010, 352, 3022–3030. [Google Scholar] [CrossRef]
- Phan, N.T.; Le, K.K.; Phan, T.D. MOF-5 as an efficient heterogeneous catalyst for Friedel-Crafts alkylation reactions. Appl. Catal. A Gen. 2010, 382, 246–253. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.N.; Jang, H.G.; Seo, G.; Ahn, W.S. CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal. A Gen. 2013, 453, 175–180. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Alvaro, M.; Garcia, H. Metal organic frameworks as catalysts in solvent-free or ionic liquid assisted conditions. Green Chem. 2018, 20, 86–107. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, C.; Lin, W. Metal-organic frameworks for light harvesting and photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Aryanejad, S.; Bagherzade, G.; Moudi, M. Design and development of novel Co-MOF nanostructures as an excellent catalyst for alcohol oxidation and Henry reaction, with a potential antibacterial activity. Appl. Organomet. Chem. 2019, 33, e4820. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, Y.; Li, J.; Yue, C.; Jiang, H. Enhanced Activity and Enantioselectivity of Henry Reaction by the Postsynthetic Reduction Modification for a Chiral Cu (salen)-Based Metal-Organic Framework. Inorg. Chem. 2018, 57, 11986–11994. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, H.; Fang, Y.; Xiao, Z.; Wang, K.; Wu, X.; Niu, H.; Zhu, C.; Zhou, H.C. Bottom-Up Assembly of a Highly Efficient Metal−Organic Framework for Cooperative Catalysis. Inorg. Chem. 2018, 57, 13912–13919. [Google Scholar] [CrossRef]
- Ugale, B.; Dhankhar, S.S.; Nagaraja, C.M. Construction of 3D homochiral metal-organic frameworks (MOFs) of Cd (II): Selective CO2 adsorption and catalytic properties for Knoevenagel and Henry reaction. Inorg. Chem. Front. 2017, 4, 348–359. [Google Scholar] [CrossRef]
- Lin, X.M.; Li, T.T.; Wang, Y.W.; Zhang, L.; Su, C.Y. Two ZnII Metal-Organic Frameworks with Coordinatively Unsaturated Metal Sites: Structures, Adsorption, and Catalysis. Chem. Asian J. 2012, 7, 2796–2804. [Google Scholar] [CrossRef]
- Joharian, M.; Morsali, A. Ultrasound-assisted synthesis of two new fluorinated metal-organic frameworks (F-MOFs) with the high surface area to improve the catalytic activity. J. Solid State Chem. 2019, 270, 135–146. [Google Scholar] [CrossRef]
- Shi, L.X.; Wu, C.D. A nanoporous metal-organic framework with accessible Cu2+ sites for the catalytic Henry reaction. Chem. Commun. 2011, 47, 2928–2930. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Gavrilenko, K.S.; Lytvynenko, A.S.; Kolotilov, S.V. Catalytic activity of copper (II) benzenetricarboxylate (HKUST-1) in reactions of aromatic aldehydes condensation with nitromethane: Kinetic and diffusion study. Inorg. Chim. Acta 2015, 426, 119–125. [Google Scholar] [CrossRef]
- Gupta, A.K.; De, D.; Bharadwaj, P.K. A NbO type Cu (II) metal-organic framework showing efficient catalytic activity in Friedländer and Henry reactions. Dalton Trans. 2017, 46, 7782–7790. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, F.; Luo, S.; Xiao, J.; Li, L.; Xi, H. Facile synthesis of hierarchical porous metal-organic frameworks with enhanced catalytic activity. Chem. Eng. J. 2018, 334, 1477–1483. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, J.; Li, J.; Li, F.; Duan, C.; Xi, H. Hierarchically porous metal-organic frameworks with single-crystal structures and their enhanced catalytic properties. CrystEngComm 2018, 20, 5754–5759. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Chernyshev, V.V.; Fomkin, A.A.; Shkolin, A.V.; Veselovsky, V.V.; Kapustin, G.I.; Sokolova, N.A.; Kustov, L.M. Preparation of novel hybrid catalyst with an hierarchical micro-/mesoporous structure by direct growth of the HKUST-1 nanoparticles inside mesoporous silica matrix (MMS). Microporous Mesoporous Mater. 2020, 300, 110136. [Google Scholar] [CrossRef]
- Arai, T.; Kawasaki, N.; Kanoh, H. Magnetically Separable Cu-Carboxylate MOF Catalyst for the Henry Reaction. Synlett 2012, 23, 1549–1553. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; da Silva, M.F.; Paul, A.; Pombeiro, A.J. Nanoporous lanthanide metal-organic frameworks as efficient heterogeneous catalysts for the Henry reaction. CrystEngComm 2016, 18, 1337–1349. [Google Scholar] [CrossRef]
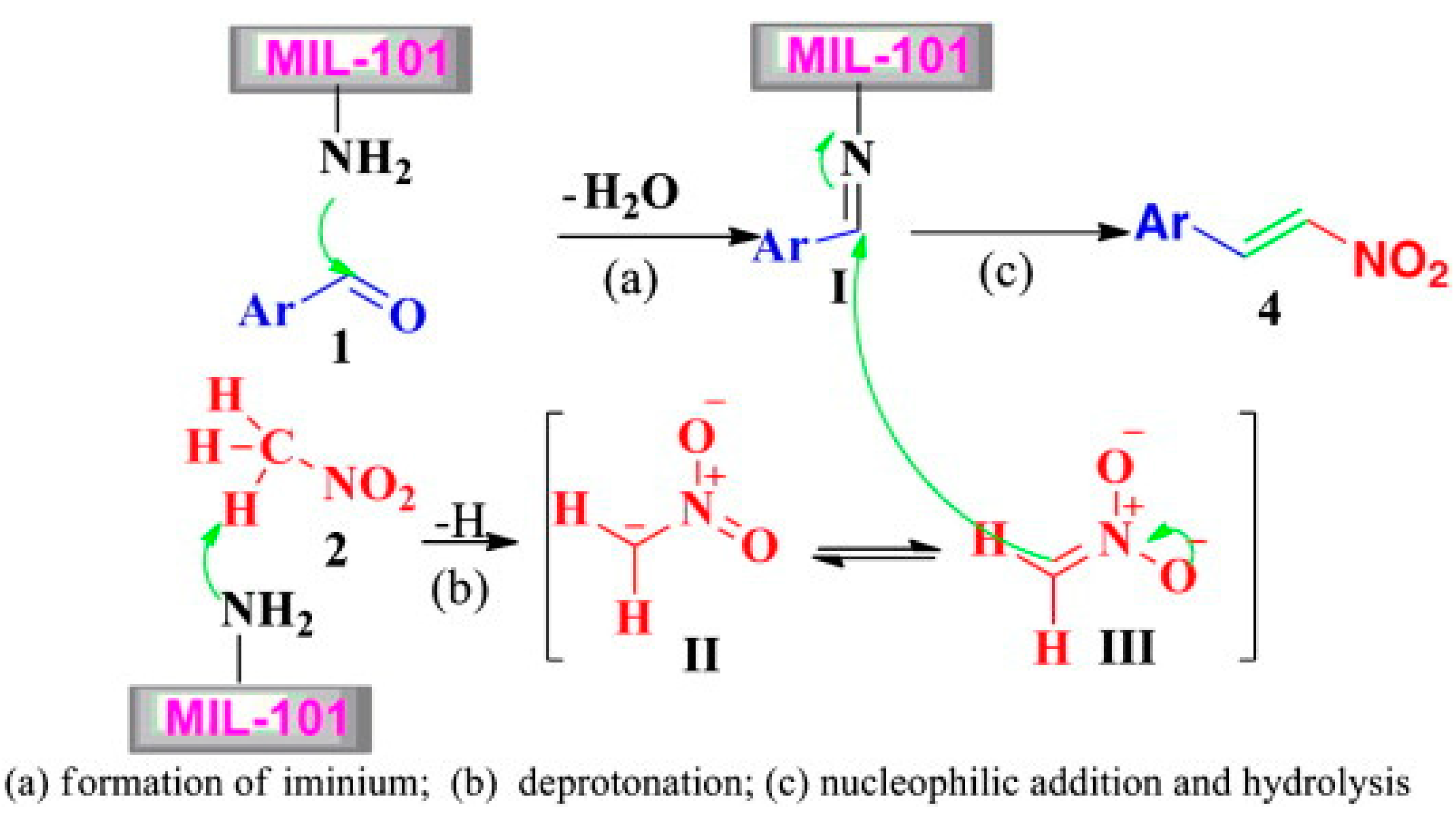
- Yu, H.; Xie, J.; Zhong, Y.; Zhang, F.; Zhu, W. One-pot synthesis of nitroalkenes via the Henry reaction over amino-functionalized MIL-101 catalysts. Catal. Commun. 2012, 29, 101–104. [Google Scholar] [CrossRef]
- Li, X.; Mao, Y.; Leng, K.; Ye, G.; Sun, Y.; Xu, W. Synthesis of amino-functionalized MIL-101 (Cr) with large surface area. Mater. Lett. 2017, 197, 192–195. [Google Scholar] [CrossRef]
- Cirujano, F.G.; López-Maya, E.; Rodríguez-Albelo, M.; Barea, E.; Navarro, J.A.; De Vos, D.E. Selective One-Pot Two-Step C-C Bond Formation using Metal-Organic Frameworks with Mild Basicity as Heterogeneous Catalysts. ChemCatChem 2017, 9, 4019–4023. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Tao, L.; Zhu, H.; Hu, G. Synthesis, characterization and heterogeneous base catalysis of amino functionalized lanthanide metal-organic frameworks. J. Mol. Struct. 2017, 1146, 853–860. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Martín, N.; Fu, G.; Jia, C.; Vos, D.E. Cooperative acid-base bifunctional ordered porous solids in sequential multi-step reactions: MOF vs. mesoporous silica. Catal. Sci. Technol. 2020, 10, 1796–1802. [Google Scholar] [CrossRef]
- Luan, Y.; Qi, Y.; Gao, H.; Andriamitantsoa, R.S.; Zheng, N.; Wang, G. A general post-synthetic modification approach of amino-tagged metal-organic frameworks to access efficient catalysts for the Knoevenagel condensation reaction. J. Mater. Chem. A 2015, 3, 17320–17331. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.; Hazra, S.; Guedes da Silva, M.F.; Pombeiro, A.J. Metal-Organic Frameworks with Pyridyl-Based Isophthalic Acid and Their Catalytic Applications in Microwave Assisted Peroxidative Oxidation of Alcohols and Henry Reaction. Cryst. Growth Des. 2016, 16, 1837–1849. [Google Scholar] [CrossRef]
- Gu, J.M.; Kim, W.S.; Huh, S. Size-dependent catalysis by DABCO-functionalized Zn-MOF with one-dimensional channels. Dalton Trans. 2011, 40, 10826–10829. [Google Scholar] [CrossRef]
- Müller, P.; Bon, V.; Senkovska, I.; Nguyen, K.D.; Kaskel, S. A bifunctional metal-organic framework platform for catalytic applications. Polyhedron 2019, 159, 382–386. [Google Scholar] [CrossRef]
- Karmakar, A.; da Silva, M.F.; Pombeiro, A.J. Zinc metal-organic frameworks: Efficient catalysts for the diastereoselective Henry reaction and transesterification. Dalton Trans. 2014, 43, 7795–7810. [Google Scholar] [CrossRef]
- Paul, A.; Karmakar, A.; da Silva, M.F.; Pombeiro, A.J. Amide functionalized metal-organic frameworks for diastereoselective nitroaldol (Henry) reaction in aqueous medium. RSC Adv. 2015, 5, 87400–87410. [Google Scholar] [CrossRef]
- Paul, A.; Martins, L.M.; Karmakar, A.; Kuznetsov, M.L.; Novikov, A.S.; da Silva, M.F.; Pombeiro, A.J. Environmentally benign benzyl alcohol oxidation and C-C coupling catalysed by amide functionalized 3D Co (II) and Zn (II) metal organic frameworks. J. Catal. 2020, 385, 324–337. [Google Scholar] [CrossRef]
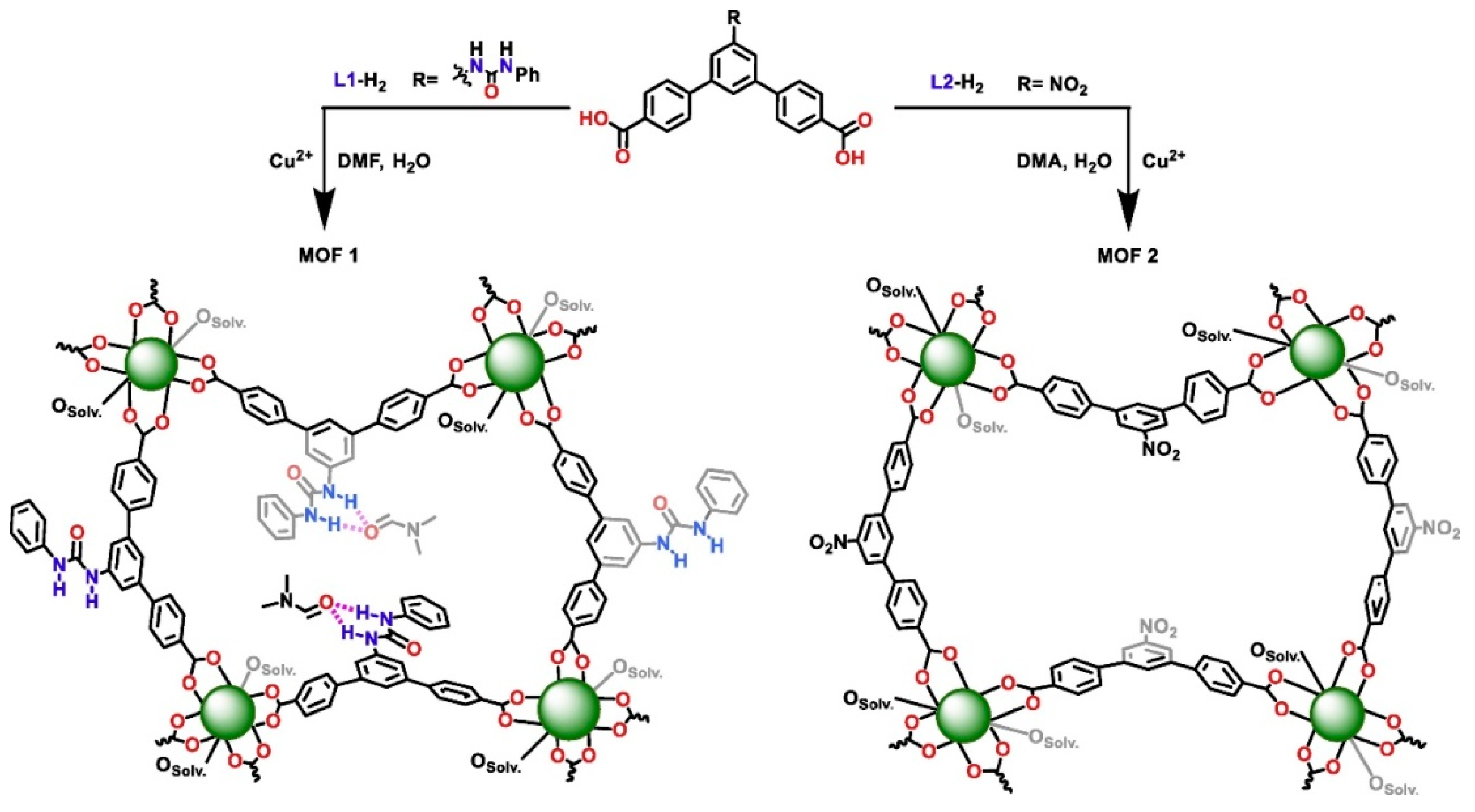
- Karmakar, A.; Oliver, C.L.; Roy, S.; Öhrströma, L. The synthesis, structure, topology and catalytic application of a novel cubane-based copper(ii) metal-organic framework derived from a flexible amido tripodal acid. Dalton Trans. 2015, 44, 10156–10165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Tang, H.; Yang, K.; Wu, X.; Luo, Y.; Wang, J.; Li, Y. A urea-containing metal-organic framework as a multifunctional heterogeneous hydrogen bond-donating catalyst. Catal. Commun. 2020, 135, 105837. [Google Scholar] [CrossRef]
- Siu, P.W.; Brown, Z.J.; Farha, O.K.; Hupp, J.T.; Scheidt, K.A. A mixed dicarboxylate strut approach to enhancing catalytic activity of a de novo urea derivative of metal-organic framework UiO-67. Chem. Commun. 2013, 49, 10920–10922. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.W.; Wang, L.; Zhao, X.; Li, Q.Y.; Wang, X.J. Microwave-assisted synthesis of urea-containing zirconium metal-organic frameworks for heterogeneous catalysis of Henry reactions. CrystEngComm 2019, 21, 1358–1362. [Google Scholar] [CrossRef]




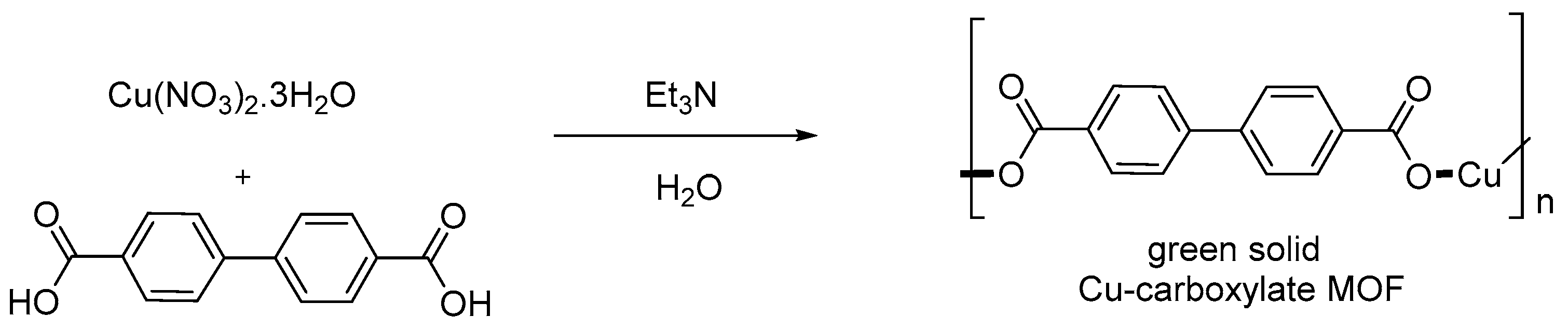
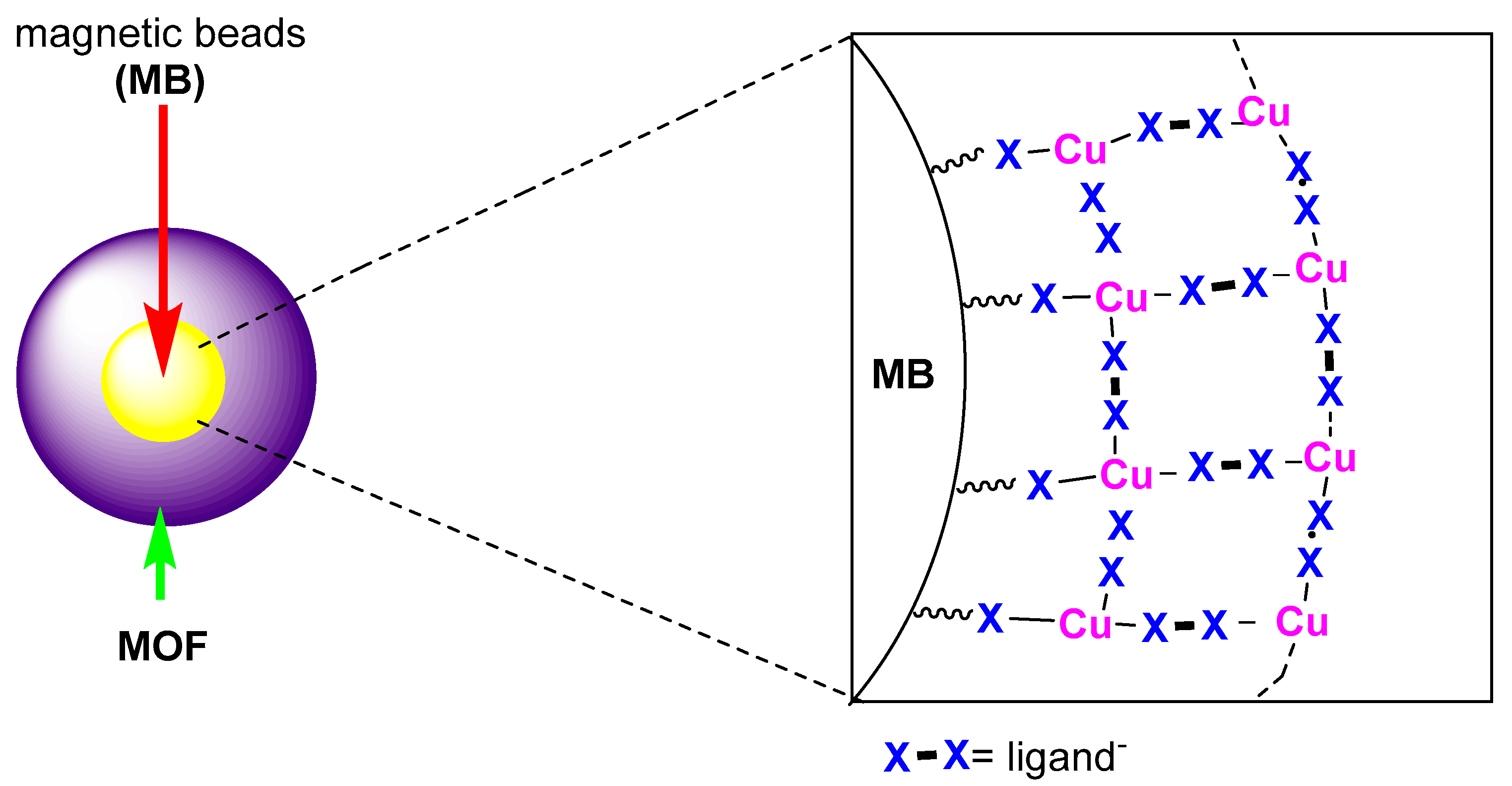
| MOF | Active Site | T (°C), Solvent | TON | Ref. |
|---|---|---|---|---|
| Co2(bdda)1.5(OAc)1·5H2O | Co | 70, water | 27.7 | 30 |
| [Cd2(Cu(salen))(DMF)3]·DMF·3H2O | Cd/Cu | 45, methanol | 70 | 31 |
| Cd3(CuL)8·(DMF)6·(H2O)8·(NMe2)2 | Cd/Cu | 60, methanol | 86 | 32 |
| {Cd2(Lglu)2(bpe)3(H2O)}·2H2O | Cd | RT, methanol a | 35.6 | 33 |
| [Zn3(TCPB)2·2H2O]·2H2O·4DMF | Zn | 70, neat b | 8 | 34 |
| [Zn2(hfipbb)2(4-bpdh)].(DMF)0.5 | Zn | 60, methanol | 32.3 | 35 |
| [Cu3(pdtc)L2(H2O)3].2DMF.10H2O | Cu | 70, neat | 5.9 | 36 |
| HKUST-1 | Cu | 100, toluene | 5.6 | 37 |
| NbO type-Cu MOF | Cu | 50, neat | 13.4 | 38 |
| Cu-BTC | Cu | 70, neat b | 4 | 39 |
| HP-MOF | Cu | - | - | 40 |
| HKUST-1@MMS | Cu | 75, water b | 18.2 | 41 |
| MOF-MB II | Cu | RT, ethanol b | 19.8 | 42 |
| [Sm(L1)2]n·1n(HCONH2)H·2n(HCONH2) | Sm | 70, water | 23.8 | 43 |
| MIL-101-NH2 NiBDP-OH NH2-Tb-MOF | NH2 | 80, toluene | 9.4 | 44 |
| OH | 100, 2-butanol | 2.0 | 46 | |
| NH2 | 90, neat | 6.7 | 47 | |
| MIL-101-NHR Cu-amide MOF | NHR | RT, toluene | 100 | 49 |
| NHCOR | 75, water | 42 | 50 | |
| Zn-MOF-DABCO Zn-amide MOF Cu-urea MOF UiO-67-urea | NHR | 60, neat | 48 | 51 |
| NHCOR | 70, methanol | 32 | 53 | |
| NHCONH | 60, methanol | 55 | 57 | |
| NHCONH | RT, THF | 14 | 58 | |
| UiO-68-urea | NHCONH | RT, THF | 18 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirujano, F.G.; Luque, R.; Dhakshinamoorthy, A. Metal-Organic Frameworks as Versatile Heterogeneous Solid Catalysts for Henry Reactions. Molecules 2021, 26, 1445. https://doi.org/10.3390/molecules26051445
Cirujano FG, Luque R, Dhakshinamoorthy A. Metal-Organic Frameworks as Versatile Heterogeneous Solid Catalysts for Henry Reactions. Molecules. 2021; 26(5):1445. https://doi.org/10.3390/molecules26051445
Chicago/Turabian StyleCirujano, Francisco G., Rafael Luque, and Amarajothi Dhakshinamoorthy. 2021. "Metal-Organic Frameworks as Versatile Heterogeneous Solid Catalysts for Henry Reactions" Molecules 26, no. 5: 1445. https://doi.org/10.3390/molecules26051445