Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification
Abstract
:1. Introduction
2. The Potential Anti-Tumor Mechanisms of β-Elemene
2.1. The Inhibition of Tumor Cell Proliferation and Growth
2.2. The Induction of Cell Apoptosis
2.3. The Inhibition of Tumor Cell Invasion and Metastasis
2.4. The Reverse of Multidrug Resistance
2.5. Enhancement of the Chemoradiotherapy Sensitization
2.6. The Activation of Protective Autophagy
2.7. The Regulation of the Immune System
3. Structural Modification of β-Elemene
3.1. Reduction and Oxidation Derivatives of β-Elemene
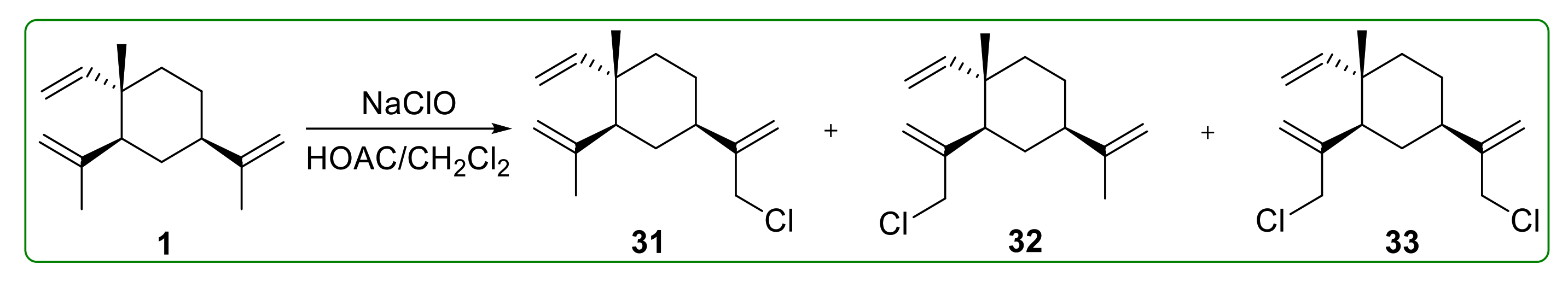
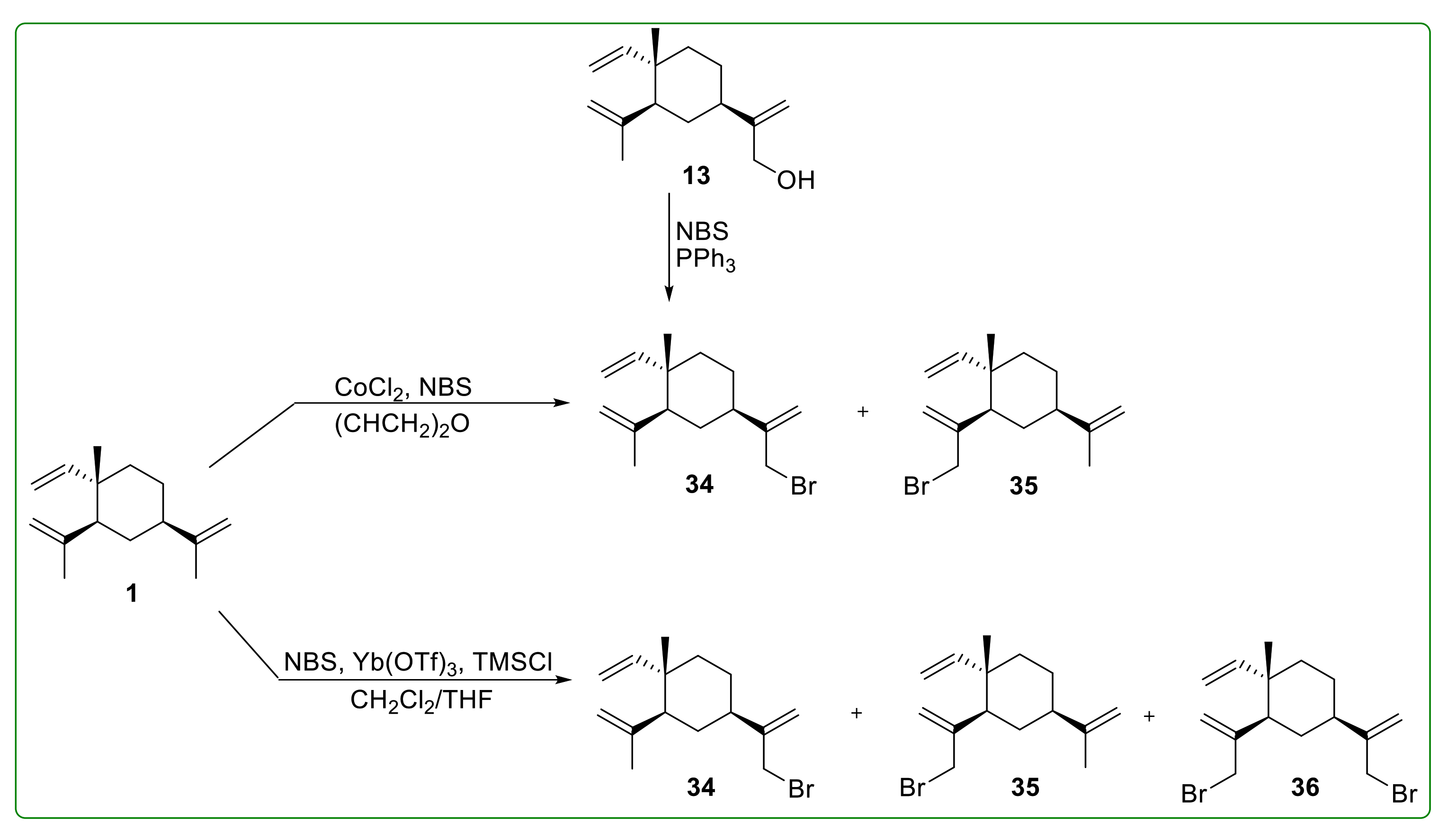
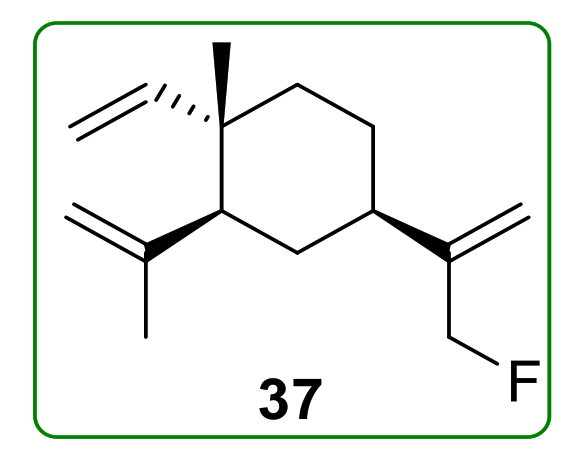
3.2. Halogenated Derivatives of β-Elemene
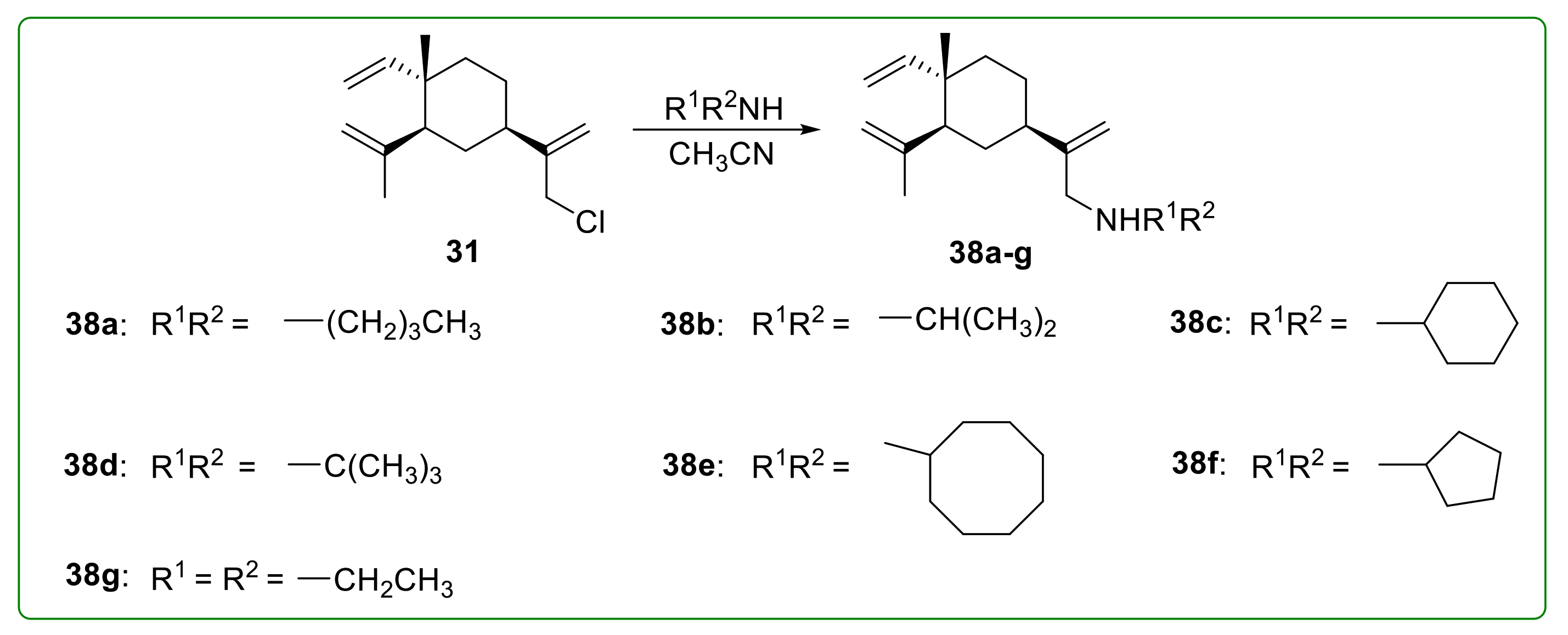
3.3. Amine Derivatives of Β-Elemene
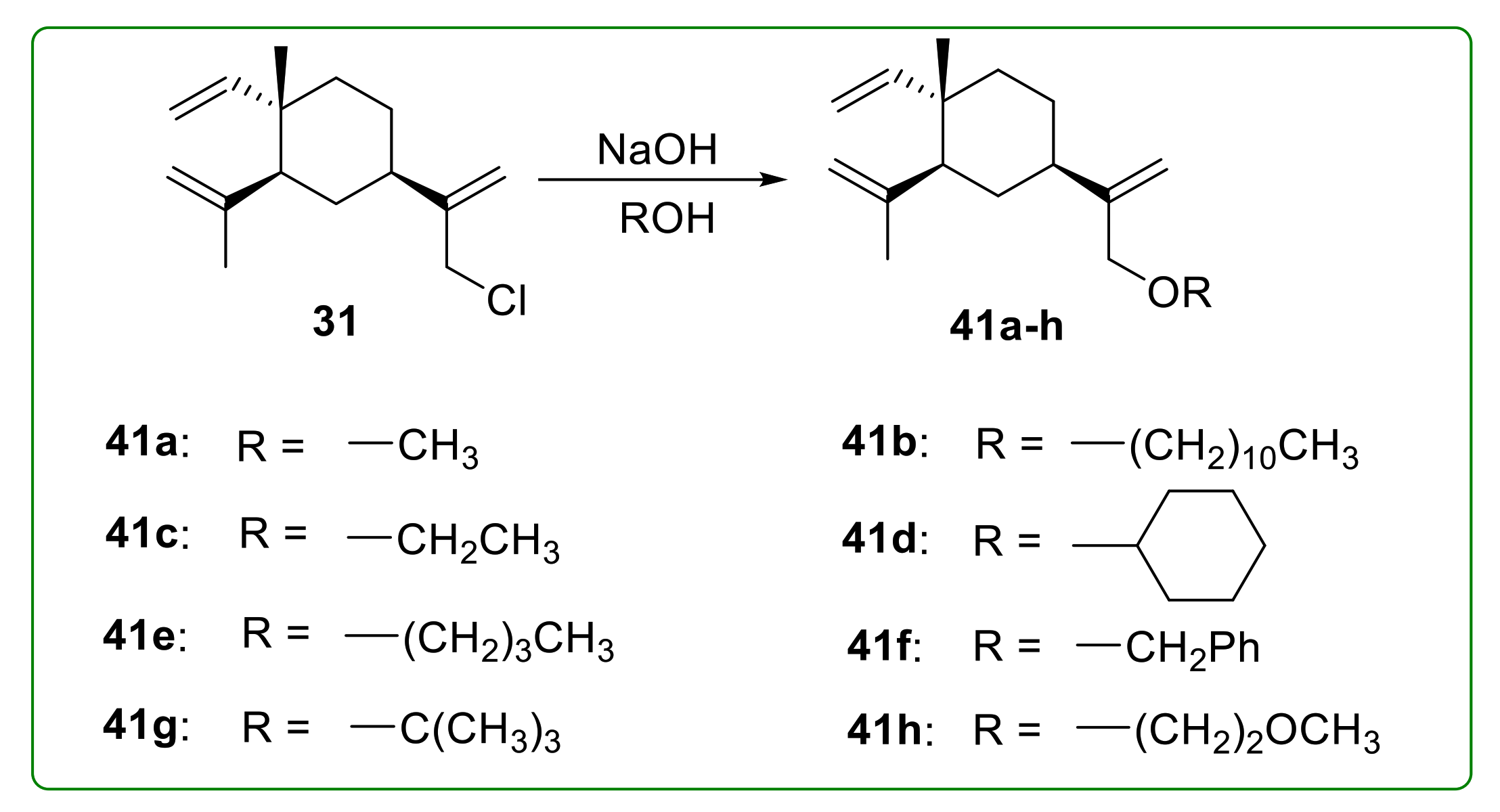
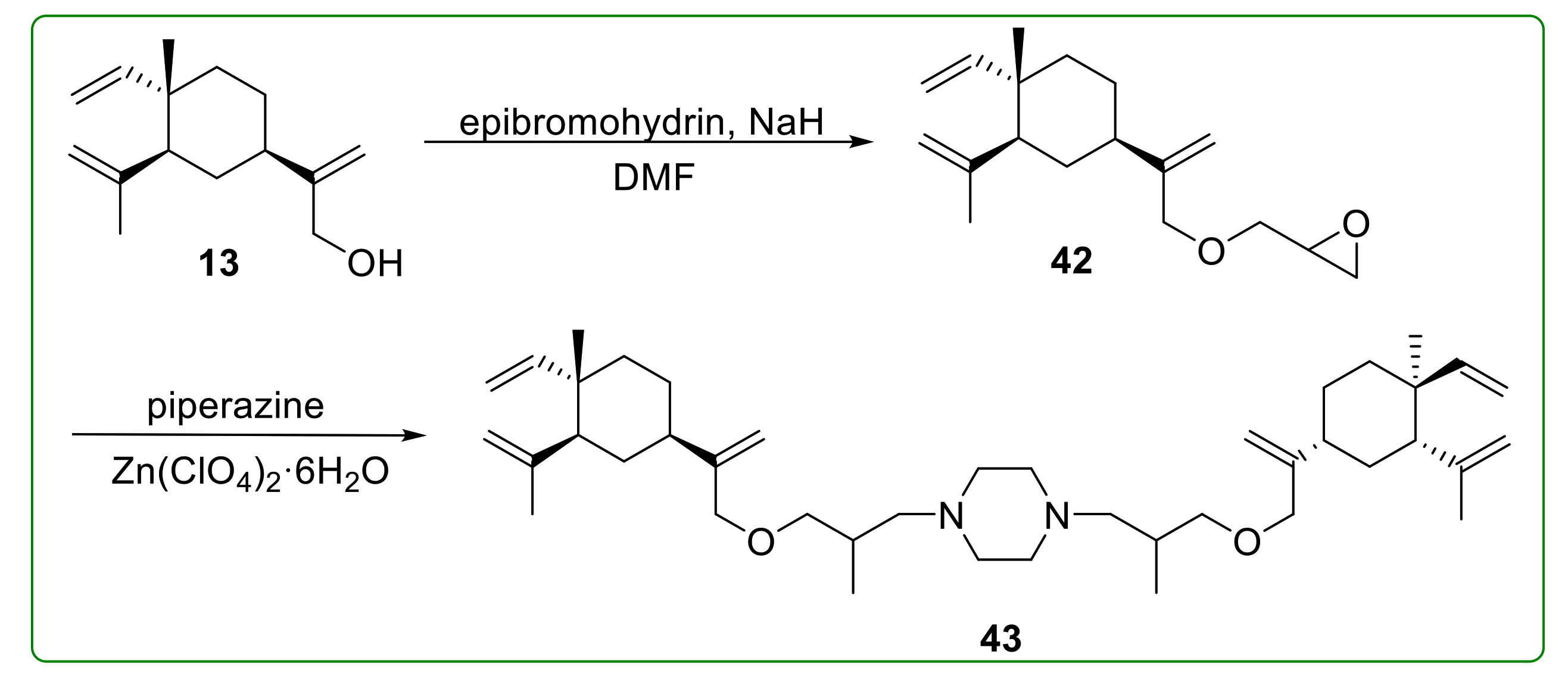
3.4. Ether Derivatives of β-Elemene
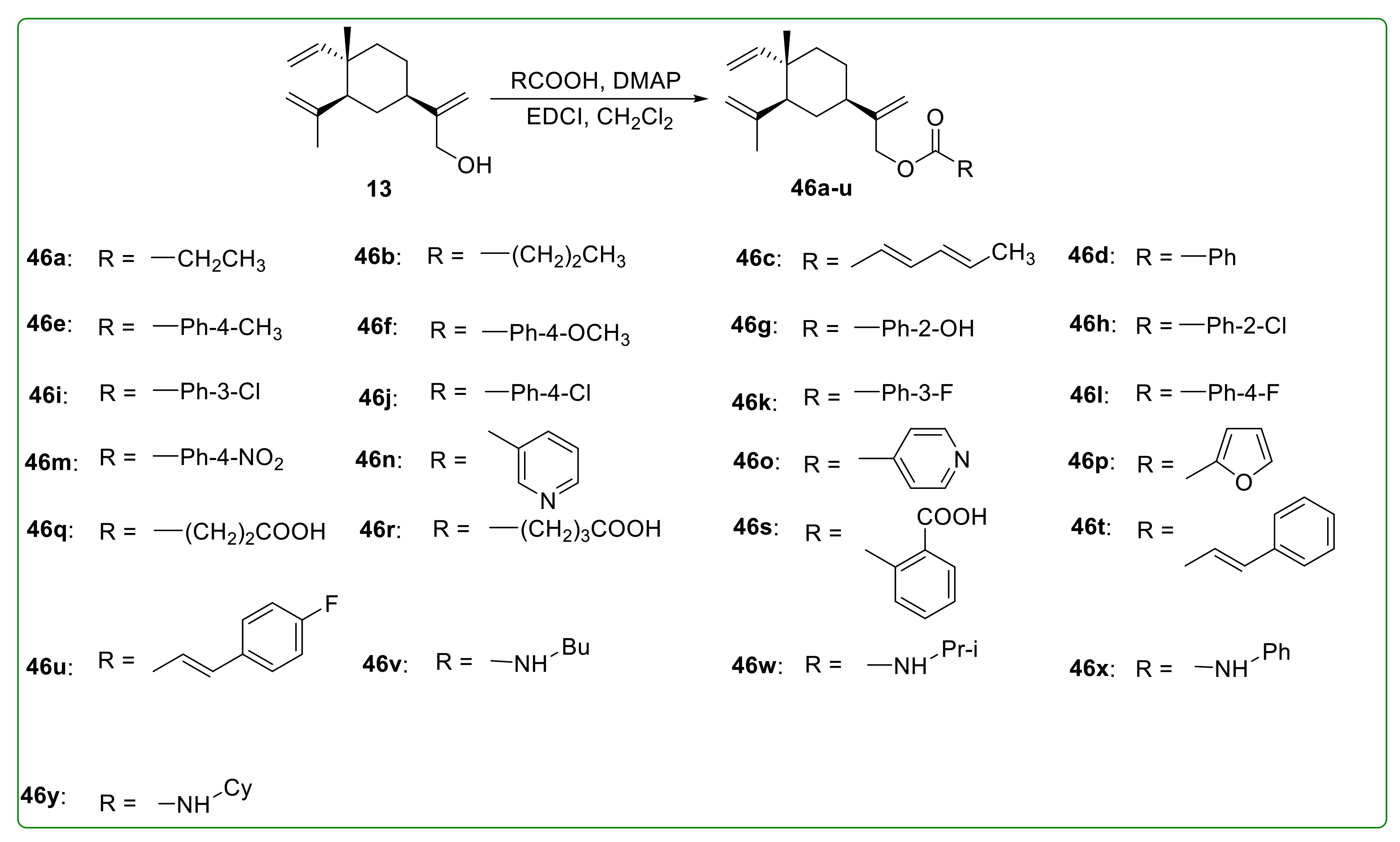
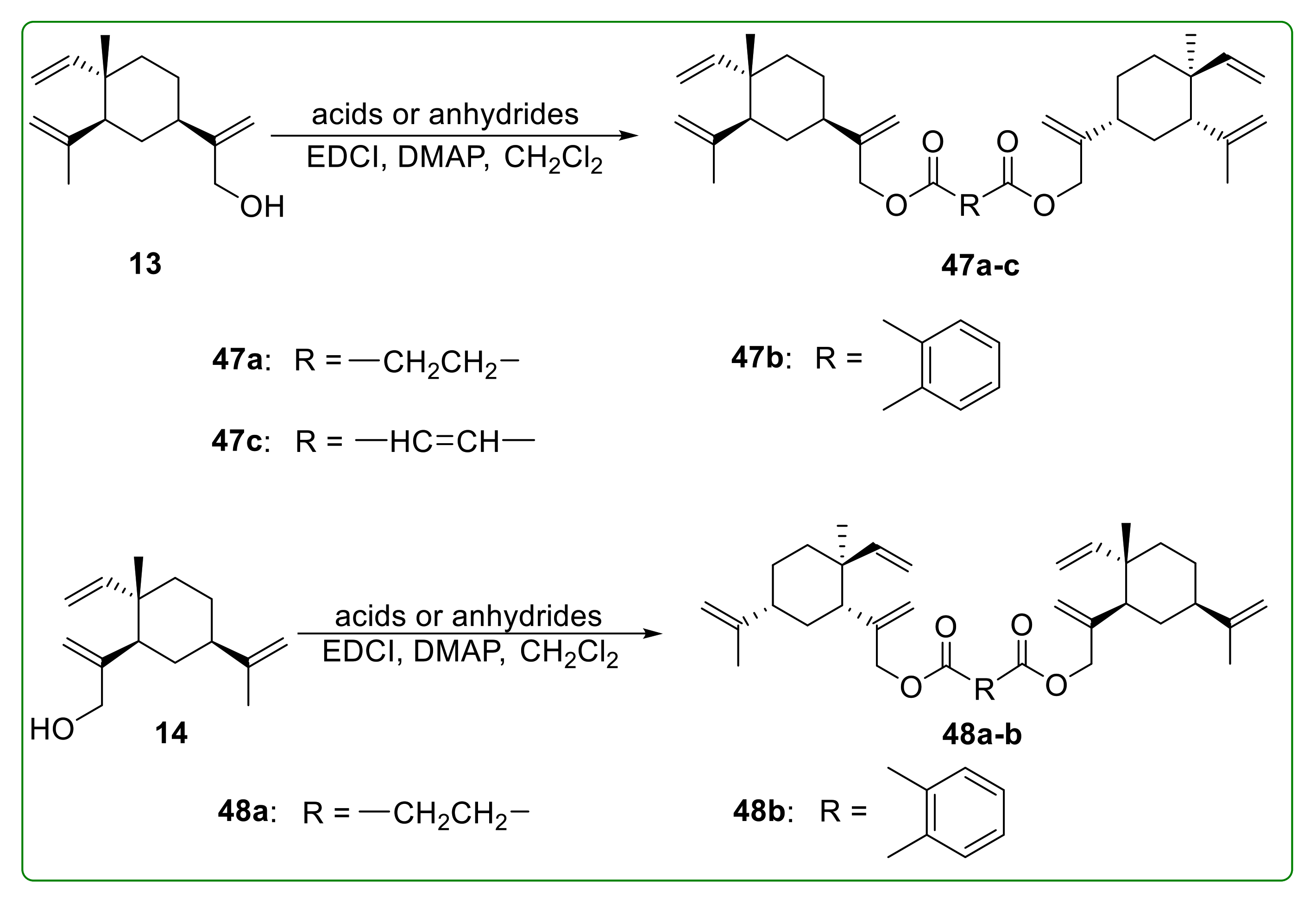
3.5. Ester Derivatives of β-Elemene
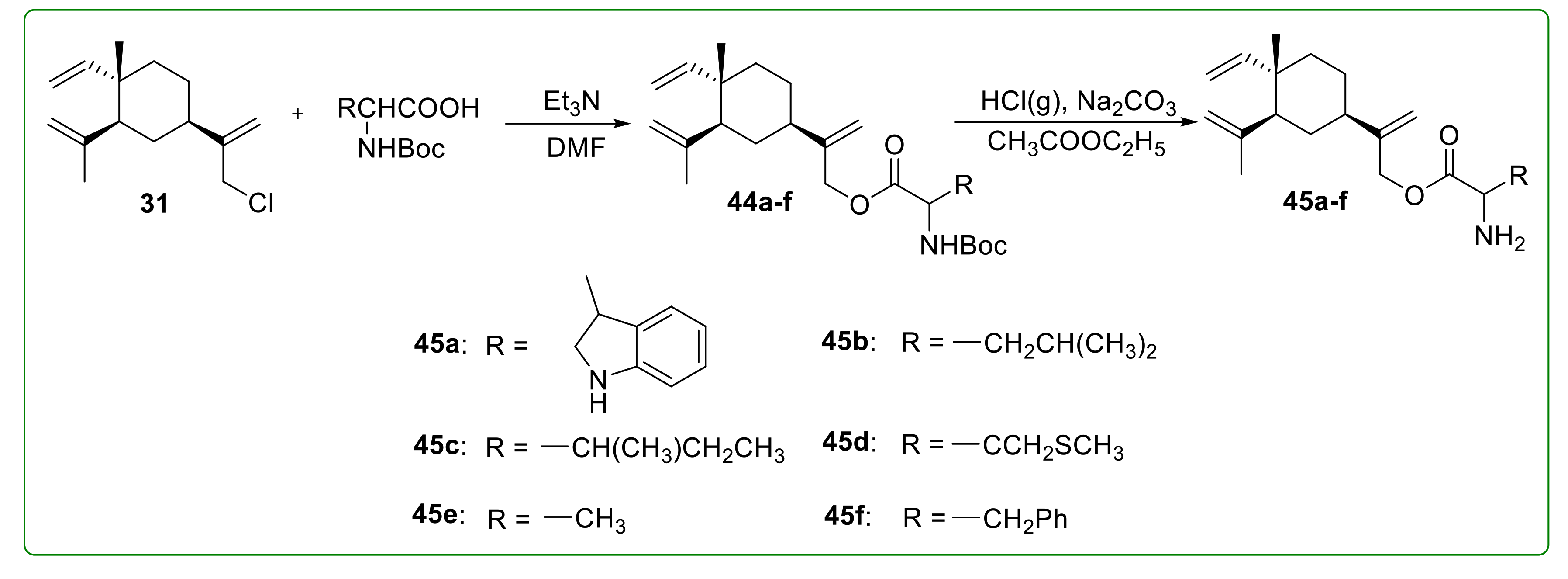
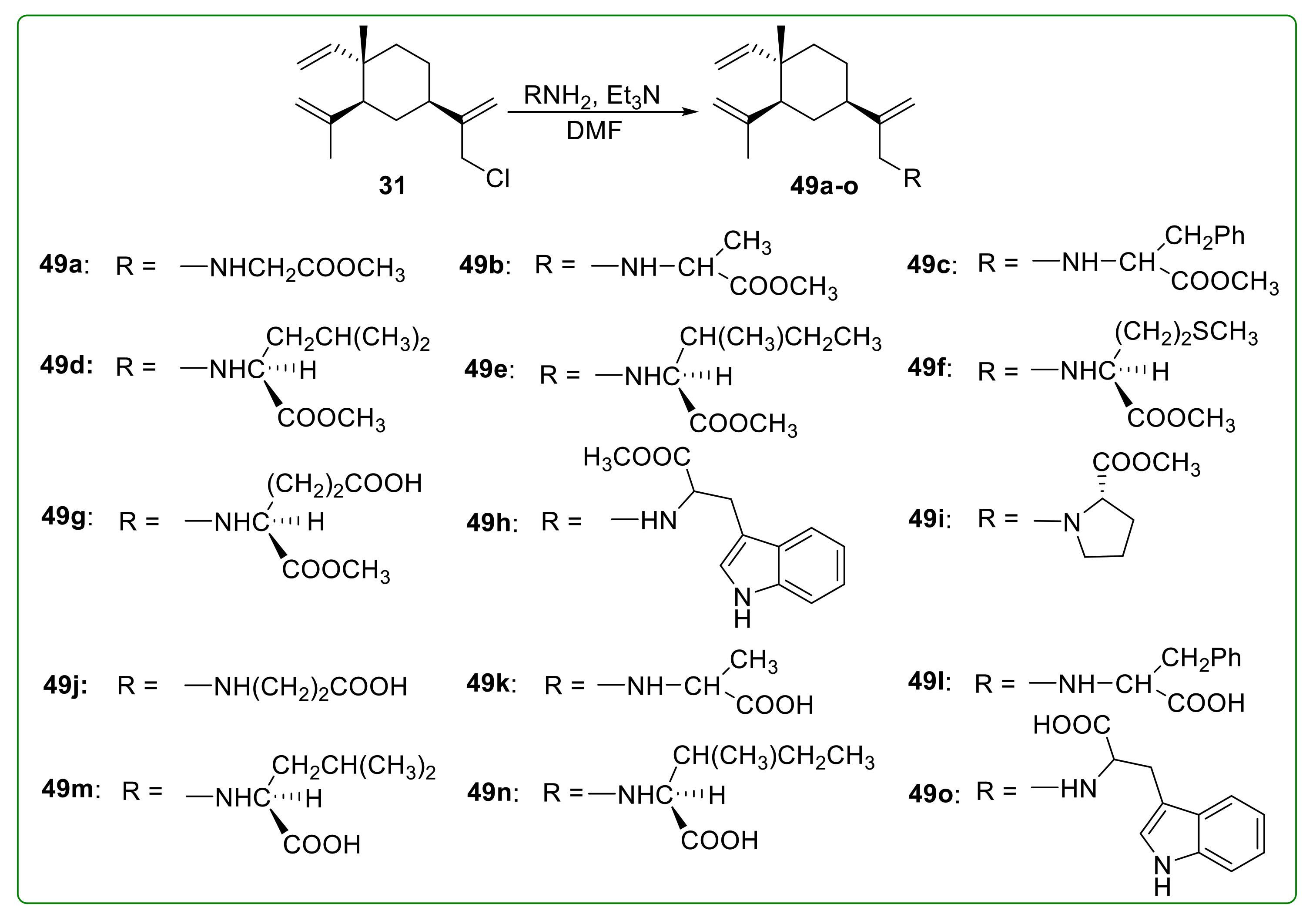
3.6. Amino Acid Derivatives of β-Elemene
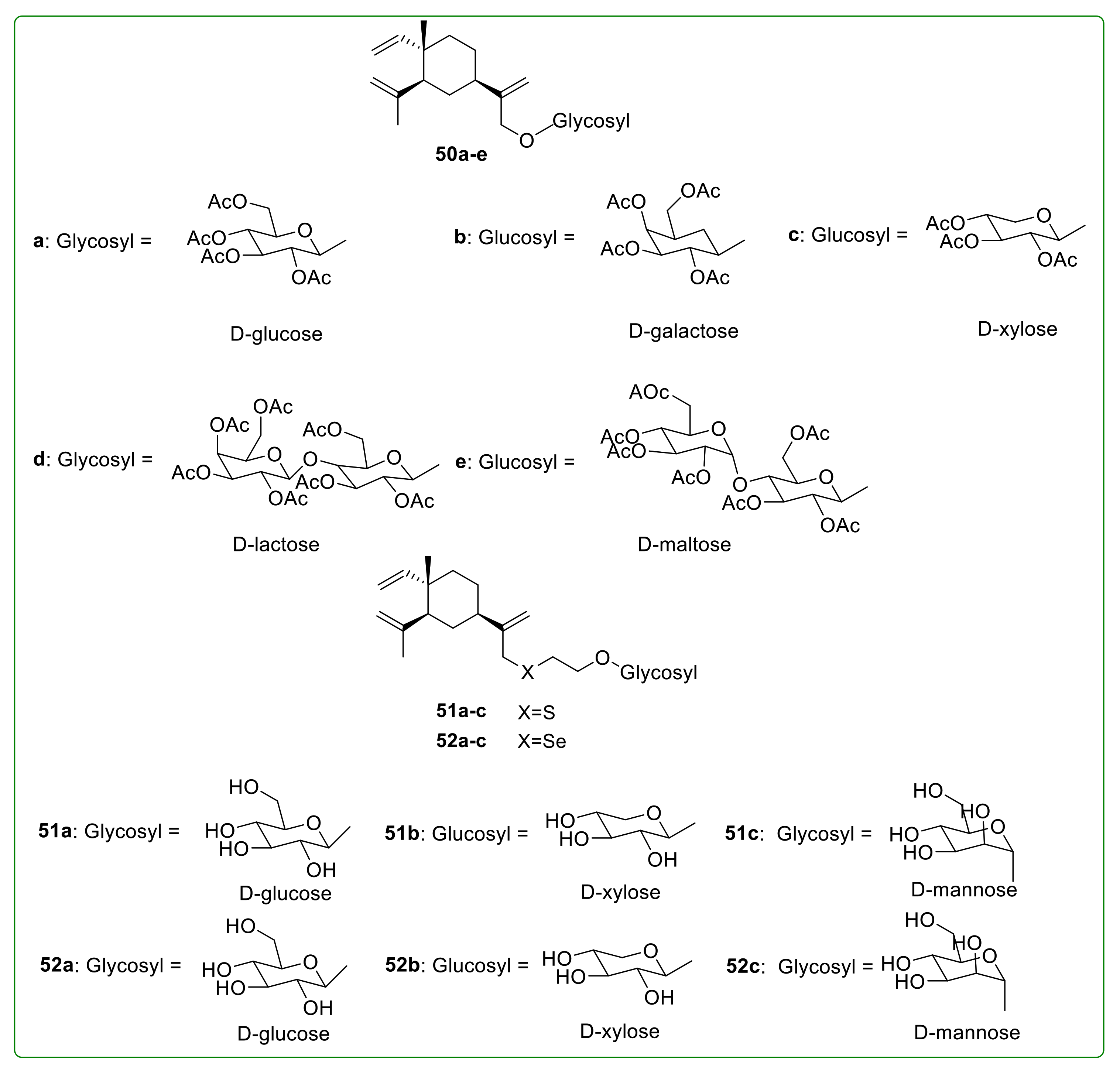
3.7. Glycoside Derivatives of β-Elemene
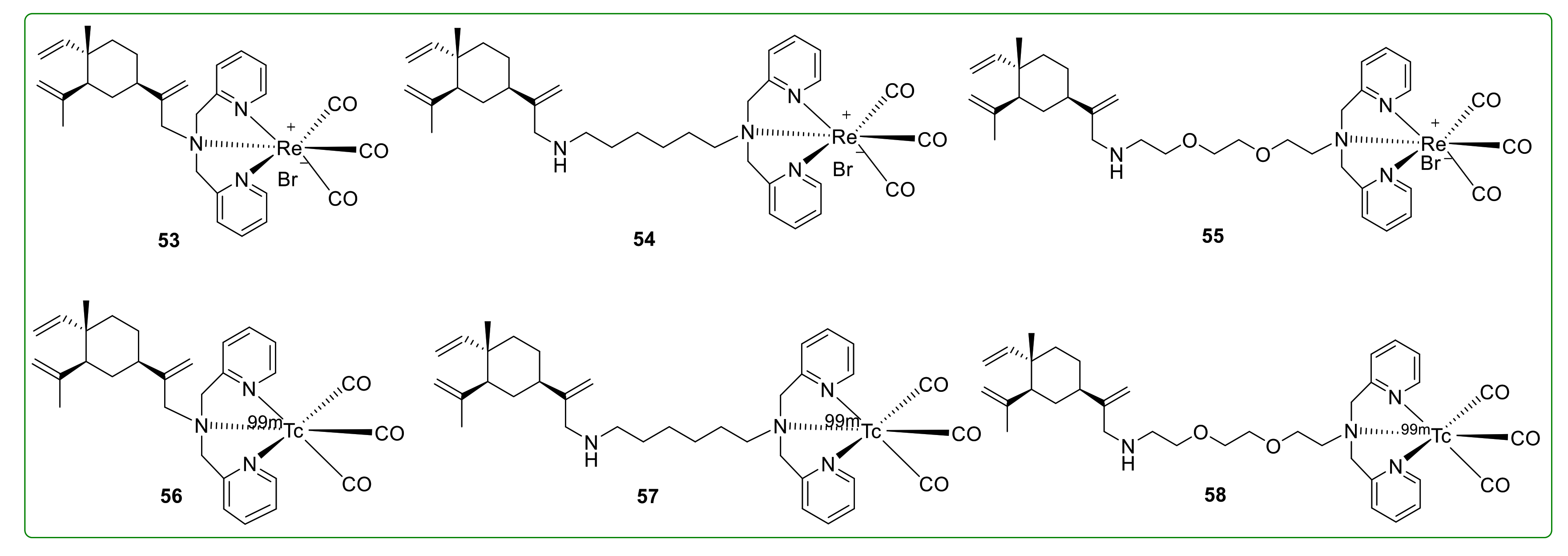
3.8. Radioactive Derivatives of β-Elemene
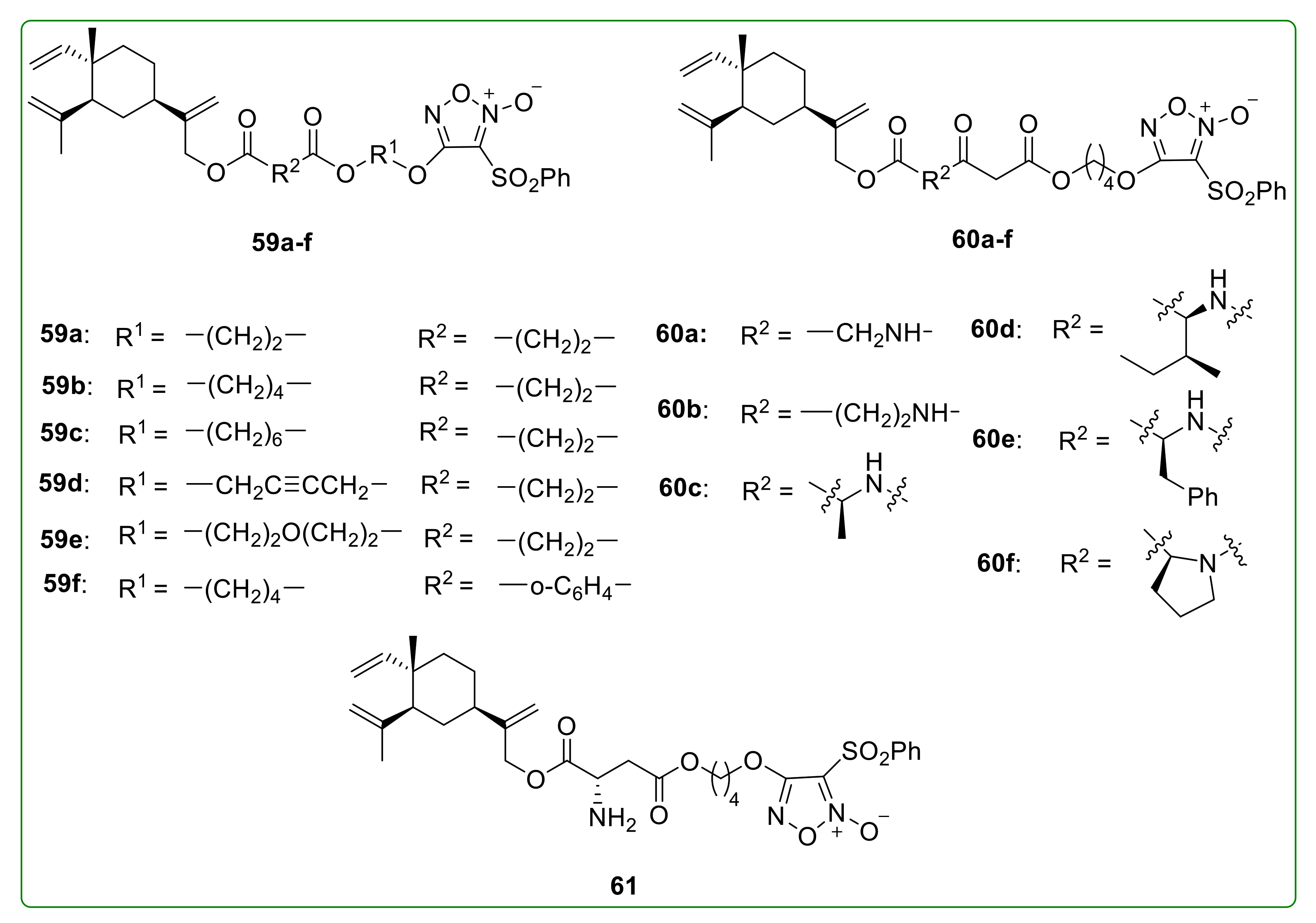
3.9. NO-Donating Derivatives of β-Elemene
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Focusing on the cell biology of cancer. Nat. Cell Biol. 2013, 15, 1. [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, X.; Tan, M.; Gong, J.; Tan, W.; Bian, B.; Chen, M.; Wang, Y. Fighting fire with fire: Poisonous Chinese herbal medicine for cancer therapy. J. Ethnopharmacol. 2012, 140, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.holleykingkong.com/cpzs/cpzs1/2017-11-24/144.html (accessed on 4 February 2021).
- Available online: http://www.holleykingkong.com/cpzs/cpzs1/2017-11-24/143.html (accessed on 4 February 2021).
- Wang, J.; Zhang, H.; Sun, Y. Results of phase II clinical trials of elemene emulsion in the management of advanced malignancies. Chin. J. New Drugs 1995, 4, 26–30. [Google Scholar]
- Coffman, J.A. Cell Cycle Development. Dev. Cell 2004, 6, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol. Life Sci. 2005, 62, 881–893. [Google Scholar] [CrossRef]
- Delgado, J.L.; Hsieh, C.-M.; Chan, N.-L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef]
- Dai, Z.-J.; Tang, W.; Lu, W.-F.; Gao, J.; Kang, H.-F.; Ma, X.-B.; Min, W.-L.; Wang, X.-J.; Wu, W.-Y. Antiproliferative and apoptotic effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell Int. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Liu, Y.; Zhang, J.; Gao, Y.-J.; Zhai, P.-P.; Su, X.; Li, X.; Li, Y.; Hou, L.; Cui, X.-N. β-Elemene Inhibits Cell Proliferation by Regulating the Expression and Activity of Topoisomerases I and IIalpha in Human Hepatocarcinoma HepG-2 Cells. Biomed. Res. Int. 2015, 2015, 153987. [Google Scholar] [CrossRef] [Green Version]
- Bao, F.; Qiu, J.; Zhang, H. Potential role of β-elemene on histone H1 in the H22 ascites hepatoma cell line. Mol. Med. Rep. 2012, 6, 185–190. [Google Scholar]
- Qin, Y.; Guo, Y.; Wei, W.; Wang, B.; Jin, H.; Sun, J.; Qi, X.; Ren, S.; Zuo, Y. Anti-tumor effect of β-elemene in murine hepatocellular carcinoma cell line H22 depends on the level of c-Met downregulation. Biomed. Prev. Nutr. 2012, 2, 91–98. [Google Scholar] [CrossRef]
- Yao, Y.-Q.; Ding, X.; Jia, Y.-C.; Huang, C.-X.; Wang, Y.-Z.; Xu, Y.-H. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef]
- Zhu, T.; Xu, Y.; Dong, B.; Zhang, J.; Wei, Z.; Xu, Y.; Yao, Y. β-elemene inhibits proliferation of human glioblastoma cells through the activation of glia maturation factor β and induces sensitization to cisplatin. Oncol. Rep. 2011, 26, 405–413. [Google Scholar]
- Zhu, T.-Z.; Li, X.-M.; Luo, L.-H.; Xu, Y.-H.; Cao, P.; Liu, Y.; Liang, G.-B. β-Elemene inhibits proliferation through crosstalk between glia maturation factor beta and extracellular signalregulated kinase 1/2 and impairs drug resistance to temozolomide in glioblastoma cells. Mol. Med. Rep. 2014, 10, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, H.; Li, Y.; Hou, Q.; Wang, Y.; Li, S.; Xia, B.; Wu, S. β-Elemene inhibits the proliferation of esophageal squamous cell carcinoma by regulating long noncoding RNA-mediated inhibition of hTERT expression. Anti-Cancer Drugs 2015, 26, 531–539. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar] [CrossRef]
- Wu, J.J.; Tang, Q.; Yang, L.J.; Chen, Y.Q.; Zheng, F.; Hann, S.S. Interplay of DNA methyltransferase 1 and EZH2 through inactivation of Stat3 contributes to beta-elemene-inhibited growth of nasopharyngeal carcinoma cells. Sci. Rep. 2017, 7, 509. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.Y.; Wu, J.; Zheng, F.; Tang, Q.; Yang, L.J.; Li, L.; Wu, W.Y. β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKα signalling pathways in human lung cancer cells: The role of Sp1. J. Cell. Mol. Med. 2015, 19, 630–641. [Google Scholar] [CrossRef]
- Zheng, F.; Tang, Q.; Zheng, X.-H.; Wu, J.J.; Huang, H.D.; Zhang, H.; Hann, S.S. Inactivation of Stat3 and crosstalk of miRNA155-5p and FOXO3a contribute to the induction of IGFBP1 expression by beta-elemene in human lung cancer. Exp. Mol. Med. 2018, 50, 121. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.-B.; Wang, J.; Jiang, H.-R.; Mei, X.; Zhao, Y.-Y.; Chen, F.-R.; Qu, Y.; Sai, K.; Guo, C.-C.; Yang, Q.-Y.; et al. β-Elemene Selectively Inhibits the Proliferation of Glioma Stem-Like Cells Through the Downregulation of Notch1. Stem Cells Transl. Med. 2017, 6, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Luo, H.; Qiu, W.; Han, H.; Chen, X.; Yang, L. β-elemene inhibits the proliferation of T24 bladder carcinoma cells through upregulation of the expression of Smad4. Mol. Med. Rep. 2013, 7, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Chong, F.L.Z.Z.; Maiese, K. Winding through the WNT pathway during cellular development and demise. Histol. Histopathol. 2006, 21, 103–124. [Google Scholar]
- Wang, L.; Zhao, Y.; Wu, Q.; Guan, Y.; Wu, X. Therapeutic effects of β-elemene via attenuation of the Wnt/β-catenin signaling pathway in cervical cancer cells. Mol. Med. Rep. 2018, 17, 4299–4306. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Zhang, J.; Hou, L.; Cui, X. The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells. Chin. J. Cancer Res. 2013, 25, 770–776. [Google Scholar]
- Qiao, L.; Wong, B.C.Y. Targeting apoptosis as an approach for gastrointestinal cancer therapy. Drug Resist. Updates 2009, 12, 55–64. [Google Scholar] [CrossRef]
- Kaufmann, T.; Strasser, A.; Jost, P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Emi, M.; Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 2006, 57, 545–553. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011, 278, 403–413. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.-Y.; Zhou, Y.-L.; Qiu, H.-H.; Wang, G.; Luo, Y.; Liu, J.-B.; Liu, X.-W.; Bu, W.-Q.; Song, J.; et al. β-elemene regulates endoplasmic reticulum stress to induce the apoptosis of NSCLC cells through PERK/IRE1alpha/ATF6 pathway. Biomed. Pharmacother. 2017, 93, 490–497. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Qu, X.; Zhao, M.; Yu, P.; Kang, J.; Liu, Y.; Hu, X. Cbl-regulated Akt and ERK signals are involved in beta-elemene-induced cell apoptosis in lung cancer cells. Mol. Med. Rep. 2011, 4, 1243–1246. [Google Scholar] [PubMed]
- Liu, J.-S.; He, S.-C.; Zhang, Z.-L.; Chen, R.; Fan, L.; Qiu, G.-L.; Chang, S.; Li, L.; Che, X.-M. Anticancer effects of beta-elemene in gastric cancer cells and its potential underlying proteins: A proteomic study. Oncol. Rep. 2014, 32, 2635–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Garcia, D.; Manero-Ruperez, N.; Quesada, R.; Korrodi-Gregorio, L.; Soto-Cerrato, V. Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 2019, 39, 887–909. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xie, T.; Jin, H.; Shi, L. β-elemene induces glioma cell apoptosis by downregulating survivin and its interaction with hepatitis B X-interacting protein. Oncol. Rep. 2012, 28, 2083–2090. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-S.; Zhu, T.-Z.; Chen, Y.-W.; Yao, Y.-Q.; Wu, C.-M.; Wei, Z.-Q.; Wang, W.; Xu, Y.-H. Beta-elemene inhibits Hsp90/Raf-1 molecular complex inducing apoptosis of glioblastoma cells. J. Neurooncol. 2012, 107, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Luo, H.; Luo, Z.; Zhang, T.; Yang, N.; Long, X.; Xie, H.; Qiu, W.; Zhang, B.; et al. β-elemene acts as an antitumor factor and downregulates the expression of survivin, Bcl-xL and Mta-1. Mol. Med. Rep. 2012, 6, 989–995. [Google Scholar] [PubMed] [Green Version]
- Spender, L.S.; Carter, M.J.; O’Brien, D.I.; Clark, L.J.; Yu, J.; Michalak, E.M.; Happo, L.; Cragg, M.S.; Inman, G.J. Transforming growth factor-beta directly induces p53-up-regulated modulator of apoptosis PUMA during the rapid induction of apoptosis in myc-driven B-cell lymphomas. J. Biol. Chem. 2013, 288, 5198–5209. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Gao, Y. β-elemene against Burkitt’s lymphoma via activation of PUMA mediated apoptotic pathway. Biomed. Pharmacother. 2018, 106, 1557–1562. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Liu, L.; Liu, L.; Geng, J.; Zhou, Y.; Chen, L. β-Elemene inhibits the metastasis of B16F10 melanoma cells by downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9. Melanoma Res. 2014, 24, 99–107. [Google Scholar] [CrossRef]
- Shi, H.; Liu, L.; Liu, L.-M.; Geng, J.; Chen, L. Inhibition of tumor growth by beta-elemene through downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9 in a murine intraocular melanoma model. Melanoma Res. 2015, 25, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, Y.; Feng, S.; Lv, C.; Xiu, L.; Zhang, Y.; Shi, J.; Li, Y.; Wei, P.; Qin, Z. β -Elemene-Attenuated Tumor Angiogenesis by Targeting Notch-1 in Gastric Cancer Stem-Like Cells. Evid-Based Compl. Alt. Med. 2013, 2013, 268468. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Zhang, Y.; Liu, B.; Chen, Y.; Song, H.; Yu, R.; Che, X.; Qu, X.; Liu, Y.; Hu, X.; et al. β-Elemene inhibits peritoneal metastasis of gastric cancer cells by modulating FAK/Claudin-1 signaling. Phytother. Res. 2019, 33, 2448–2456. [Google Scholar] [CrossRef]
- Deng, M.; Liu, B.; Song, H.; Yu, R.; Zou, D.; Chen, Y.; Ma, Y.; Lv, F.; Xu, L.; Zhang, Z.; et al. β-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway. Phytomedicine 2020, 69, 153184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, Y. β-elemene decreases cell invasion by upregulating E-cadherin expression in MCF-7 human breast cancer cells. Oncol. Rep. 2013, 30, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Y.; Zhang, Y.; Song, J.; Wang, Q.; Zheng, L.; Liu, D. Beta-elemene blocks epithelial-mesenchymal transition in human breast cancer cell line MCF-7 through Smad3-mediated down-regulation of nuclear transcription factors. PLoS ONE 2013, 8, e58719. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Wang, W.; Huang, S.; Ni, W.; Wei, Z.; Cao, Y.; Yu, S.; Jia, Q.; Wu, Y.; Chai, C.; et al. Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation. J. Cell Mol. Med. 2019, 23, 6846–6858. [Google Scholar] [CrossRef]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Yao, C.; Jiang, J.; Tu, Y.; Ye, S.; Du, H.; Zhang, Y. β-elemene reverses the drug resistance of A549/DDP lung cancer cells by activating intracellular redox system, decreasing mitochondrial membrane potential and P-glycoprotein expression, and inducing apoptosis. Thorac. Cancer 2014, 5, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, X.-D.; Li, E.-Z.; Luo, Y.; Song, N.; Qu, X.-J.; Hu, X.-J.; Liu, Y.-P. The role of E3 ubiquitin ligase Cbl proteins in beta-elemene reversing multi-drug resistance of human gastric adenocarcinoma cells. Int. J. Mol. Sci. 2013, 14, 10075–10089. [Google Scholar] [CrossRef]
- Guo, H.-Q.; Zhang, G.-N.; Wang, Y.-J.; Zhang, Y.-K.; Sodani, K.; Talele, T.T.; Ashby, C.R.; Chen, Z.-S., Jr. β-Elemene, a compound derived from Rhizoma zedoariae, reverses multidrug resistance mediated by the ABCB1 transporter. Oncol. Rep. 2014, 31, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, G.; Zhao, J.; Ding, H.; Cunningham, C.; Chen, F.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antiproliferative effect of beta-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol. Life Sci. 2005, 62, 894–904. [Google Scholar] [CrossRef]
- Lee, R.X.; Li, Q.Q.; Reed, E. β-Elemene Effectively Suppresses the Growth and Survival of Both Platinum-sensitive and -resistant Ovarian Tumor Cells. Anticancer Res. 2012, 32, 3103–3113. [Google Scholar] [PubMed]
- Dong, Y.; Li, L.; Wang, L.; Zhou, T.; Liu, J.W.; Gao, Y.J. Preliminary study of the effects of β-elemene on MCF-7/ADM breast cancer stem cells. Genet. Mol. Res. 2015, 14, 2347–2355. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Zhu, L.-X.; Yu, J.-D.; Chen, Z.; Gu, M.-C.; Mu, C.-F.; Liu, Q.; Xiong, Y. Effect of beta-elemene on the kinetics of intracellular transport of d-luciferin potassium salt ABC substrate in doxorubicin-resistant breast cancer cells and the associated molecular mechanism. Eur. J. Pharm. Sci. 2018, 120, 20–29. [Google Scholar] [CrossRef]
- Xu, H.-B.; Li, L.; Fu, J.; Mao, X.-P.; Xu, L.-Z. Reversion of multidrug resistance in a chemoresistant human breast cancer cell line by β-elemene. Pharmacology 2012, 89, 303–312. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.-D.; Yao, Y.-F.; Zhong, S.-L.; Zhao, J.H.; Tang, J.H. β-Elemene Reverses Chemoresistance of Breast Cancer Cells by Reducing Resistance Transmission via Exosomes. Cell Physiol. Biochem. 2015, 36, 2274–2286. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.-C.; Tu, Y.-R.; Jiang, J.; Ye, S.-F.; Du, H.-X.; Zhang, Y. β-elemene reverses the drug resistance of lung cancer A549/DDP cells via the mitochondrial apoptosis pathway. Oncol. Rep. 2014, 31, 2131–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Pan, T.; Xiang, Y.; Zhang, M.; Feng, J.; Liu, S.; Duan, T.; Chen, P.; Zhai, B.; Chen, X.; et al. β-Elemene Reverses the Resistance of p53-Deficient Colorectal Cancer Cells to 5-Fluorouracil by Inducing Pro-death Autophagy and Cyclin D3-Dependent Cycle Arrest. Front. Bioeng. Biotechnol. 2020, 8, 378. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Zhao, Y.; Yuan, Y. β-elemene enhances both radiosensitivity and chemosensitivity of glioblastoma cells through the inhibition of the ATM signaling pathway. Oncol. Rep. 2015, 34, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Wang, T.; Chen, Y.; Tang, X.; Yuan, Y.; Zhao, Y. β-Elemene enhances the efficacy of gefitinib on glioblastoma multiforme cells through the inhibition of the EGFR signaling pathway. Int. J. Oncol. 2016, 49, 1427–1436. [Google Scholar] [CrossRef]
- Li, Q.Q.; Lee, R.X.; Liang, H.; Wang, G.; Li, J.M.; Zhong, Y.; Reed, E. β-Elemene enhances susceptibility to cisplatin in resistant ovarian carcinoma cells via downregulation of ERCC-1 and XIAP and inactivation of JNK. Int. J. Oncol. 2013, 43, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Q.; Lee, R.X.; Liang, H.; Zhong, Y.; Reed, E. Enhancement of cisplatin-induced apoptosis by beta-elemene in resistant human ovarian cancer cells. Med. Oncol. 2013, 30, 424. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Ma, S.; Feng, J. In vitro study of radiosensitization by β-Elemene in A549 cell line from adenocarcinoma of lung. Chin. Ger. J. Clin. Oncol. 2009, 8, 12–15. [Google Scholar] [CrossRef]
- Li, L.-J.; Zhong, L.-F.; Jiang, L.-P.; Geng, C.-Y.; Zou, L.-J. β-Elemene radiosensitizes lung cancer A549 cells by enhancing DNA damage and inhibiting DNA repair. Phytother. Res. 2011, 25, 1095–1097. [Google Scholar] [CrossRef]
- Tong, E.; Xu, Y.; Li, G.; Zou, K.; Zou, L. The effects of beta-elemene on the expression of mTOR, HIF-1A, survivin in lung adenocarcinoma A549 cell. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 18–23. [Google Scholar]
- Li, G.; Xie, B.; Li, X.; Chen, Y.; Wang, Q.; Xu, Y.; Xu-Welliver, M.; Zou, L. Down-regulation of survivin and hypoxia-inducible factor-1 alpha by beta-elemene enhances the radiosensitivity of lung adenocarcinoma xenograft. Cancer Biother. Radiopharm. 2012, 27, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, B.; Li, X.; Chen, Y.; Xu, Y.; Xu-Welliver, M.; Zou, L. Downregulation of peroxiredoxin-1 by beta-elemene enhances the radiosensitivity of lung adenocarcinoma xenografts. Oncol. Rep. 2015, 33, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Q.; Wang, G.; Huang, F.; Li, J.M.; Cuff, C.F.; Reed, E. Sensitization of lung cancer cells to cisplatin by beta-elemene is mediated through blockade of cell cycle progression: Antitumor efficacies of beta-elemene and its synthetic analogs. Med. Oncol. 2013, 30, 488. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Zhang, M.; Cuff, C.F.; Huang, L.; Reed, E. beta-Elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol. Rep. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Gan, D.; He, W.; Yin, H.; Gou, X. β-elemene enhances cisplatin-induced apoptosis in bladder cancer cells through the ROS-AMPK signaling pathway. Oncol. Lett. 2020, 19, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wang, G.; Liang, H.; Li, J.M.; Huang, F.; Agarwal, P.K.; Zhong, Y.; Reed, E. β-Elemene Promotes Cisplatin-induced Cell Death in Human Bladder Cancer and Other Carcinomas. Anticancer Res. 2013, 33, 1421–1428. [Google Scholar] [PubMed]
- Liu, J.-S.; Che, X.-M.; Chang, S.; Qiu, G.-L.; He, S.-C.; Fan, L.; Zhao, W.; Zhang, Z.-L.; Wang, S.-F. β-elemene enhances the radiosensitivity of gastric cancer cells by inhibiting Pak1 activation. World J. Gastroenterol. 2015, 21, 9945–9956. [Google Scholar] [CrossRef]
- Su, P.; Ahmad, B.; Zou, K.; Zou, L. β-Elemene Enhances the Chemotherapeutic Effect of 5-Fluorouracil in Triple-Negative Breast Cancer via PI3K/AKT, RAF-MEK-ErK, and NF-kappaB Signaling Pathways. Onco. Targets Ther. 2020, 13, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yu, Y. Synergistic Cytotoxicity of β-Elemene and Cisplatin in Gingival Squamous Cell Carcinoma by Inhibition of STAT3 Signaling Pathway. Med. Sci. Monit. 2017, 23, 1507–1513. [Google Scholar] [CrossRef] [Green Version]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The Use of β-Elemene to Enhance Radio Sensitization of A375 Human Melanoma Cells. Cell J. 2020, 21, 419–425. [Google Scholar] [PubMed]
- Guo, Z.; Liu, Z.; Yue, H.; Wang, J. Beta-elemene increases chemosensitivity to 5-fluorouracil through down-regulating microRNA-191 expression in colorectal carcinoma cells. J. Cell Biochem. 2018, 119, 7032–7039. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Reed, E.; Huang, L.; Cuff, C.F. Evaluation of cisplatin in combination with beta-elemene as a regimen for prostate cancer chemotherapy. Basic Clin. Pharmacol. Toxicol. 2010, 107, 868–876. [Google Scholar]
- Li, X.; Lin, Z.; Zhang, B.; Guo, L.; Liu, S.; Li, H.; Zhang, J.; Ye, Q. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 2016, 6, 21010. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Yu, D.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 2007, 17, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Tsurita, G.; Kawai, K.; Okaji, Y.; Nishikawa, T.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 2010, 10, 370. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Qu, J.; Xu, L.; Hou, K.; Zhang, J.; Qu, X.; Liu, Y. β-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer 2011, 11, 183. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, X.-J.; Jin, B.; Qu, X.-J.; Hou, K.-Z.; Liu, Y.-P. β-Elemene induces apoptosis as well as protective autophagy in human non-small-cell lung cancer A549 cells. J. Pharm. Pharmacol. 2012, 64, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.-H.; Liu, J.; Qu, X.-J.; Hou, K.-Z.; Wang, K.-F.; Liu, Y.-P.; Wu, B. β-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/ mTOR signalling pathways. Asian Pac. J. Cancer Prev. 2012, 13, 2739–2744. [Google Scholar] [CrossRef] [Green Version]
- Guan, C.; Liu, W.; Yue, Y.; Jin, H.; Wang, X.; Wang, X.-J. Inhibitory effect of β-elemene on human breast cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 3948–3956. [Google Scholar] [PubMed]
- Lin, Y.; Wang, K.; Hu, C.; Lin, L.; Qin, S.; Cai, X. Elemene injection induced autophagy protects human hepatoma cancer cells from starvation and undergoing apoptosis. Evid. Based Complement. Alternat. Med. 2014, 2014, 637528. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Guo, T.; Qu, X.; Hu, X.; Zhang, Y.; Che, X.; Song, H.; Gong, J.; Ma, R.; Li, C.; et al. β-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts. Cell Biol. Int. 2018, 42, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, M.; Li, N.; Li, Z.; Li, H.; Shao, S.; Zou, K.; Zou, L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem. Biophys. Res. Commun. 2017, 490, 514–520. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zhang, Y.; Xu, M.; Che, Y.; Tian, X.; Wang, R.; Zou, K.; Zou, L. β-elemene inhibits radiation and hypoxia-induced macrophages infiltration via Prx-1/NF-kappaB/HIF-1alpha signaling pathway. Onco. Targets Ther. 2019, 12, 4203–4211. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Wang, Z.; Che, L.; Yin, S. Immunotherapeutic effects on murine pancreatic carcinoma by beta-elemene combined with dendritic cells modified with genes encoding interleukin-23. Front. Med. China 2007, 1, 41–45. [Google Scholar] [CrossRef] [PubMed]
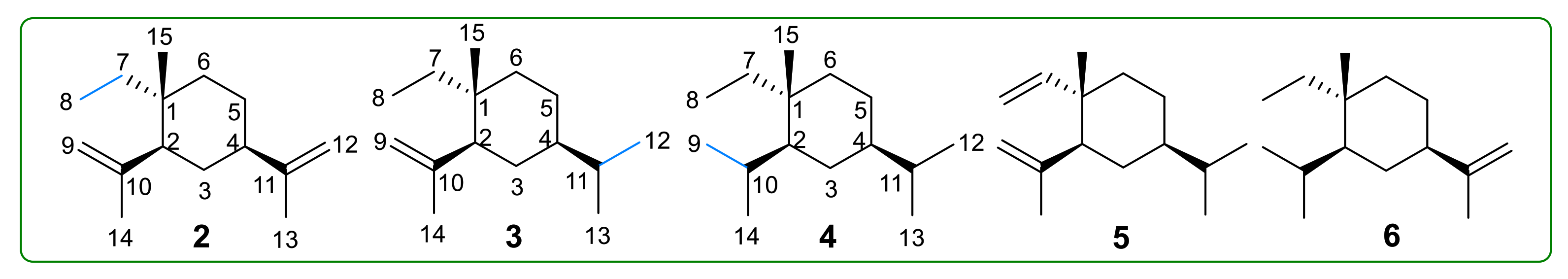
- Dong, J.; Cheng, G.; Hu, J. Study on catalytic hydrogenation of β-elemene. Chem. Bull. 1996, 10, 36–37. [Google Scholar]
- Hu, J.; Cheng, G.; Gong, G.; Wang, W.; Xu, Y. 13C NMR spectra of the product of the catalytic hydrogenation of β-elemene. Chin. J. Magn. Reso. 1997, 14, 491–493. [Google Scholar]
- Thomas, A.F.; Vial, C.; Ozainne, M.; Ohloff, G. The oxidation of the double bonds of β-elemene. Helv. Chim. Acta 1973, 56, 2270–2279. [Google Scholar] [CrossRef]
- Maurer, B.; Grieder, A. Sesquiterpenoids from Costus Root Oil Suussureu luppu CLARK. Helv. Chim. Acta 1977, 60, 2177–2190. [Google Scholar] [CrossRef]
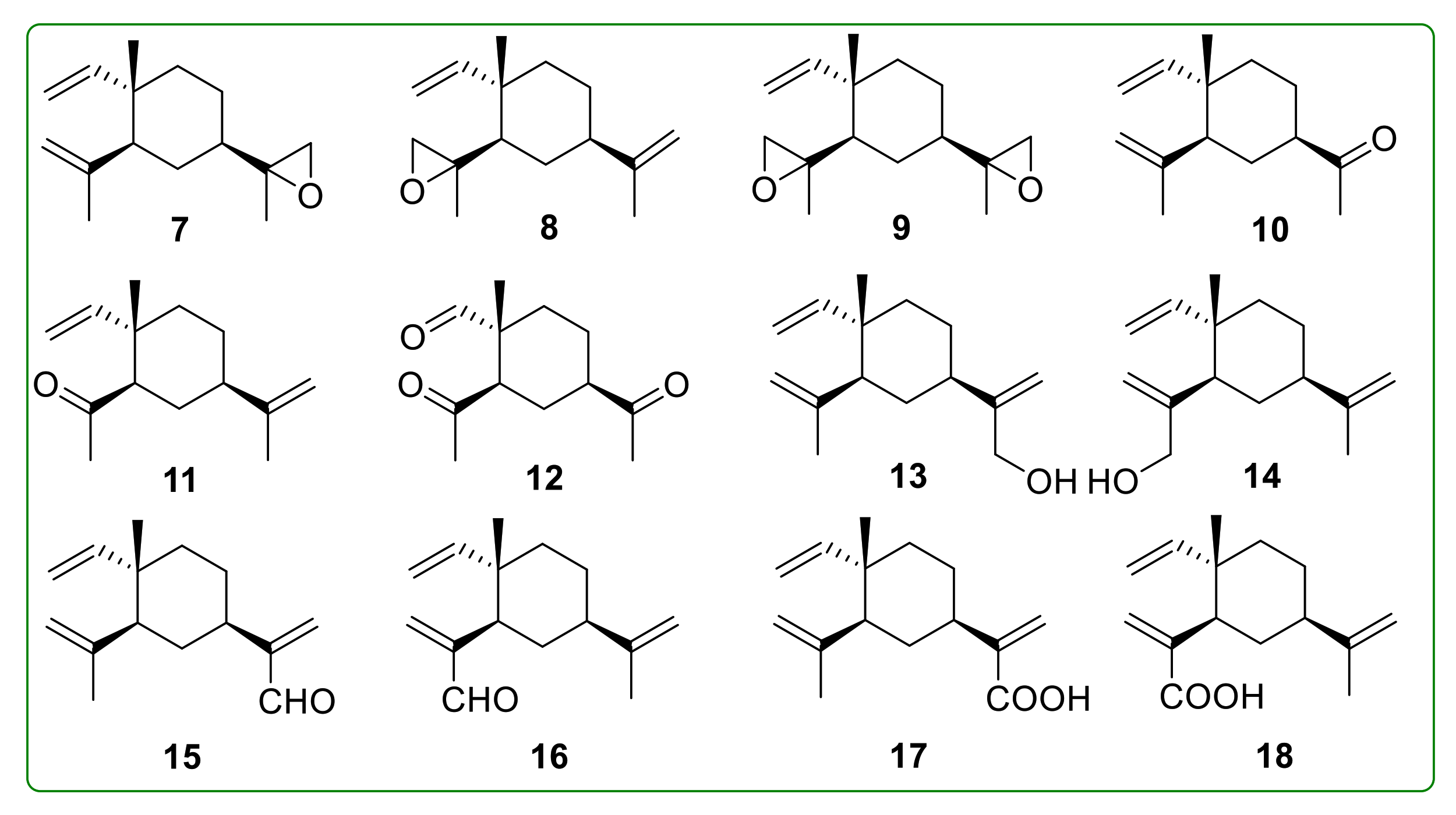
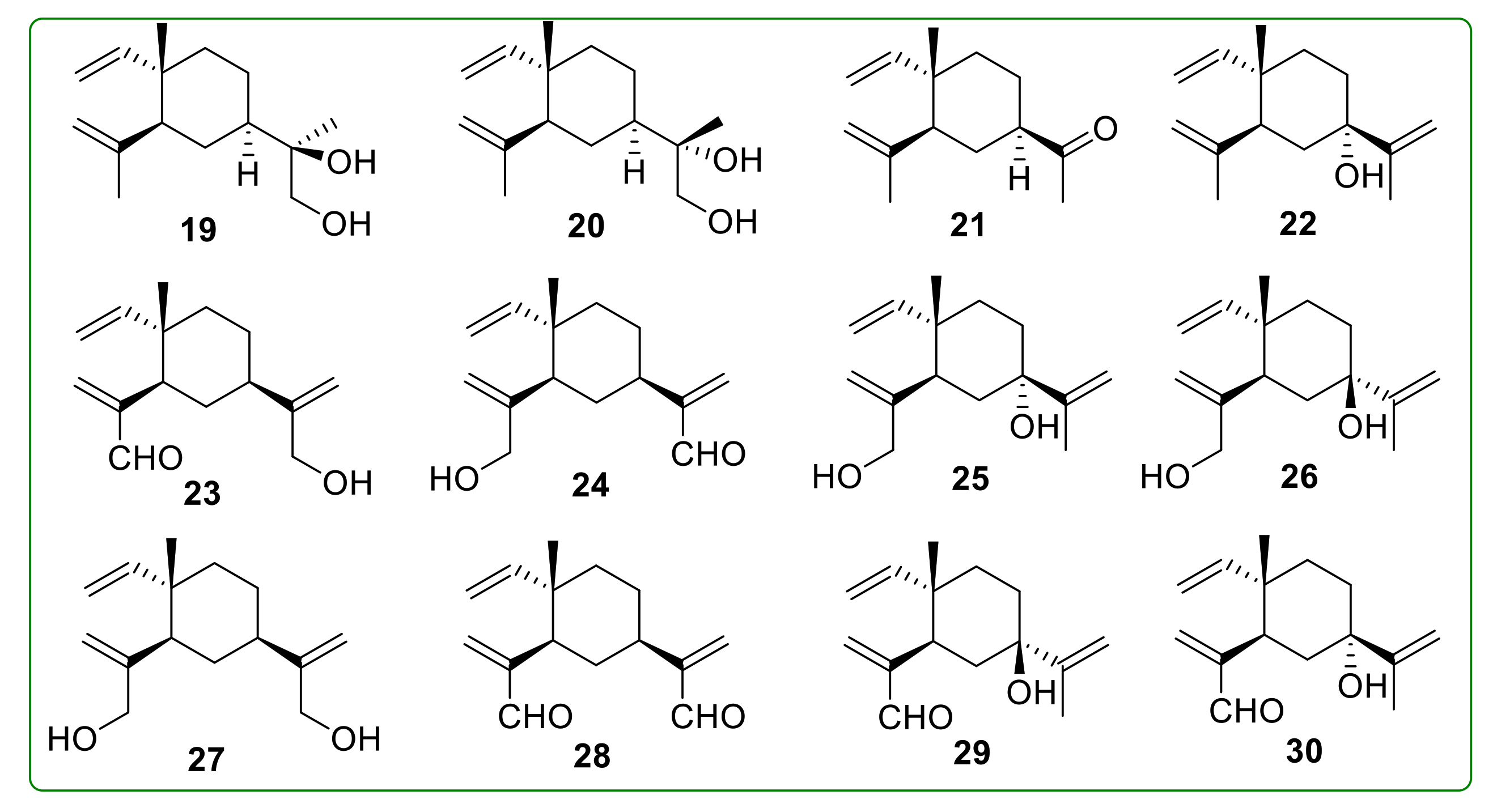
- Bai, R.; Jie, X.; Salgado, E.; Sun, J.; Zhu, Y.; Pickel, T.; Xie, Y.; Chen, J.; Xu, J. Rational Design, Synthesis and Biological Evaluation of Novel Derivatives Based on In vivo Metabolism of Natural Product β-elemene. Lett. Drug Des. Discov. 2018, 15, 905–912. [Google Scholar] [CrossRef]
- Li, Q.Q.; Lee, R.X.; Liang, H.; Zhong, Y. Anticancer activity of β-Elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res. 2013, 33, 65–76. [Google Scholar]
- He, X.; Zhuo, X.-T.; Gao, Y.; Bai, R.; Ye, X.; Xie, T. β-Elemene derivatives produced from SeO2-mediated oxidation reaction. R. Soc. Open Sci. 2020, 7, 200038. [Google Scholar] [CrossRef]
- Hui, Z.; Li, Z.; Gao, Y.; Jiang, S.; Qi, X.; Ye, X.; Xie, T. Research progress on synthesis of intermediates in derivatization of β-elemene. Chin. Tradit. Herb. Drugs 2020, 51, 4547–4558. [Google Scholar]
- Chen, J.; Wang, R.; Wang, T.; Ding, Q.; Khalil, A.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; et al. Antioxidant Properties of Novel Dimers Derived from Natural β-Elemene through Inhibiting H2O2-induced Apoptosis. Acs Med. Chem. Lett. 2017, 8, 443–448. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, Y.; Zhu, H.; Ren, Y.; Shen, Y.-M. Synthesis and in vitro anti-proliferative activity of beta-elemene monosubstituted derivatives in HeLa cells mediated through arrest of cell cycle at the G1 phase. Bioorg. Med. Chem. 2009, 17, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Tao, S.; Wang, X.; Yu, Z.; Wang, M.; Chen, D.; Jing, Y.; Dong, J. The synthesis and anti-proliferative effects of beta-elemene derivatives with mTOR inhibition activity. Bioorg. Med. Chem. 2006, 14, 5351–5356. [Google Scholar] [CrossRef]
- Ding, X.-F.; Shen, M.; Xu, L.-Y.; Dong, J.-H.; Chen, G. 13,14-biscis-3,5-dimethyl-1-piperazinyl-β-elemene, a novel β-elemene derivative, shows potent antitumor activities via inhibition of mTOR in human breast cancer cells. Oncol. Lett. 2013, 5, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, T.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Novel hybrids of natural β-elemene bearing isopropanolamine moieties: Synthesis, enhanced anticancer profile, and improved aqueous solubility. Fitoterapia 2017, 120, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Xu, L.-Y.; Tao, S.-J.; Wang, X.-M.; Chen, G.; Wang, M.-W.; Dong, J.-H. Synthesis and antitumor activity of β-elemene-13-yl esters. Chin. J. Med. Chem. 2007, 17, 13–17. [Google Scholar]
- Chen, J.-C.; Duan, W.-L.; Bai, R.-R.; Yao, H.Q.; Wu, X.-M.; Shang, J.; Xu, J.-Y. Synthesis of 13-β-elemene ester derivatives and evaluation of their antioxidant activity in human umbilical vein endothelial cells. Chin. J. Nat. Med. 2015, 13, 0618–0627. [Google Scholar] [CrossRef]
- Liu, G.; Kong, Z.; Shen, Y. Synthesis, characterization, and in vitro antiproliferative activity of novel β-elemene monosubstituted derivatives. Med. Chem. Res. 2013, 22, 3536–3540. [Google Scholar] [CrossRef]
- Chen, J.; Duan, W.; Bai, R.; Yao, H.; Shang, J.; Xu, J. Design, synthesis and antioxidant activity evaluation of novel β-elemene derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 3407–3411. [Google Scholar] [CrossRef]
- Xu, L.-Y.; Zhang, X.-Z.; Du, H.-L.; Tao, S.-J.; Wang, X.-M.; Chen, G.; Dong, J.-H. Synthesis and antitumor activity of β-elemene derivatives bearing amino acid moiety. Chin. J. Med. Chem. 2013, 23, 169–175. [Google Scholar]
- Yu, Z.; Wu, F.; Chen, L.; Li, Q.; Wang, C.; Dong, J.; Xie, S.-Q. ETME, a novel β-elemene derivative, synergizes with arsenic trioxide in inducing apoptosis and cell cycle arrest in hepatocarcinoma cells via a p53-dependent pathway. Acta Pharm. Sin. B 2014, 4, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, L.; Vosseller, K.; Hart, G.W. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science 2001, 291, 2376–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.-Y.; Zhang, S.-J.; Zheng, X.-F.; Yin, H.-X. Synthesis of β-Elemene Glycoside Derivatives. Chin. J. Org. Chem. 2008, 28, 1797–1802. [Google Scholar]
- Yang, L.-Y.; Zhang, S.-J.; Zheng, X.-F.; Yin, H.-X. Design and Synthesis of β-Elemene Glycoside Derivatives Containing heteratom S or Se. Chem. J. Chinese U. 2008, 29, 2187–2190. [Google Scholar]
- Sun, Y.; Ren, Y.; Zhu, H.; Zhang, Y.; Liu, G.; Zhang, C.; Huang, L.; Xu, J.; Qi, Y.; Shen, Y.-M. Radioactive synthesis and biodistribution study of beta-elemene-99mTcCO3 conjugates. J. Biol. Inorg. Chem. 2009, 14, 899–904. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xu, C.; Huang, J.; Wang, C.; Wang, X.; He, L.; Ling, Y. Synthesis and biological evaluation of nitric oxide-releasing hybrids from gemcitabine and phenylsulfonyl furoxans as anti-tumor agents. MedChemComm 2015, 6, 1130–1136. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, M.; Cerecetto, H.; Fernández, M.; González, M.; León, E.; Pintos, C.; Raymondo, S.; Arredondo, C.; Pacheco, J.P.; et al. Furoxan derivatives as cytotoxic agents: Preliminary in vivo antitumoral activity studies. Pharmazie 2006, 61, 54–59. [Google Scholar]
- Chen, J.; Wang, T.; Xu, S.; Zhang, P.; Lin, A.; Wu, L.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Discovery of novel antitumor nitric oxide-donating β-elemene hybrids through inhibiting the PI3K/Akt pathway. Eur. J. Med. Chem. 2017, 135, 414–423. [Google Scholar] [CrossRef]




| Elemene Oral Emulsion | Elemene Injection | ||
|---|---|---|---|
| Ingredient list | 85% β-elemene and 15% γ- and δ-elemene | 85% β-elemene and 15% γ- and δ-elemene | |
| Description | opalescent homogeneous emulsion | opalescent homogeneous emulsion | |
| Indication | for adjuvant treatment of esophageal cancer and gastric cancer to improve symptoms | lung cancer, liver cancer, esophageal cancer, nasopharyngeal cancer, brain cancer, and bone metastasis; intervention, intracavitary chemotherapy, and carcinoma hydrothorax treatment | |
| Side effects | digestive tract reactions: nausea, vomiting, diarrhea, and occasionally loss of appetite; decreased hemoglobin; decreased leukocytes | phlebitis; fever; local pain; allergic reaction; mild digestive tract reaction | |
| Specification | 20 mL: 176 mg | 20 mL: 88 mg | |
| Pharmacokinetics [5,6] | t1/2 = 65 min (i.v.) t1/2 = 126 min (i.p.) | bioavailability = 18.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.-Y.; Bai, R.; Xie, T. Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification. Molecules 2021, 26, 1499. https://doi.org/10.3390/molecules26061499
Bai Z, Yao C, Zhu J, Xie Y, Ye X-Y, Bai R, Xie T. Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification. Molecules. 2021; 26(6):1499. https://doi.org/10.3390/molecules26061499
Chicago/Turabian StyleBai, Ziqiang, Chuansheng Yao, Junlong Zhu, Yuanyuan Xie, Xiang-Yang Ye, Renren Bai, and Tian Xie. 2021. "Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification" Molecules 26, no. 6: 1499. https://doi.org/10.3390/molecules26061499









