Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders
Abstract
:1. Introduction
2. Research Methodology
3. Physico-Chemical Properties of GT
3.1. Chemical Composition and Structure of GT

3.2. Degradation of GT
3.3. Modification of GT
4. GT in Green Chemistry
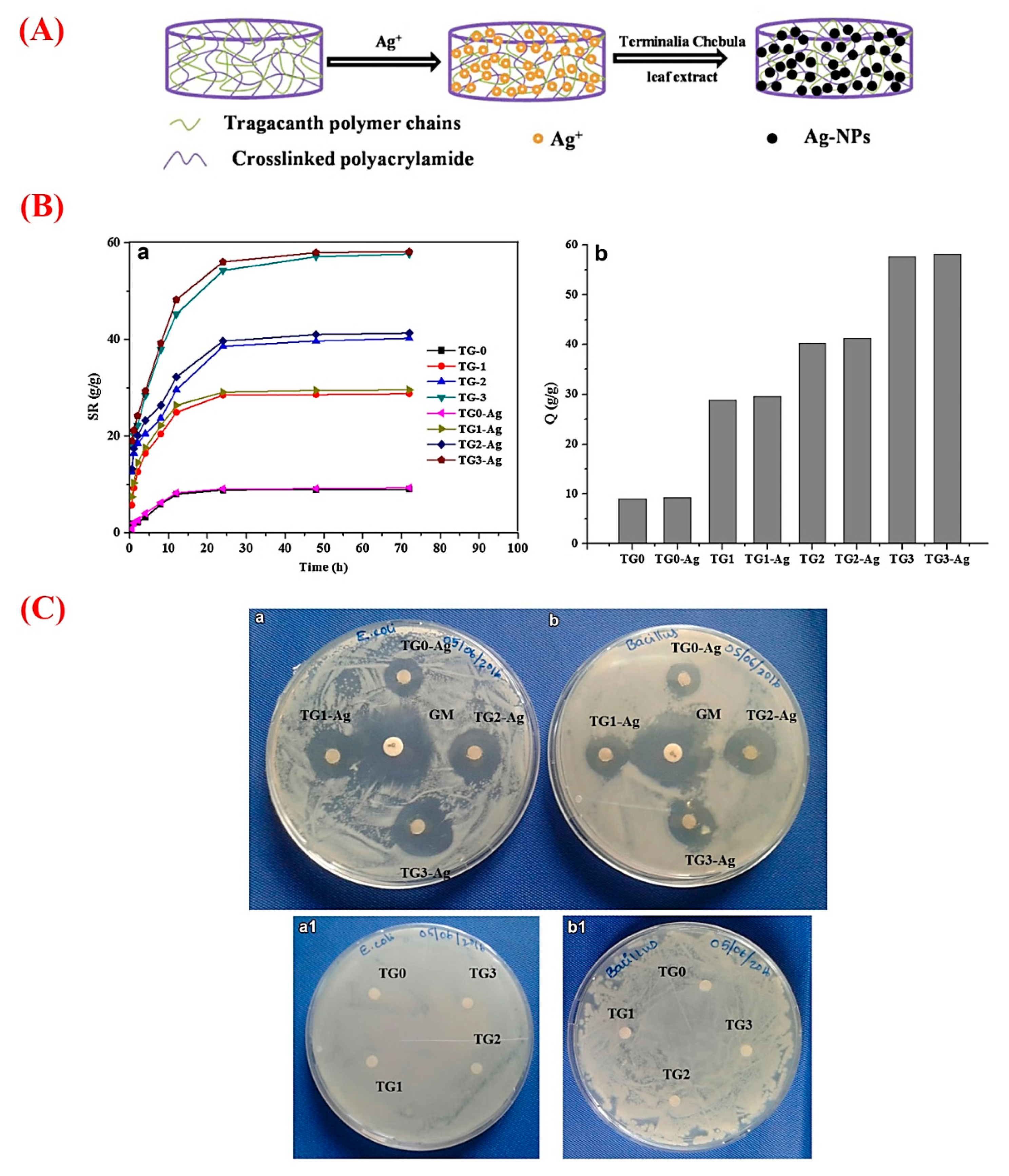
5. GT for Wastewater Treatment
6. GT for Drug Delivery Strategies
7. GT in Tissue Engineering and Regenerative Medicine
7.1. In Vitro Cell Interactions
7.2. Hard Tissue Regeneration
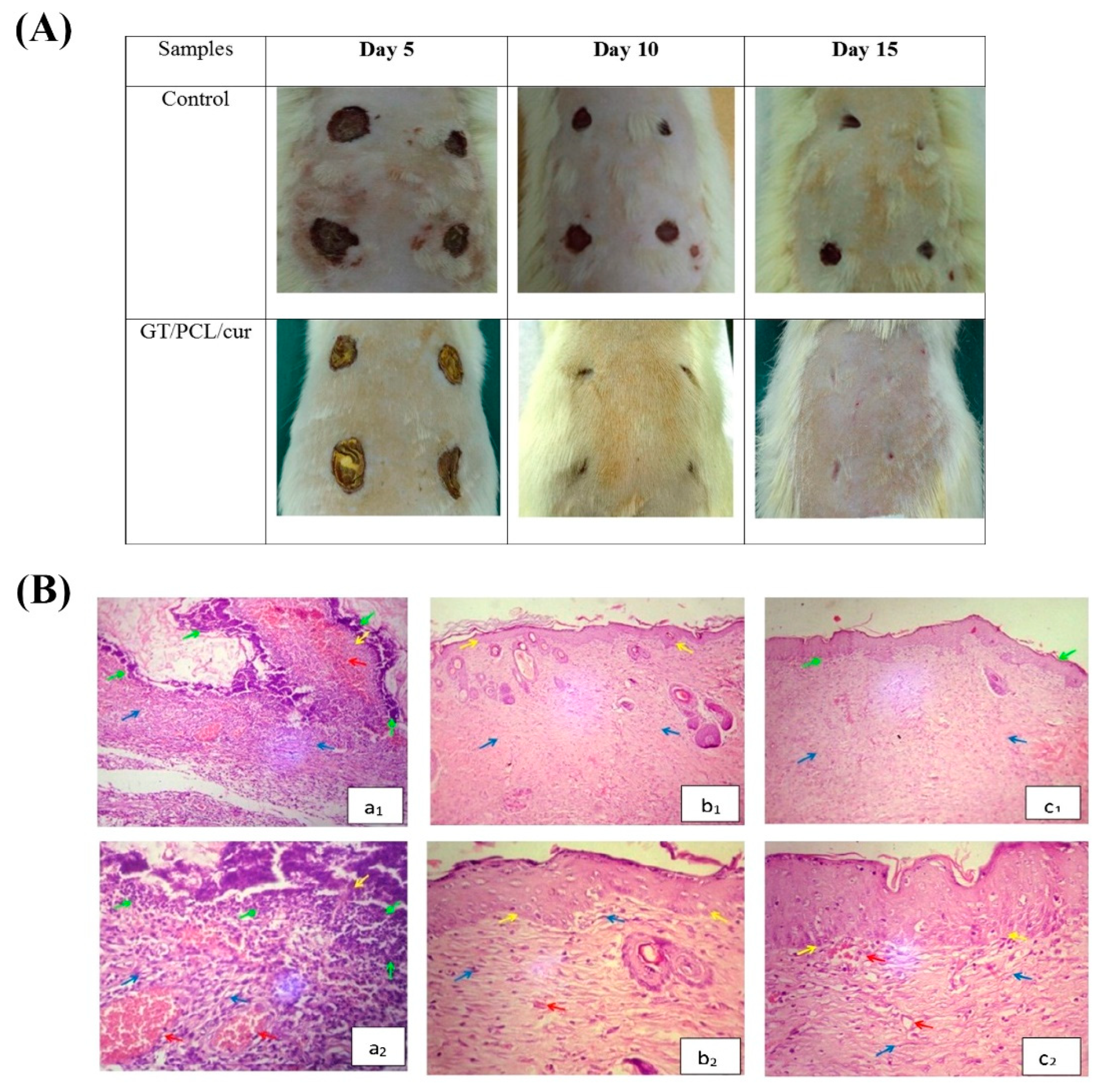
7.3. Soft Tissue Healing
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, D.; Grant, D. The chemical characterization of some Astragalus gum exudates. Food Hydrocoll. 1988, 2, 417–423. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R.; TaghavizadehYazdi, M.E. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad, Iran. Iran. J. Pharm. Res. 2014, 13, 157. [Google Scholar] [PubMed]
- Gentry, H.S. Gum tragacanth in Iran. Econ. Bot. 1957, 11, 40–63. [Google Scholar] [CrossRef]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A.; Gavlighi, H.A.; Mohammadi, M. Compositional analysis and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Hydrocoll. 2011, 25, 1775–1784. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Taneja, S. Exudate gums: Chemistry, properties and food applications—A review. J. Sci. Food Agric. 2020, 100, 2828–2835. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R.; Nadaf, M.; Nasseh, Y. Ethnobotanical knowledge of Astragalus spp.: The world′s largest genus of vascular plants. Avicenna J. Phytomed. 2020, 10, 128–142. [Google Scholar] [PubMed]
- Zarshenas, M.M.; Arabzadeh, A.; Tafti, M.A.; Kordafshari, G.; Zargaran, A.; Mohagheghzadeh, A. Application of herbal exudates in traditional Persian medicine. Galen Med. J. 2013, 1, 78–83. [Google Scholar]
- Whistler, R.L. Exudate gums. In Industrial Gums; Elsevier: Amsterdam, The Netherlands, 1993; pp. 309–339. [Google Scholar]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Razavi-Nouri, M. Tragacanth gum-graft-polyacrylonitrile: Synthesis, characterization and hydrolysis. J. Polym. Res. 2008, 15, 173–180. [Google Scholar] [CrossRef]
- Nazarzadeh, E.Z.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Hamedi, A.; Yousefi, G.; Farjadian, S.; Bour, M.S.B.; Parhizkar, E. Physicochemical and Immunomodulatory Properties of Gum Exudates Obtained from Astragalus myriacanthus and Some of Its Isolated Carbohydrate Biopolymers. Iran. J. Pharm. Res. 2017, 16, 1520. [Google Scholar]
- Kaith, B.S.; Jindal, R.; Kumar, V. Biodegradation of Gum tragacanth acrylic acid based hydrogel and its impact on soil fertility. Polym. Degrad. Stab. 2015, 115, 24–31. [Google Scholar]
- Singh, B.; Varshney, L.; Francis, S. Synthesis and characterization of tragacanth gum based hydrogels by radiation method for use in wound dressing application. Radiat. Phys. Chem. 2017, 135, 94–105. [Google Scholar] [CrossRef]
- Singh, B.; Varshney, L.; Francis, S. Designing tragacanth gum based sterile hydrogel by radiation method for use in drug delivery and wound dressing applications. Int. J. Biol. Macromol. 2016, 88, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Haeri, S.M.J.; Sadeghi, Y.; Salehi, M.; Farahani, R.M.; Mohsen, N. Osteogenic differentiation of human adipose-derived mesenchymal stem cells on gum tragacanth hydrogel. Biologicals 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kulanthaivel, S.; Sharan Rathnam, V.S.; Agarwal, T.; Pradhan, S.; Pal, K.; Giri, S.; Maiti, T.K.; Banerjee, I. Gum tragacanth–alginate beads as proangiogenic–osteogenic cell encapsulation systems for bone tissue engineering. J. Mater. Chem. B 2017, 5, 4177–4189. [Google Scholar] [CrossRef]
- Podlech, D.; Zarre, S. A Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World; Vienna Natural History Museum/Naturhistorisches Museum Wien: Vienna, Austria, 2013; Volume 2, pp. 1039–1150. [Google Scholar]
- Gavlighi, H.A.; Meyer, A.S.; Mikkelsen, J.D. Tragacanth gum: Functionality and prebiotic potential. Agro Food Ind. Hi Tech 2013, 24, 46–48. [Google Scholar]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A. Physicochemical and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Biophys. 2010, 5, 59–71. [Google Scholar] [CrossRef]
- Gorji, S.G.; Gorji, E.G.; Mohammadifar, M.A.; Zargaraan, A. Complexation of sodium caseinate with gum tragacanth: Effect of various species and rheology of coacervates. Int. J. Biol. Macromol. 2014, 67, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, A.; Sadrjavadi, K.; Golozar, M.A.; Varshosaz, J.; Fathi, M.-H.; Mirmohammad-Sadeghi, H. Preparation and characterization of oligochitosan–tragacanth nanoparticles as a novel gene carrier. Carbohydr. Polym. 2013, 97, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum Tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Chiantore, O.; Riedo, C.; Scalarone, D. Gas chromatography–mass spectrometric analysis of products from on-line pyrolysis/silylation of plant gums used as binding media. Int. J. Mass Spectrom. 2009, 284, 35–41. [Google Scholar] [CrossRef]
- Kurt, A. Physicochemical, rheological and structural characteristics of alcohol precipitated fraction of gum tragacanth. Food Health 2018, 4, 183–193. [Google Scholar] [CrossRef]
- Tischer, C.A.; Iacomini, M.; Gorin, P.A. Structure of the arabinogalactan from gum tragacanth (Astralagus gummifer). Carbohydr. Res. 2002, 337, 1647–1655. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Meyer, A.S.; Zaidel, D.N.; Mohammadifar, M.A.; Mikkelsen, J.D. Stabilization of emulsions by gum tragacanth (Astragalus spp.) correlates to the galacturonic acid content and methoxylation degree of the gum. Food Hydrocoll. 2013, 31, 5–14. [Google Scholar] [CrossRef]
- Zohuriaan, M.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Silva, C.; Torres, M.; Chenlo, F.; Moreira, R. Rheology of aqueous mixtures of tragacanth and guar gums: Effects of temperature and polymer ratio. Food Hydrocoll. 2017, 69, 293–300. [Google Scholar] [CrossRef]
- Torres, M.D.; Moreira, R.; Chenlo, F.; Vázquez, M.J. Water adsorption isotherms of carboxymethyl cellulose, guar, locust bean, tragacanth and xanthan gums. Carbohydr. Polym. 2012, 89, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, M.; Moghadam, P.N. Synthesis of a new superabsorbent copolymer based on acrylic acid grafted onto carboxymethyl tragacanth. Carbohydr. Polym. 2018, 202, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.; Dehshiri, S.; Vasheghani-Farahani, E. Electron beam irradiation crosslinked hydrogels based on tyramine conjugated gum tragacanth. Carbohydr. Polym. 2016, 152, 504–509. [Google Scholar] [CrossRef]
- Tavakol, M.; Vasheghani-Farahani, E.; Mohammadifar, M.A.; Soleimani, M.; Hashemi-Najafabadi, S. Synthesis and characterization of an in situ forming hydrogel using tyramine conjugated high methoxyl gum tragacanth. J. Biomater. Appl. 2016, 30, 1016–1025. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Influence of polymer network parameters of tragacanth gum-based pH responsive hydrogels on drug delivery. Carbohydr. Polym. 2014, 101, 928–940. [Google Scholar] [CrossRef]
- Wang, W. Tragacanth and karaya. In Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2000; pp. 231–246. [Google Scholar]
- Kaith, B.S.; Jindal, R.; Sharma, R. Study of ionic charge dependent salt resistant swelling behavior and removal of colloidal particles using reduced gum rosin-poly (acrylamide)-based green flocculant. Iran. Polym. J. 2016, 25, 349–362. [Google Scholar] [CrossRef]
- Kolangi, F.; Memariani, Z.; Bozorgi, M.; Mozaffarpur, S.A.; Mirzapour, M. Herbs with Potential Nephrotoxic Effects According to the Traditional Persian Medicine: Review and Assessment of Scientific Evidence. Curr. Drug Metab. 2018, 19, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Abari, A.; Emtiazi, G.; Jazini, M.; Kim, J.; Kim, B.G. LC/MS detection of oligogalacturonic acids obtained from tragacanth degradation by pectinase producing bacteria. J. Basic Microbiol. 2019, 59, 249–255. [Google Scholar] [CrossRef]
- Raoufi, N.; Kadkhodaee, R.; Fang, Y.; Phillips, G.O. Ultrasonic degradation of Persian gum and gum tragacanth: Effect on chain conformation and molecular properties. Ultrason. Sonochem. 2019, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Gavlighi, H.A.; Michalak, M.; Meyer, A.S.; Mikkelsen, J.D. Enzymatic depolymerization of gum tragacanth: Bifidogenic potential of low molecular weight oligosaccharides. J. Agric. Food Chem. 2013, 61, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alijani, S.; Balaghi, S.; Mohammadifar, M.A. Effect of gamma irradiation on rheological properties of polysaccharides exuded by A. fluccosus and A. gossypinus. Int. J. Biol. Macromol. 2011, 49, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Sahraei, R.; Ghaemy, M. Preparation of spherical porous hydrogel beads based on ion-crosslinked gum tragacanth and graphene oxide: Study of drug delivery behavior. Carbohydr. Polym. 2018, 194, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Novel superabsorbent biosensor nanohydrogel based on gum tragacanth polysaccharide for optical detection of glucose. Int. J. Biol. Macromol. 2020, 151, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Shahbazi, M.; Asempour, H. Hydrogel membranes based on gum tragacanth with tunable structure and properties. I. Preparation method using Taguchi experimental design. J. Appl. Polym. Sci. 2012, 124, 99–108. [Google Scholar] [CrossRef]
- Niknia, N.; Kadkhodaee, R. Factors affecting microstructure, physicochemical and textural properties of a novel Gum tragacanth-PVA blend cryogel. Carbohydr. Polym. 2017, 155, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Verma, C.; Negi, P.; Tyagi, I.; Asif, M.; Kumar, N.S.; Al-Ghurabi, E.H.; Agarwal, S.; Gupta, V.K. Novel nanohydrogel based on itaconic acid grafted tragacanth gum for controlled release of ampicillin. Carbohydr. Polym. 2018, 196, 262–271. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Taghavi Fardood, S.; Ramazani, A.; Golfar, Z.; Joo, S.W. Green synthesis of Ni-Cu-Zn ferrite nanoparticles using tragacanth gum and their use as an efficient catalyst for the synthesis of polyhydroquinoline derivatives. Appl. Organomet. Chem. 2017, 31, e3823. [Google Scholar] [CrossRef]
- Kora, A.J.; Arunachalam, J. Green fabrication of silver nanoparticles by gum Tragacanth (Astragalus gummifer): A dual functional reductant and stabilizer. J. Nanomater. 2012, 2012, 69. [Google Scholar] [CrossRef] [Green Version]
- Ghayempour, S.; Montazer, M.; Rad, M.M. Tragacanth gum biopolymer as reducing and stabilizing agent in biosonosynthesis of urchin-like ZnO nanorod arrays: A low cytotoxic photocatalyst with antibacterial and antifungal properties. Carbohydr. Polym. 2016, 136, 232–241. [Google Scholar] [CrossRef]
- Darroudi, M.; Sarani, M.; Oskuee, R.K.; Zak, A.K.; Amiri, M.S. Nanoceria: Gum mediated synthesis and in vitro viability assay. Ceram. Int. 2014, 40, 2863–2868. [Google Scholar] [CrossRef]
- Waghmare, P.R.; Watharkar, A.D.; Jeon, B.-H.; Govindwar, S.P. Bio-ethanol production from waste biomass of Pogonatherum crinitum phytoremediator: An eco-friendly strategy for renewable energy. 3 Biotech 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, M.; Taylor, R.; Cozens, P.; Manson-Whitton, C. Production of BioSNG from waste derived syngas: Pilot plant operation and preliminary assessment. Waste Manag. 2018, 79, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Tsiliyannis, C.A. Energy from waste: Plant design and control options for high efficiency and emissions’ compliance under waste variability. Energy 2019, 176, 34–57. [Google Scholar] [CrossRef]
- Deniz, F.; Yildiz, H. Bioremediation potential of a widespread industrial biowaste as renewable and sustainable biosorbent for synthetic dye pollution. Int. J. Phytoremediat. 2019, 21, 259–267. [Google Scholar] [CrossRef]
- Hemmati, K.; Masoumi, A.; Ghaemy, M. Tragacanth gum-based nanogel as a superparamagnetic molecularly imprinted polymer for quercetin recognition and controlled release. Carbohydr. Polym. 2016, 136, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, A.; Ghaemy, M. Removal of metal ions from water using nanohydrogel tragacanth gum-g-polyamidoxime: Isotherm and kinetic study. Carbohydr. Polym. 2014, 108, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Rahimdokht, M.; Pajootan, E. Low cost hydrogels based on gum Tragacanth and TiO2 nanoparticles: Characterization and RBFNN modelling of methylene blue dye removal. Int. J. Biol. Macromol. 2019, 134, 967–975. [Google Scholar] [CrossRef]
- Mohammadian, M.; Sahraei, R.; Ghaemy, M. Synthesis and fabrication of antibacterial hydrogel beads based on modified-gum tragacanth/poly (vinyl alcohol)/Ag0 highly efficient sorbent for hard water softening. Chemosphere 2019, 225, 259–269. [Google Scholar] [CrossRef]
- Kumar, V.; Mittal, H.; Alhassan, S.M. Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. Int. J. Biol. Macromol. 2019, 132, 1252–1261. [Google Scholar]
- Shojaipour, M.; Ghaemy, M.; Amininasab, S.M. Removal of NO3− ions from water using bioadsorbent based on gum tragacanth carbohydrate biopolymer. Carbohydr. Polym. 2020, 227, 115367. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Tavakol, M. Synthesis of composite hydrogel of glutamic acid, gum tragacanth, and anionic polyacrylamide by electron beam irradiation for uranium (VI) removal from aqueous samples: Equilibrium, kinetics, and thermodynamic studies. Carbohydr. Polym. 2019, 206, 352–361. [Google Scholar] [CrossRef]
- Sahraei, R.; Pour, Z.S.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Jazi, M.E.; Allahrasani, A.; Ganjali, M.R.; Badiei, A. Removal of acid dyes from aqueous solutions using a new eco-friendly nanocomposite of CoFe2O4 modified with Tragacanth gum. J. Appl. Polym. Sci. 2020, 137, 48605. [Google Scholar] [CrossRef]
- Malviya, R.; Srivastava, P.; Kulkarni, G. Applications of mucilages in drug delivery-A review. Adv. Biol. Res. 2011, 5, 1–7. [Google Scholar]
- Hosseini, M.S.; Hemmati, K.; Ghaemy, M. Synthesis of nanohydrogels based on tragacanth gum biopolymer and investigation of swelling and drug delivery. Int. J. Biol. Macromol. 2016, 82, 806–815. [Google Scholar] [CrossRef]
- Cikrikci, S.; Mert, B.; Oztop, M.H. Development of pH sensitive alginate/gum tragacanth based hydrogels for oral insulin delivery. J. Agric. Food Chem. 2018, 66, 11784–11796. [Google Scholar] [CrossRef]
- Sheorain, J.; Mehra, M.; Thakur, R.; Grewal, S.; Kumari, S. In vitro anti-inflammatory and antioxidant potential of thymol loaded bipolymeric (tragacanth gum/chitosan) nanocarrier. Int. J. Biol. Macromol. 2019, 125, 1069–1074. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sood, S.; Agarwal, S.; Saini, A.K.; Pathania, D. Antioxidant activity and controlled drug delivery potential of tragacanth gum-cl-poly (lactic acid-co-itaconic acid) hydrogel. Int. J. Biol. Macromol. 2018, 107, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Ghayempour, S.; Montazer, M.; Rad, M.M. Tragacanth gum as a natural polymeric wall for producing antimicrobial nanocapsules loaded with plant extract. Int. J. Biol. Macromol. 2015, 81, 514–520. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M. Production of cotton fabrics with durable antibacterial property by using gum tragacanth and silver. Int. J. Biol. Macromol. 2018, 109, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Rao, K.S.V.K.; Haider, A.; Han, S.S. Biodegradable tragacanth gum based silver nanocomposite hydrogels and their antibacterial evaluation. J. Polym. Environ. 2018, 26, 778–788. [Google Scholar] [CrossRef]
- Verma, C.; Negi, P.; Pathania, D.; Anjum, S.; Gupta, B. Novel Tragacanth Gum-Entrapped lecithin nanogels for anticancer drug delivery. Int. J. Polym. Mater. 2019, 69, 604–609. [Google Scholar] [CrossRef]
- Apoorva, A.; Rameshbabu, A.P.; Dasgupta, S.; Dhara, S.; Padmavati, M. Novel pH-sensitive alginate hydrogel delivery system reinforced with gum tragacanth for intestinal targeting of nutraceuticals. Int. J. Biol. Macromol. 2020, 147, 675–687. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. A novel controlled release system based on Tragacanth nanofibers loaded Peppermint oil. Carbohydr. Polym. 2019, 205, 589–595. [Google Scholar] [CrossRef]
- Dehcheshmeh, M.A.; Fathi, M. Production of core-shell nanofibers from zein and tragacanth for encapsulation of saffron extract. Int. J. Biol. Macromol. 2019, 122, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Cipolla, L. Glycomics: New challenges and opportunities in regenerative medicine. Chem. Eur. J. 2016, 22, 13380–13388. [Google Scholar] [CrossRef]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for tissue engineering: Current landscape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive—A review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Hagiwara, A.; Boonyaphiphat, P.; Kawabe, M.; Naito, H.; Shirai, T.; Ito, N. Lack of carcinogenicity of tragacanth gum in B6C3F1 mice. Food Chem. Toxicol. 1992, 30, 673–679. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, K.; Dutt, S. Dietary fiber tragacanth gum based hydrogels for use in drug delivery applications. Bioact. Carbohydr. Diet. Fibre 2020, 21, 100208. [Google Scholar] [CrossRef]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Yadava, N.; Chambers, J.J.; Thayumanavan, S. Anionic polymers promote mitochondrial targeting of delocalized lipophilic cations. Bioconjug. Chem. 2020, 31, 1344–1353. [Google Scholar] [CrossRef]
- Wang, T.; Jones, J.D.; Niyonshuti, I.I.; Agrawal, S.; Gundampati, R.K.; Kumar, T.K.S.; Quinn, K.P.; Chen, J. Biocompatible, Injectable Anionic Hydrogels Based on Poly (Oligo Ethylene Glycol Monoacrylate-co-Acrylic Acid) for Protein Delivery. Adv. Ther. 2019, 2, 1900092. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, H.; Qiao, F.; Huang, W.; Bao, X.; Wang, Z.; Hu, Q. Fabrication of alginate-P (SBMA-co-AAm) hydrogels with ultrastretchability, strain sensitivity, self-adhesiveness, biocompatibility, and self-cleaning function for strain sensors. J. Appl. Polym. Sci. 2021, 138, 49697. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Brouki Milan, P.; Kim, H.-W.; Baino, F. Bone tissue engineering using human cells: A comprehensive review on recent trends, current prospects, and recommendations. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Seifalian, A.; Ghanbari, H. Polyurethane-polycaprolactone blend patches: Scaffold characterization and cardiomyoblast adhesion, proliferation, and function. ACS Biomater. Sci. Eng. 2018, 4, 4299–4310. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Verdi, J.; Asadpour, S.; Mozafari, M. Curcumin: Footprints on cardiac tissue engineering. Expert Opin. Biol. Ther. 2019, 19, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.; Kim, H.W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. BioFactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- López-Cebral, R.; Civantos, A.; Ramos, V.; Seijo, B.; López-Lacomba, J.L.; Sanz-Casado, J.V.; Sanchez, A. Gellan gum based physical hydrogels incorporating highly valuable endogen molecules and associating bmp-2 as bone formation platforms. Carbohydr. Polym. 2017, 167, 345–355. [Google Scholar] [CrossRef]
- Maia, F.R.; Musson, D.S.; Naot, D.; da Silva, L.P.; Bastos, A.R.; Costa, J.B.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Cornish, J. Differentiation of osteoclast precursors on gellan gum-based spongy-like hydrogels for bone tissue engineering. Biomed. Mater. 2018, 13, 035012. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.A.; Sundareswari, M.; Ravichandran, K.; Latha, B.; Sagadevan, S. Fabrication and characterization of porous scaffolds for bone replacements using gum tragacanth. Mater. Sci. Eng. C 2019, 96, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef]
- Ranjbar Mohammadi, M.; Kargozar, S.; Bahrami, S.H.; Rabbani, S. An excellent nanofibrous matrix based on gum tragacanth-poly (Ɛ-caprolactone)-poly (vinyl alcohol) for application in diabetic wound healing. Polym. Degrad. Stab. 2020, 174, 109105. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Gum tragacanth/poly (l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016, 140, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fayazzadeh, E.; Rahimpour, S.; Ahmadi, S.M.; Farzampour, S.; Anvari, M.S.; Boroumand, M.A.; Ahmadi, S.H. Acceleration of skin wound healing with tragacanth (Astragalus) preparation: An experimental pilot study in rats. Acta Med. Iran. 2014, 52, 3–8. [Google Scholar] [PubMed]
- Zarekhalili, Z.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int. J. Biol. Macromol. 2017, 94, 679–690. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Pathania, D.; Anjum, S.; Gupta, B. Smart Designing of Tragacanth Gum by Graft Functionalization for Advanced Materials. Macromol. Mater. Eng. 2020, 305, 1900762. [Google Scholar] [CrossRef]
- Anderson, D. Evidence for the safety of gum tragacanth (Asiatic Astragalus spp.) and modern criteria for the evaluation of food additives. Food Addit. Contam. 1989, 6, 1–12. [Google Scholar] [CrossRef]
- Heydary, H.A.; Karamian, E.; Poorazizi, E.; Heydaripour, J.; Khandan, A. Electrospun of polymer/bioceramic nanocomposite as a new soft tissue for biomedical applications. J. Asian Ceram. Soc. 2015, 3, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Novikova, E.A.; Raab, M.; Discher, D.E.; Storm, C. Persistence driven durotaxis: Generic, directed motility in rigidity gradients. Phys. Rev. Lett. 2017, 118, 078103. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavizadeh Yazdi, M.E.; Nazarnezhad, S.; Mousavi, S.H.; Sadegh Amiri, M.; Darroudi, M.; Baino, F.; Kargozar, S. Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders. Molecules 2021, 26, 1510. https://doi.org/10.3390/molecules26061510
Taghavizadeh Yazdi ME, Nazarnezhad S, Mousavi SH, Sadegh Amiri M, Darroudi M, Baino F, Kargozar S. Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders. Molecules. 2021; 26(6):1510. https://doi.org/10.3390/molecules26061510
Chicago/Turabian StyleTaghavizadeh Yazdi, Mohammad Ehsan, Simin Nazarnezhad, Seyed Hadi Mousavi, Mohammad Sadegh Amiri, Majid Darroudi, Francesco Baino, and Saeid Kargozar. 2021. "Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders" Molecules 26, no. 6: 1510. https://doi.org/10.3390/molecules26061510
APA StyleTaghavizadeh Yazdi, M. E., Nazarnezhad, S., Mousavi, S. H., Sadegh Amiri, M., Darroudi, M., Baino, F., & Kargozar, S. (2021). Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders. Molecules, 26(6), 1510. https://doi.org/10.3390/molecules26061510






