Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression of SMO
2.2. High Cell Density Fermentation
2.3. Isolation and Characterisation of Recombinant SMO
2.3.1. Purification of Recombinant SMO
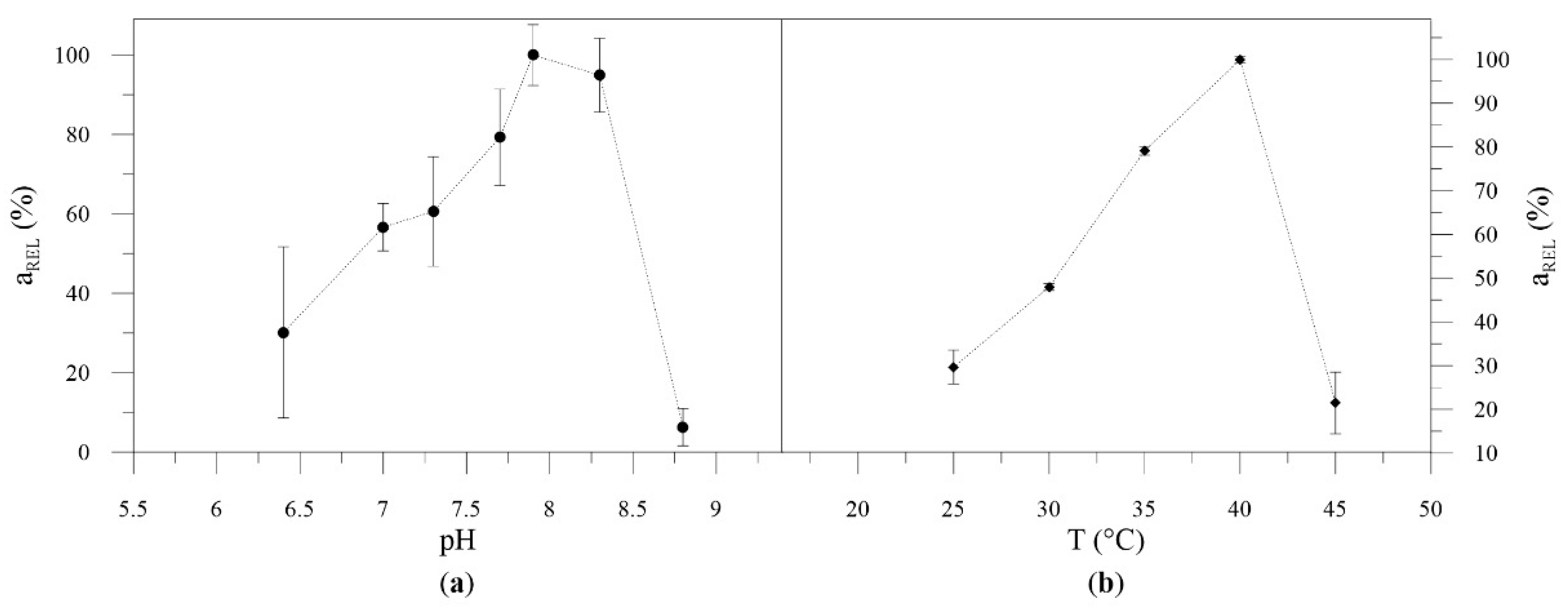
2.3.2. Determination of Optimal pH, Temperature, and Storage Conditions
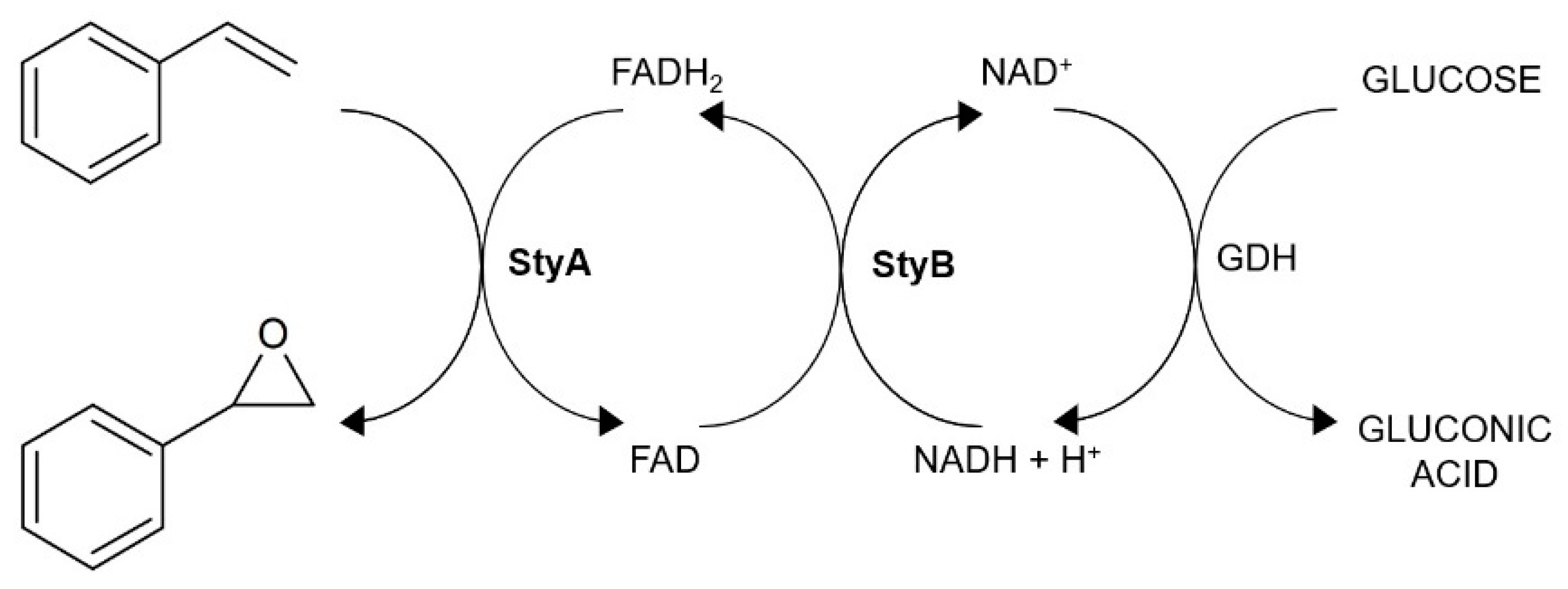
2.4. Biotransformation of Alkenes
2.5. Upscale Production of Chiral Epoxides
Evaluation of Chiral Epoxides
3. Materials and Methods
3.1. Chemicals and Media
3.2. Construction of Expression Vectors
3.3. Expression of SMO
3.4. High Cell Density Fermentation
3.5. Activity Assay
3.6. Purification of Recombinant SMO
pH Profile and Temperature Profile
3.7. Biotransformation of Alkenes
3.8. Analytics
3.8.1. High-Performance Liquid Chromatography
3.8.2. Gas Chromatography
3.9. Isolation and Characterisation of Chiral Epoxides
3.9.1. General Information
3.9.2. General Procedure for the Isolation of Chiral Epoxides. Isolation of (S)-4-Chlorostyrene Oxide


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Oelschlagel, M.; Zimmerling, J.; Tischler, D. A Review: The Styrene Metabolizing Cascade of Side-Chain Oxygenation as Biotechnological Basis to Gain Various Valuable Compounds. Front. Microbiol. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Cui, C.; Guo, C.; Wu, Z.-L. Characterization of two styrene monooxygenases from marine microbes. Enzym. Microb. Technol. 2018, 112, 29–34. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, M.; Jeong, Y.J.; Park, S.; Lee, S.-G. Colorimetric monitoring of the activity of recombinant Escherichia coli expressing styrene monooxygenase. J. Ind. Eng. Chem. 2009, 15, 520–523. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, X.; Zhu, Z.; Xu, M.; Yang, T.; Osire, T.; Yang, S.; Rao, Z. Asp305Gly mutation improved the activity and stability of the styrene monooxygenase for efficient epoxide production in Pseudomonas putida KT2440. Microb. Cell Factories 2019, 18, 12. [Google Scholar] [CrossRef]
- Qaed, A.A.; Lin, H.; Tang, D.F.; Wu, Z.L. Rational design of styrene monooxygenase mutants with altered substrate preference. Biotechnol. Lett. 2011, 33, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.-C.; Wu, Z.-L. Asymmetric bio-epoxidation catalyzed with the styrene monooxygenase from Pseudomonas sp. LQ26. Bioresour. Bioprocess. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Heine, T.; van Berkel, W.J.H.; Gassner, G.; van Pee, K.H.; Tischler, D. Two-Component FAD-Dependent Monooxygenases: Current Knowledge and Biotechnological Opportunities. Biology 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panke, S.; Witholt, B.; Schmid, A.; Wubbolts, M.G. Towards a biocatalyst for (S)-styrene oxide production: Characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl. Environ. Microbiol. 1998, 64, 2032–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panke, S.; de Lorenzo, V.; Kaiser, A.; Witholt, B.; Wubbolts, M.G. Engineering of a stable whole-cell biocatalyst capable of (S)-styrene oxide formation for continuous two-liquid-phase applications. Appl. Environ. Microbiol. 1999, 65, 5619–5623. [Google Scholar] [CrossRef] [Green Version]
- Panke, S.; Wubbolts, M.G.; Schmid, A.; Witholt, B. Production of enantiopure styrene oxide by recombinant Escherichia coli synthesizing a two-component styrene monooxygenase. Biotechnol. Bioeng. 2000, 69, 91–100. [Google Scholar] [CrossRef]
- Panke, S.; Held, M.; Wubbolts, M.G.; Witholt, B.; Schmid, A. Pilot-scale production of (S)-styrene oxide from styrene by recombinant Escherichia coli synthesizing styrene monooxygenase. Biotechnol. Bioeng. 2002, 80, 33–41. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Colmegna, A.; Galli, E.; Sello, G.; Pelizzoni, F.; Bestetti, G. A new biocatalyst for production of optically pure aryl epoxides by styrene monooxygenase from Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 1999, 65, 2794–2797. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Qiao, J.; Liu, Y.; Wu, Z.-L. Styrene monooxygenase from Pseudomonas sp. LQ26 catalyzes the asymmetric epoxidation of both conjugated and unconjugated alkenes. J. Mol. Catal. B Enzym. 2010, 67, 236–241. [Google Scholar] [CrossRef]
- Lin, H.; Xu, M.-Y.; Liu, Y.; Wu, Z.-L. Biocatalytic Epoxidation for Green Synthesis. Green Biocatal. 2016. [Google Scholar] [CrossRef]
- Otto, K.; Hofstetter, K.; Rothlisberger, M.; Witholt, B.; Schmid, A. Biochemical characterization of StyAB from Pseudomonas sp. strain VLB120 as a two-component flavin-diffusible monooxygenase. J. Bacteriol. 2004, 186, 5292–5302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, T.; Tucker, K.; Okonkwo, N.; Assefa, B.; Conrad, C.; Scholtissek, A.; Schlomann, M.; Gassner, G.; Tischler, D. Engineering Styrene Monooxygenase for Biocatalysis: Reductase-Epoxidase Fusion Proteins. Appl. Biochem. Biotechnol. 2017, 181, 1590–1610. [Google Scholar] [CrossRef] [PubMed]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Kesseler, M.; Sturmer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem. 2004, 43, 788–824. [Google Scholar] [CrossRef]
- Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J.J. Asymmetric Synthesis of Active Pharmaceutical Ingredients. Chem. Rev. 2006, 106, 2734–2793. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.E.; Dobson, A.D.; Hartmans, S. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl. Environ. Microbiol. 1997, 63, 4287–4291. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Young, K.D. A new suite of tnaA mutants suggests that Escherichia coli tryptophanase is regulated by intracellular sequestration and by occlusion of its active site. Bmc Microbiol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gursky, L.J.; Nikodinovic-Runic, J.; Feenstra, K.A.; O’Connor, K.E. In vitro evolution of styrene monooxygenase from Pseudomonas putida CA-3 for improved epoxide synthesis. Appl. Microbiol. Biotechnol. 2010, 85, 995–1004. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, D.; Chen, S.; Chen, J.; Wu, J. High-level extracellular production of alpha-cyclodextrin glycosyltransferase with recombinant Escherichia coli BL21 (DE3). J. Agric. Food Chem. 2011, 59, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.J.; Lee, S.Y. High-level production of human leptin by fed-batch cultivation of recombinant Escherichia coli and its purification. Appl. Environ. Microbiol. 1999, 65, 3027–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Z.; Chen, X.; Hou, Y.; Gao, B.; Zhao, C.; Yang, S.; Zeng, R. Enhanced a novel β-agarase production in recombinant Escherichia coli BL21 (DE3) through induction mode optimization and glycerol feeding strategy. Acta Oceanol. Sin. 2018, 37, 110–118. [Google Scholar] [CrossRef]
- Sohoni, S.V.; Nelapati, D.; Sathe, S.; Javadekar-Subhedar, V.; Gaikaiwari, R.P.; Wangikar, P.P. Optimization of high cell density fermentation process for recombinant nitrilase production in E. coli. Bioresour. Technol. 2015, 188, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Petrovičová, T.; Markošová, K.; Hegyi, Z.; Smonou, I.; Rosenberg, M.; Rebroš, M. Co-Immobilization of Ketoreductase and Glucose Dehydrogenase. Catalysts 2018, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Oster, T.; Boddupalli, S.S.; Peterson, J.A. Expression, purification, and properties of the flavoprotein domain of cytochrome P-450BM-3. Evidence for the importance of the amino-terminal region for FMN binding. J. Biol. Chem. 1991, 266, 22718–22725. [Google Scholar] [CrossRef]
- Tischler, D.; Eulberg, D.; Lakner, S.; Kaschabek, S.R.; van Berkel, W.J.; Schlomann, M. Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J. Bacteriol. 2009, 191, 4996–5009. [Google Scholar] [CrossRef] [Green Version]
- Toda, H.; Imae, R.; Komio, T.; Itoh, N. Expression and characterization of styrene monooxygenases of Rhodococcus sp. ST-5 and ST-10 for synthesizing enantiopure (S)-epoxides. Appl. Microbiol. Biotechnol. 2012, 96, 407–418. [Google Scholar] [CrossRef]
- Feenstra, K.A.; Hofstetter, K.; Bosch, R.; Schmid, A.; Commandeur, J.N.; Vermeulen, N.P. Enantioselective substrate binding in a monooxygenase protein model by molecular dynamics and docking. Biophys. J. 2006, 91, 3206–3216. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-C.; Guo, C.; Liu, Y.; Wang, H.-B.; Wu, Z.-L. Enzymatic cascades for the stereo-complementary epimerisation of in situ generated epoxy alcohols. Org. Biomol. Chem. 2017, 15, 2562–2568. [Google Scholar] [CrossRef]
- Toda, H.; Imae, R.; Itoh, N. Bioproduction of Chiral Epoxyalkanes using Styrene Monooxygenase from Rhodococcus sp. ST-10 (RhSMO). Adv. Synth. Catal. 2014, 356, 3443–3450. [Google Scholar] [CrossRef]
- Ghotekar, G.S.; More, D.A.; Nalla, V.; Muthukrishnan, M. A new enantioselective synthesis of antiobesity drug lorcaserin. New J. Chem. 2019, 43, 16876–16880. [Google Scholar] [CrossRef]
- Choi, W.J.; Choi, C.Y. Production of chiral epoxides: Epoxide hydrolase-catalyzed enantioselective hydrolysis. Biotechnol. Bioprocess Eng. 2005, 10, 167. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Manoj, K.M.; Archelas, A.; Baratti, J.; Furstoss, R. Microbiological transformations. Part 45: A green chemistry preparative scale synthesis of enantiopure building blocks of Eliprodil: Elaboration of a high substrate concentration epoxide hydrolase-catalyzed hydrolytic kinetic resolution process. Tetrahedron 2001, 57, 695–701. [Google Scholar] [CrossRef]
- Huang, K.; Wang, H.; Stepanenko, V.; De Jesús, M.; Torruellas, C.; Correa, W.; Ortiz-Marciales, M. Chiral Epoxides via Borane Reduction of 2-Haloketones Catalyzed by Spiroborate Ester: Application to the Synthesis of Optically Pure 1,2-Hydroxy Ethers and 1,2-Azido Alcohols. J. Org. Chem. 2011, 76, 1883–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondekar, N.B.; Kumar, P. Synthesis of (R)-Selegiline via Hydrolytic Kinetic Resolution. Synth. Commun. 2011, 41, 1301–1308. [Google Scholar] [CrossRef]
- Takeda, S.; Hayashi, S.; Noji, M.; Takanami, T. Chiroptical Protocol for the Absolute Configurational Assignment of Alkyl-Substituted Epoxides Using Bis(zinc porphyrin) as a CD-Sensitive Bidentate Host. J. Org. Chem. 2019, 84, 645–652. [Google Scholar] [CrossRef]
- Li, X.; Burrell, C.E.; Staples, R.J.; Borhan, B. Absolute Configuration for 1,n-Glycols: A Nonempirical Approach to Long-Range Stereochemical Determination. J. Am. Chem. Soc. 2012, 134, 9026–9029. [Google Scholar] [CrossRef]
- Machinaga, N.; Kibayashi, C. General entry to the 3,5-disubstituted indolizidine class of dendrobatid alkaloids. Total syntheses of both enantiomers of indolizidines 195B, 223AB, 239AB, and 239CD from a common chiral synthon. J. Org. Chem. 1992, 57, 5178–5189. [Google Scholar] [CrossRef]
- Florence, G.J.; Cadou, R. Efficient access to 2,5-substituted tetrahydrofurans via a one-pot cyclization of di- and triepoxides. Tetrahedron Lett. 2008, 49, 6784–6786. [Google Scholar] [CrossRef]
- Yudin, A.K.; Chiang, J.P.; Adolfsson, H.; Copéret, C. Olefin Epoxidation with Bis(trimethylsilyl) Peroxide Catalyzed by Inorganic Oxorhenium Derivatives. Controlled Release of Hydrogen Peroxide. J. Org. Chem. 2001, 66, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhang, G.; Barinka, C.; Eubanks, J.H.; Kozikowski, A.P. Design and Synthesis of Mercaptoacetamides as Potent, Selective, and Brain Permeable Histone Deacetylase 6 Inhibitors. Acs Med. Chem. Lett. 2017, 8, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
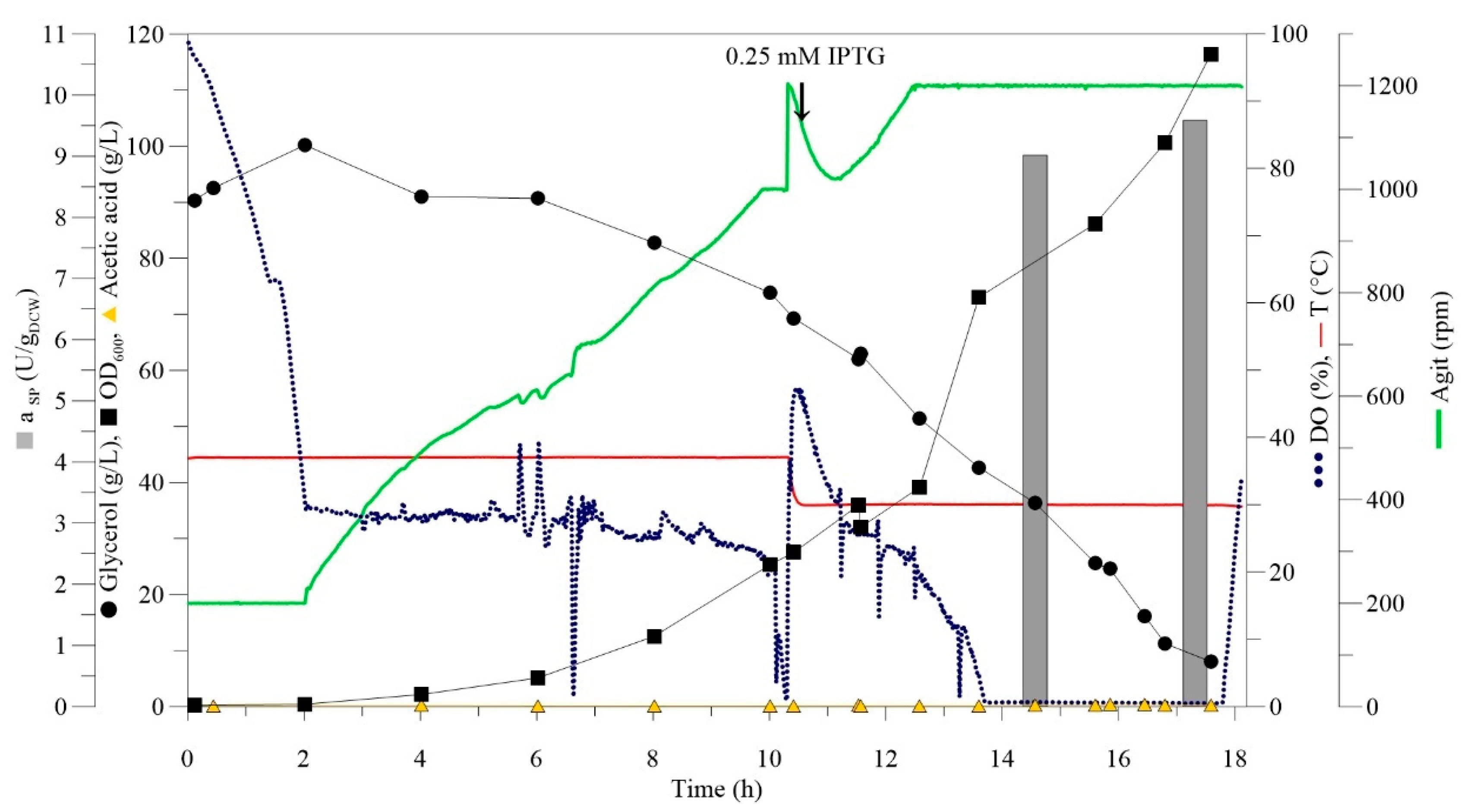
| Final Volume (L) | Cell Concentration (gDCW/L) | Total Dry Cell Weight (gDCW) | Enzyme Activity (U/gDCW) | Total Activity (U) |
|---|---|---|---|---|
| 0.5 L | 31 | 15.5 | 10.5 | 162.8 |
| 1.5 L | 35 | 52.5 | 9.6 | 504 |
| Structure | Substituents | Entry | Activity (U/gDCW) | Conversion (%) |
|---|---|---|---|---|
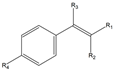 | R1, R2, R3, R4 = H | 1a | 7 | 99 |
| R1, R2, R4 = H; R3 = Br | 1b | - | - | |
| R1 = CHO, R2, R3, R4 = H | 1c | - | - | |
| R1 = CHO, R2, R3 = H, R4 = OCH3 | 1d | - | - | |
| R1 = CHO, R2, R3 = H, R4 = NO2 | 1e | - | - | |
| R1, R2, R3 = H, R4 = Cl | 1f | 12 | 90 | |
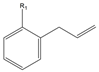 | R1 = H | 2a | 23 | 87 |
| R1 = OH | 2b | - | - | |
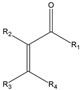 | R1, R2, R3, R4 = H | 3a | - | - |
| R1, R3, R4 = H; R2 = CH3 | 3b | 13 | 100 | |
| R1 = CH3; R2, R3, R4 = H | 3c | - | - | |
| R1, R2, R3 = H; R4 = (CH2)2CH3 | 3d | - | - | |
| R1 = Cl; R2, R3, R4 = H | 3e | - | - | |
 | R1 = OH; R2, R3, R4 = H | 4a | - | - |
| R1 = CH3; R2, R3, R4 = H | 4b | - | - | |
| R1, R2 = CH3; R3, R4 = H | 4c | - | - | |
| R1, R3 = CH3; R2, R4 = H | 4d | - | - | |
| R1 = (CH2)3CH3; R2, R3, R4 = H | 4e | - | - | |
| R1 = CH2CH3; R2, R3 = H; R4 = CHO | 4f | - | - | |
 | R1 = H, R2 = CH2OH | 5a | - | - |
| R1 = CH3, R2 = CH2OH | 5b | - | - | |
| R1 = H, R2 = (CH2)3OH | 5c | - | - | |
| R1 = H, R2 = (CH2)4OH | 5d | 25 | 99 | |
| R1 = H, R2 = CH(OH)(CH2)3CH3 | 5e | - | - | |
| R1 = H, R2 = CH(OH)(CH2)2 | 5f | - | - | |
 | 5g | 5 | 99 | |
 | 5h | 3 | 67 | |
| R1 = Br, R2 = CH3 | 5i | - | - | |
| R1 = H, R2 = (CH2)3Br | 5j | 7 | 99 | |
| R1 = H, R2 = CN | 5k | - | - | |
| R1 = H, R2 = CH(OCH2CH3)2 | 5l | - | - | |
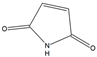 | 6 | - | - | |
 | 7 | - | - | |
 | 8 | - | - |
| Entry | Substrate | Product | Configuration | ee (%) | Conversion (%) | Yield (mg) | Reaction Volume/Yield (mL/mg) |
|---|---|---|---|---|---|---|---|
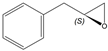
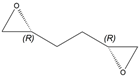
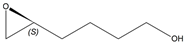
| 1f |  | 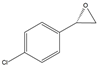 | S | >99% | 99 | 60 | 1.27 |
| 2a | 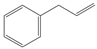 |  | S | >95% | 99 | 26 | 2.88 |
| 5g |  | 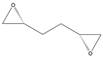 | 2-R,5-R | >97% | 99 | 157 | 1.28 |
| 5j | 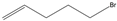 | 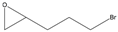 | ND1 | >99% 2 | 93 | 173 | 1.73 |
| 5d |  |  | S | >99% 3 | 99 | 76 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyuranová, D.; Štadániová, R.; Hegyi, Z.; Fischer, R.; Rebroš, M. Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase. Molecules 2021, 26, 1514. https://doi.org/10.3390/molecules26061514
Gyuranová D, Štadániová R, Hegyi Z, Fischer R, Rebroš M. Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase. Molecules. 2021; 26(6):1514. https://doi.org/10.3390/molecules26061514
Chicago/Turabian StyleGyuranová, Dominika, Radka Štadániová, Zuzana Hegyi, Róbert Fischer, and Martin Rebroš. 2021. "Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase" Molecules 26, no. 6: 1514. https://doi.org/10.3390/molecules26061514
APA StyleGyuranová, D., Štadániová, R., Hegyi, Z., Fischer, R., & Rebroš, M. (2021). Production of Enantiopure Chiral Epoxides with E. coli Expressing Styrene Monooxygenase. Molecules, 26(6), 1514. https://doi.org/10.3390/molecules26061514






