Aziridine is a nitrogen-containing three-membered ring with similar ring strain energy as other three-membered ring compounds, including cyclopropane and oxirane [1,2]. Its high ring strain energy renders various nitrogen-containing cyclic and acyclic compounds as oxirane (epoxide) does to realize oxygen-containing compounds as shown in Figure 1 [3].
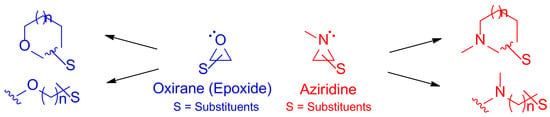
Figure 1.
Oxirane (epoxide) yields cyclic and/or acyclic compounds with oxygen in blue, while aziridine and its transformations toward cyclic and/or acyclic nitrogen-containing molecules are shown in red.
The central biomolecular structure of genetic material is composed of nitrogen-based molecules with a close chemical relationship between elementary carbon and nitrogen [4]. Unless we are not able to change the atom in one specific molecule, nitrogen is one of the essential and most abundant atoms in valuable molecules, including natural products and drugs. Many drugs and their candidates contain amines in medicinal chemistry. Building these amine-containing molecules sometimes requires multiple steps. Some of these nitrogen-containing molecules may be realized by a shortcut with the formation of aziridine as a starting material or an advanced synthetic intermediate [5]. Many synthetic organic chemists have explored “aziridine chemistry” including efficient synthesis of valuable aziridines and their utilization as key synthetic intermediates in cooperation with nitrogen. However, aziridine has been studied relatively less extensively. It has been used in limited cases compared to oxirane(epoxide) because its preparation and utilization are relatively arduous.
According to Scopus®, the number of publications with the search term “aziridine” is 6925 from 1948 to 1 October 2020, which is less than 13% compared to the numbers with the search term “oxirane” (or “epoxide”). Aziridine was called the “Ugly-Cousin of Epoxide” by Sweeny in his review article [6] compared to three-member heterocycle epoxide with similar ring strain. However, the chemistry of aziridine is sometimes laborious with some difficulties to carry on. Its handling is also quite messy to deal with. Amine is trivalent with the formation of three different bonds, at which points aziridine is more complicated compared to divalent epoxide. In addition, amine is more nucleophilic and more reactive than alcohols and water. Reactions of amines with acyl derivatives are faster than corresponding transformations with alcohols.
The stability and diversity of aziridines including ring-opening by nucleophiles are dependent on substituents at the ring nitrogen (whether they are electron-withdrawing or electron-donating) [7,8]. Aziridines are bifurcated into “activated” ones bearing electron-withdrawing substituents at the ring nitrogen and “non-activated” ones with electron-donating substituents. Successful tailing of aziridines with various substituents would warrant a streamlined synthesis of valuable and biologically active amines and heterocycles by regio- and stereoselective aziridine-ring openings and ring transformations [9,10].
Like the fairytale story “Beauty and the Beast”, a curse of an Enchantress made a handsome prince into a terrible beast because of his selfishness. He should find true love before the last rose petal falls. We are able to adopt this story to aziridine, with more difficulties in carrying out its chemistry and handling compared to epoxide. We have to be a good chemist to overcome the difficulties of aziridine as Belle did with the selfish beast, for the prince came back to his original handsome figure. With good tools to handle aziridine, including its efficient preparation and chemical transformation, aziridine can serve as a valuable molecule containing the essential nitrogen element in cyclic and acyclic forms. To change “the Ugly Aziridine” to “the Beauty Aziridine”, various chemical work is required. From the point of view this Special Issue, “aziridine chemistry” is needed more than ever for the development of valuable nitrogen-compounds. This Special Issue includes several communications in the form of original research and review articles covering aziridine synthesis and its utilization as starting materials and synthetic intermediates. Within the scope of “aziridine chemistry”, I would like to introduce you to this Special Issue of “the Beauty Aziridine” 133 years after Gabriel’s discovery of aziridine in 1888 [11].
Short Biography of the Author
 |
Hyun-Joon Ha obtained his BS degree in Chemistry in 1982 from Seoul National University with honors, and his PhD in 1987 from Brown University under the direction of Professor David E. Cane. After a one-year postdoctoral fellowship at Stanford University, he returned to Korea in 1988, and accepted the position of senior research scientist at the Korea Institute of Science and Technology (KIST). He was involved in the chemical approach for the development of new biologically active agrochemicals. In 1991, he joined the faculty of the Chemistry Department at Hankuk University of Foreign Studies, and is now a full professor of this department. During his tenure, he took university positions such as a Dean of General Affairs (2004–2006) and Dean of Natural Science (2013–2014). In 1993, he carried out research at the Chemistry Department of Cambridge University, U.K., as a short-term visiting scholar of Emmanuel College. Academically, he served as an organizer of numerous international symposia including ICOS-17, 11-ACC, IUPAC 46th General Assembly and the 43rd IUPAC Congress, etc. He was elected as the 51st president of the Korean Chemical Society and served a two-year term in 2018–2019. His research interests include the exploitation of new methods in organic synthesis, aziridines and heterocycles, imines and iminium ions, asymmetric synthesis of biologically active molecules, and lipase-mediated reactions. He has been involved in aziridine chemistry with the industrial production of chiral aziridine-2-carboxylates from which many nitrogen-containing cyclic and acyclic molecules are prepared in optically pure forms. He has recently become interested in medicinal chemistry for drug discovery, process development for pharmaceuticals, and the design and synthesis of radiopharmaceuticals. He has been a member of the Korean and American Chemical Societies and a fellow of IUPAC. He is also a Fellow of the Royal Society of Chemistry (FRSC). Currently, he serves as an associate editor of Asian J. Org. Chem. from Wiley VCH. Prof. Ha has been awarded several honors, some of which are the ACP Lectureship Award, (ICCEOCA-6) from the Asian Core Program (ACP; 2011), the Sigma-Aldrich (Korea) Award (2012), and the Outstanding Research Award in Organic Chemistry (KCS; 2015). He holds several patents and has published over 170 peer-reviewed papers in leading journals and two books, including the recently edited “Synthesis of 4- to 7-Membered Heterocycles by Ring Expansion” with Prof. M. D’hooghe.
Conflicts of Interest
The author declares no conflict of interest.
References
- Pitzer, K.S. Strain Energies of Cyclic Hydrocarbons. Science 1945, 101, 672. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Ring Strain Energies from ab Initio Calculations. J. Am. Chem. Soc. 1998, 120, 4450–4458. [Google Scholar] [CrossRef]
- Aziridines and Epoxides in Organic Synthesis; Yudin, A.K., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Eschenmoser, A. Etiology of Potentially Primordial Biomolecular Structures: From Vitamin B12 to the Nucleic Acids and an Inquiry into the Chemistry of Life’s Origin: A Retrospective. Angew. Chem. 2011, 50, 12412–12471. [Google Scholar] [CrossRef] [PubMed]
- D’hooghe, M.; Ha, H.-J. Synthesis of 4- to 7-membered Heterocycles by Ring Expansion: Aza-, oxa and thiaheterocyclic small-ring systems. In Topics in Heterocyclic Chemistry; Springer Nature: London, UK, 2016; Volume 41. [Google Scholar]
- Stankovic, S.; D’hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Van Speybroeck, V.; De Kimpe, N.; Ha, H.-J. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-K.; Ha, H.-J. High Light of the Chemistry of Enantiomerically Pure Aziridine-2-carboxylates. Aldrichim. Acta 2003, 36, 57–63. [Google Scholar]
- Ha, H.-J.; Jung, J.-H.; Lee, W.K. Application of Regio- and Stereoselective Functional Group Transformation of Chiral Aziridine-2-carboxylate. Asian J. Org. Chem. 2014, 3, 1020–1035. [Google Scholar] [CrossRef]
- Gabriel, S. Ueber Vinylamine. Chem. Ber. 1888, 21, 1049–1057. [Google Scholar] [CrossRef]
- Gabriel, S. Ueber Vinylamin und Bromäthylamin. Chem. Ber. 1888, 21, 2664–2669. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).