Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis
Abstract
1. Introduction
2. Results
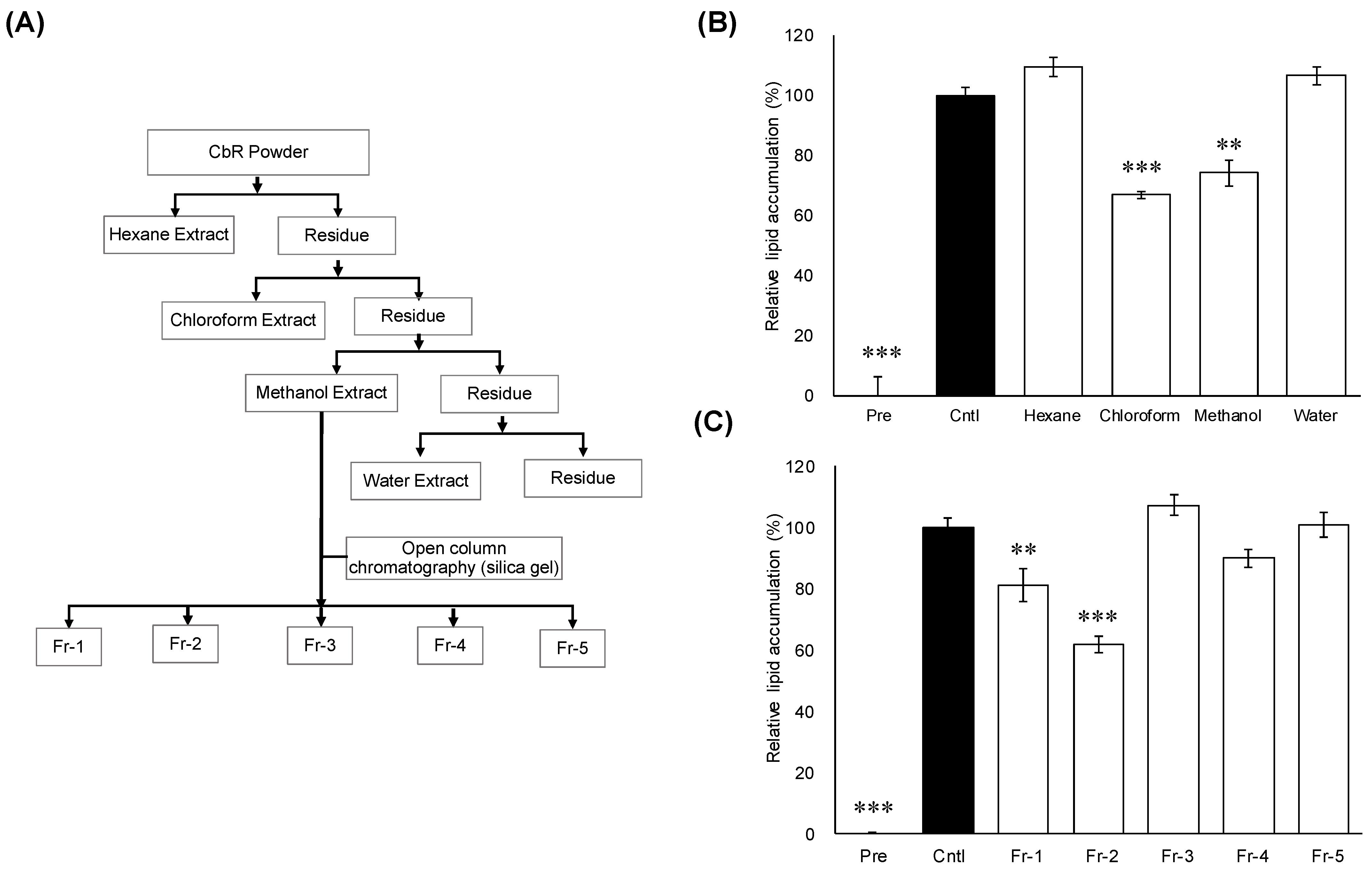
2.1. Effect of CbR Extracts Fractions on 3T3-L1 Cells
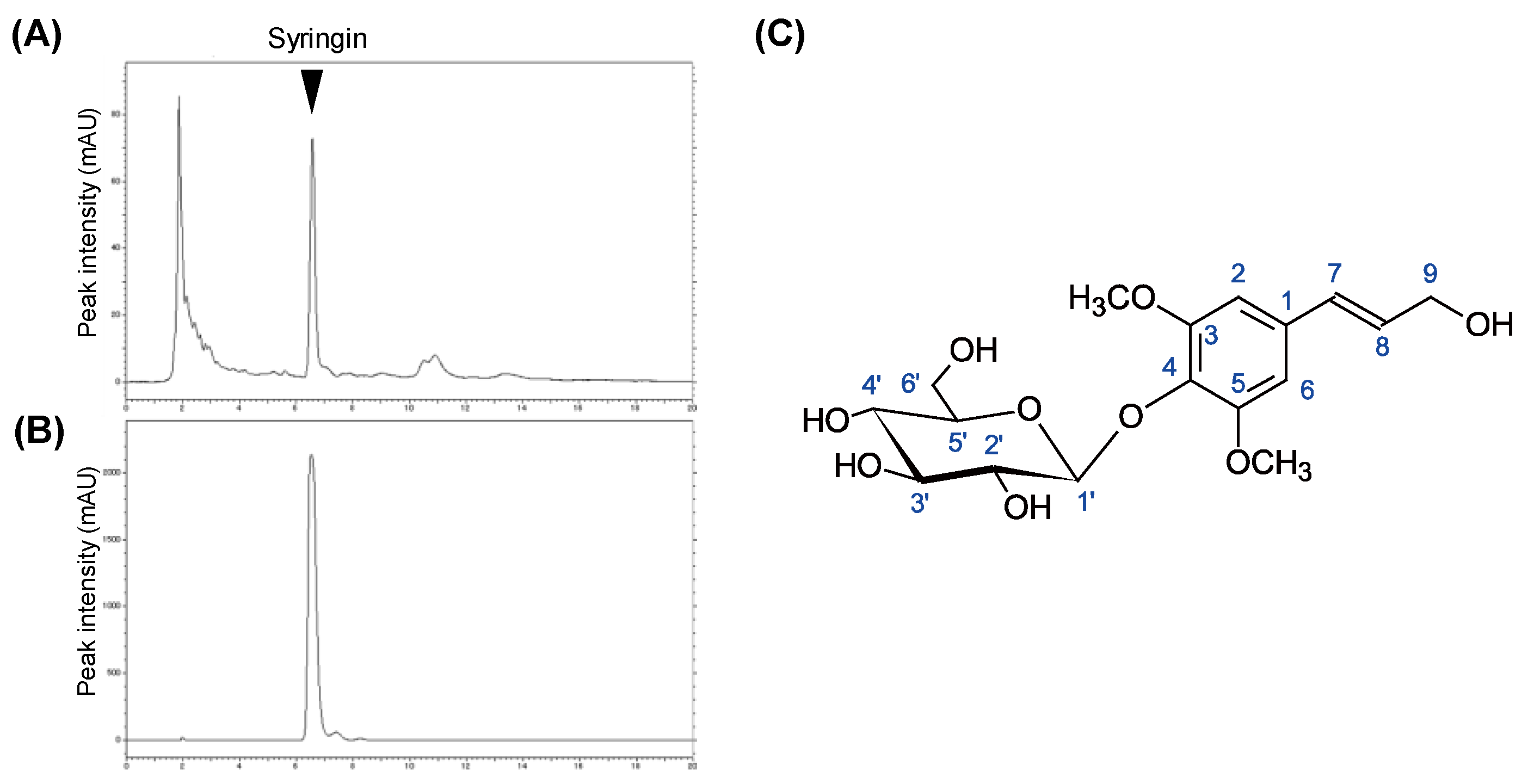
2.2. Chemical Structure Identification of Active Compound from CbR
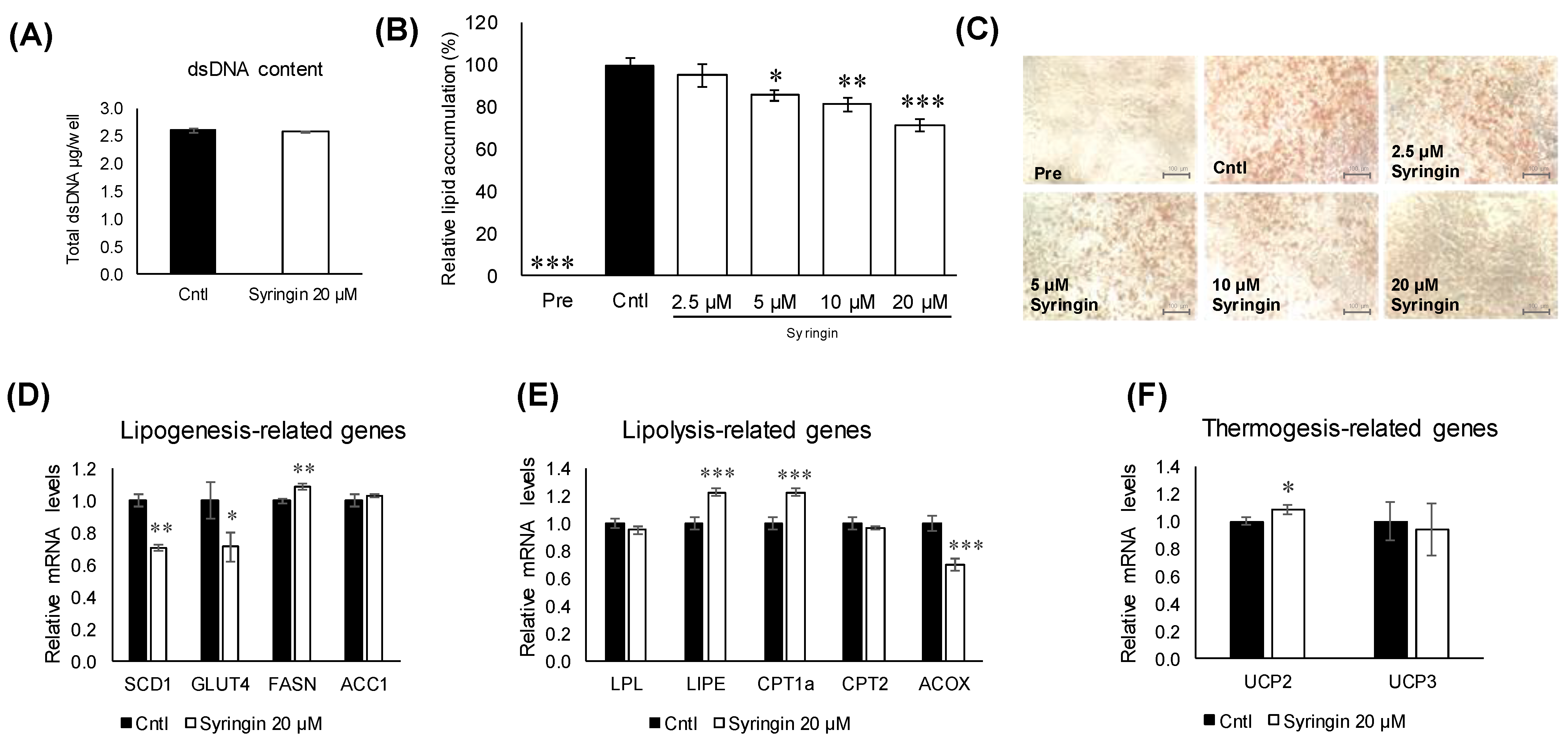
2.3. Effects of Syringin during Adipogenesis
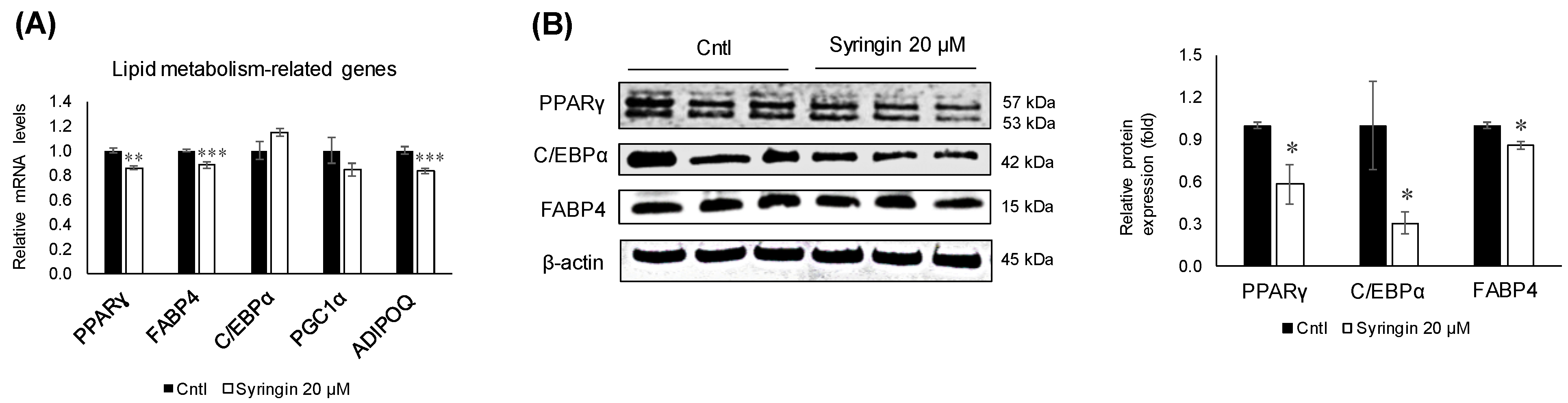
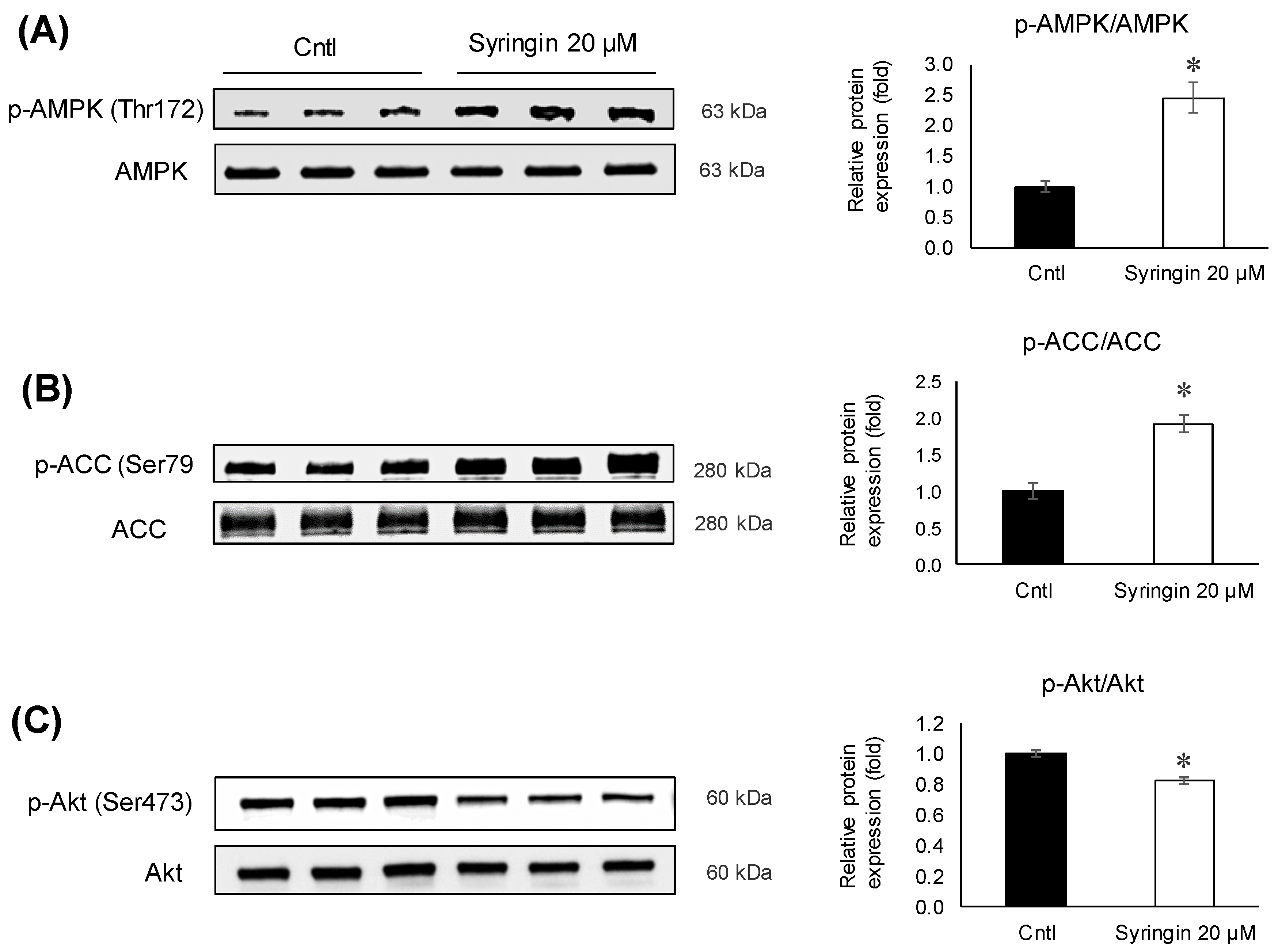
2.4. Effects of Syringin on the Adipocyte Differentiation of 3T3-L1 Cells
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of Active Compounds from CbR
4.2. Cell Culture and Treatments
4.3. Quantitative Real-Time Polymerase Chain Reaction
4.4. Western Blot Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Facts%20about%20overweight%20and%20obesity,recent%20WHO%20global%20estimates%20follow.&text=Of%20these%20over%20650%20million%20adults%20were%20obese.,women)%20were%20obese%20in%202016 (accessed on 17 February 2021).
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases Progress Monitor. WHO. 2017. Available online: https://ncdalliance.org/resources/who-ncds-progress-monitor-2017 (accessed on 20 November 2020).
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-L.; Riley, D.J.; Chen, Y.; Lee, W.-H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996, 10, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, I.; Tufekci, A.R.; Yaglioglu, A.S.; Elmastas, M. Studies on the antioxidant and antiproliferative potentials of Cirsium arvense subsp. vestitum. J. Food Biochem. 2017, 41, e12299. [Google Scholar] [CrossRef]
- Sahli, R.; Rivière, C.; Dufloer, C.; Beaufay, C.; Neut, C.; Bero, J.; Hennebelle, T.; Roumy, V.; Ksouri, R.; Quetin-Leclercq, J. Antiproliferative and antibacterial activities of Cirsium scabrum from Tunisia. Evid. Based Complementary Altern. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Jordon-Thaden, I.E.; Louda, S.M. Chemistry of Cirsium and Carduus: A role in ecological risk assessment for biological control of weeds? Biochem. Syst. Ecol. 2003, 31, 1353–1396. [Google Scholar] [CrossRef]
- He, X.-F.; He, Z.-W.; Jin, X.-J.; Pang, X.-Y.; Gao, J.-G.; Yao, X.-J.; Zhu, Y. Caryolane-type sesquiterpenes from Cirsium souliei. Phytochem. Lett. 2014, 10, 80–85. [Google Scholar] [CrossRef]
- Liao, Z.; Wu, Z.; Wu, M. Cirsium japonicum flavones enhance adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biol. Pharm. Bull. 2012, 35, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, X.; Wu, M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch. Pharm. Res. 2010, 33, 353–362. [Google Scholar] [CrossRef]
- Mori, S.; Satou, M.; Kanazawa, S.; Yoshizuka, N.; Hase, T.; Tokimitsu, I.; Takema, Y.; Nishizawa, Y.; Yada, T. Body fat mass reduction and up-regulation of uncoupling protein by novel lipolysis-promoting plant extract. Int. J. Biol. Sci. 2009, 5, 311. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.R.; Ramirez, L.M.E.; Vargas, S.R. Effect of Cirsium pascuarense on blood glucose levels of normoglycaemic and alloxan-diabetic mice. Phytother. Res. 2001, 15, 552–554. [Google Scholar] [CrossRef]
- Fernández-Martínez, E.; Jiménez-Santana, M.; Centeno-Álvarez, M.; Torres-Valencia, J.M.; Shibayama, M.; Cariño-Cortés, R. Hepatoprotective effects of nonpolar extracts from inflorescences of thistles Cirsium vulgare and Cirsium ehrenbergii on acute liver damage in rat. Pharmacogn. Mag. 2017, 13, S860. [Google Scholar] [CrossRef]
- Yin, J.; Heo, S.-I.; Wang, M.-H. Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. Nutr. Res. Pract. 2008, 2, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kadawaki, Y. Taxonomy and distribution of Cirsium brevicaule A. GRAY and its related species (Asteraceae). Mem. Natn. Sci. Mus. Tokyo 1990, 23, 51–61. [Google Scholar]
- Inafuku, M.; Nugara, R.N.; Kamiyama, Y.; Futenma, I.; Inafuku, A.; Oku, H. Cirsium brevicaule A. GRAY leaf inhibits adipogenesis in 3T3-L1 cells and C57BL/6 mice. Lipids Health Dis. 2013, 12, 124. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Ahn, J.; Jung, C.H.; Ha, T. Coumestrol modulates Akt and Wnt/β-catenin signaling during the attenuation of adipogenesis. Food Funct. 2016, 7, 4984–4991. [Google Scholar] [CrossRef]
- US, M.R.; Zin, T.; Abdurrazak, M.; Ahmad, B.A. Chemistry and pharmacology of syringin, a novel bioglycoside: A review. Asian J. Pharm. Clin. Res. 2015, 8, 20–25. [Google Scholar]
- Yin, L.; Yang, Y.-H.; Wang, M.-Y.; Zhang, X.; Duan, J.-A. Effects of syringin from Phellodendron chinensis on monosodium urate crystal-induced inflammation and intercellular adhesion molecule-1 (ICAM-1) expression. Afr. J. Pharm. Pharmacol. 2012, 6, 1515–1519. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ohsawa, K. Metabolites of orally administered Magnolia officinalis extract in rats and man and its antidepressant-like effects in mice. J. Pharm. Pharmacol. 2003, 55, 1583–1591. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Kollmann, A.; Khlifi, S.; Ducrot, P.-H. Antioxidative effect of compounds isolated from Globularia alypum L. structure–activity relationship. LWT Food Sci. Technol. 2007, 40, 1246–1252. [Google Scholar] [CrossRef]
- Krishnan, S.S.C.; Subramanian, I.P.; Subramanian, S.P. Isolation, characterization of syringin, phenylpropanoid glycoside from Musa paradisiaca tepal extract and evaluation of its antidiabetic effect in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2014, 4, 105–111. [Google Scholar] [CrossRef]
- Niu, H.-S.; Liu, I.-M.; Cheng, J.-T.; Lin, C.-L.; Hsu, F.-L. Hypoglycemic effect of syringin from Eleutherococcus senticosus in streptozotocin-induced diabetic rats. Planta Med. 2008, 74, 109–113. [Google Scholar] [CrossRef]
- Miyaichi, Y.; Matsuura, M.; Tomimori, T. Phenolic compound from the roots of Cirsium japonicum DC. Nat. Med. 1995, 49, 92–94. [Google Scholar]
- Jung, C.H.; Ahn, J.; Heo, S.H.; Ha, T.-Y. Eleutheroside E, an active compound from Eleutherococcus senticosus, regulates adipogenesis in 3T3-L1 cells. Food Sci. Biotechnol. 2014, 23, 889–893. [Google Scholar] [CrossRef]
- Evans, M.; Lin, X.; Odle, J.; McIntosh, M. Trans-10, cis-12 conjugated linoleic acid increases fatty acid oxidation in 3T3-L1 preadipocytes. J. Nutr. 2002, 132, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.; Hwang, S.G.; Hirai, S.; Matsui, T.; Yano, H.; Kawada, T.; Lim, B.O.; Park, D.K. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004, 75, 3195–3203. [Google Scholar] [CrossRef]
- Furuyashiki, T.; Nagayasu, H.; Aoki, Y.; Bessho, H.; Hashimoto, T.; Kanazawa, K.; Ashida, H. Tea catechin suppresses adipocyte differentiation accompanied by down-regulation of PPARγ2 and C/EBPα in 3T3-L1 cells. Biosci. Biotechnol. Biochem 2004, 68, 2353–2359. [Google Scholar] [CrossRef]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef]
- Morrison, R.F.; Farmer, S.R. Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 2000, 130, 3116S–3121S. [Google Scholar] [CrossRef]
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. BBA Mol. Cell Biol. Lipids 2005, 1733, 137–147. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, C.; Zhu, P.; Tian, C.; Liang, A. Protective effects of syringin against oxidative stress and inflammation in diabetic pregnant rats via TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2020, 131, 110681. [Google Scholar] [CrossRef]
- Kim, B.; Kim, M.-S.; Hyun, C.-K. Syringin attenuates insulin resistance via adiponectin-mediated suppression of low-grade chronic inflammation and ER stress in high-fat diet-fed mice. Biochem. Biophys. Res. Commun. 2017, 488, 40–45. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kong, C.-S. Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chem. Biol. Interact. 2010, 186, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Horman, S.; Browne, G.J.; Krause, U.; Patel, J.V.; Vertommen, D.; Bertrand, L.; Lavoinne, A.; Hue, L.; Proud, C.G.; Rider, M.H. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002, 12, 1419–1423. [Google Scholar] [CrossRef]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-Epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Reuss, L.; Wang, Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Baek, J.-H.; Kim, N.-J.; Song, J.-K.; Chun, K.-H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-S.; Park, H.J.; Woo, J.-H.; Kim, M.-K.; Koh, P.-O.; Min, W.; Ko, Y.-G.; Kim, C.-H.; Won, C.-K.; Cho, J.-H. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement. Altern. Med. 2012, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Choe, W.K.; Kang, B.T.; Kim, S.O. Water-extracted plum (Prunus salicina L. cv. Soldam) attenuates adipogenesis in murine 3T3-L1 adipocyte cells through the PI3K/Akt signaling pathway. Exp. Ther. Med. 2018, 15, 1608. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, L.T.; Iakova, P.; Welm, A.L.; Cai, Z.J.; Timchenko, N.A. Calreticulin interacts with C/EBPalpha and C/EBPbeta mRNAs and represses translation of C/EBP proteins. Mol. Cell. Biol. 2002, 22, 7242–7257. [Google Scholar] [CrossRef]
- Haefliger, S.; Klebig, C.; Schaubitzer, K.; Schardt, J.; Timchenko, N.; Mueller, B.U.; Pabst, T. Protein disulfide isomerase blocks CEBPA translation and is up-regulated during the unfolded protein response in AML. Blood 2011, 117, 5931–5940. [Google Scholar] [CrossRef]
- Asa Gray, M.D. Diagnostic characters of new species of phænogamous plants, collected in Japan by Charles Wright, botanist of the U.S. North Pacific exploring expedition with observations upon the relations of the Japanese flora to that of North America, and of other parts of the northern temperate zone. Mem. Am. Acad. Arts Sci. 1859, 6, 377–452. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossin, A.Y.; Inafuku, M.; Takara, K.; Nugara, R.N.; Oku, H. Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis. Molecules 2021, 26, 1531. https://doi.org/10.3390/molecules26061531
Hossin AY, Inafuku M, Takara K, Nugara RN, Oku H. Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis. Molecules. 2021; 26(6):1531. https://doi.org/10.3390/molecules26061531
Chicago/Turabian StyleHossin, Abu Yousuf, Masashi Inafuku, Kensaku Takara, Ruwani N. Nugara, and Hirosuke Oku. 2021. "Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis" Molecules 26, no. 6: 1531. https://doi.org/10.3390/molecules26061531
APA StyleHossin, A. Y., Inafuku, M., Takara, K., Nugara, R. N., & Oku, H. (2021). Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis. Molecules, 26(6), 1531. https://doi.org/10.3390/molecules26061531







