Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements
Abstract
1. Introduction
2. Results
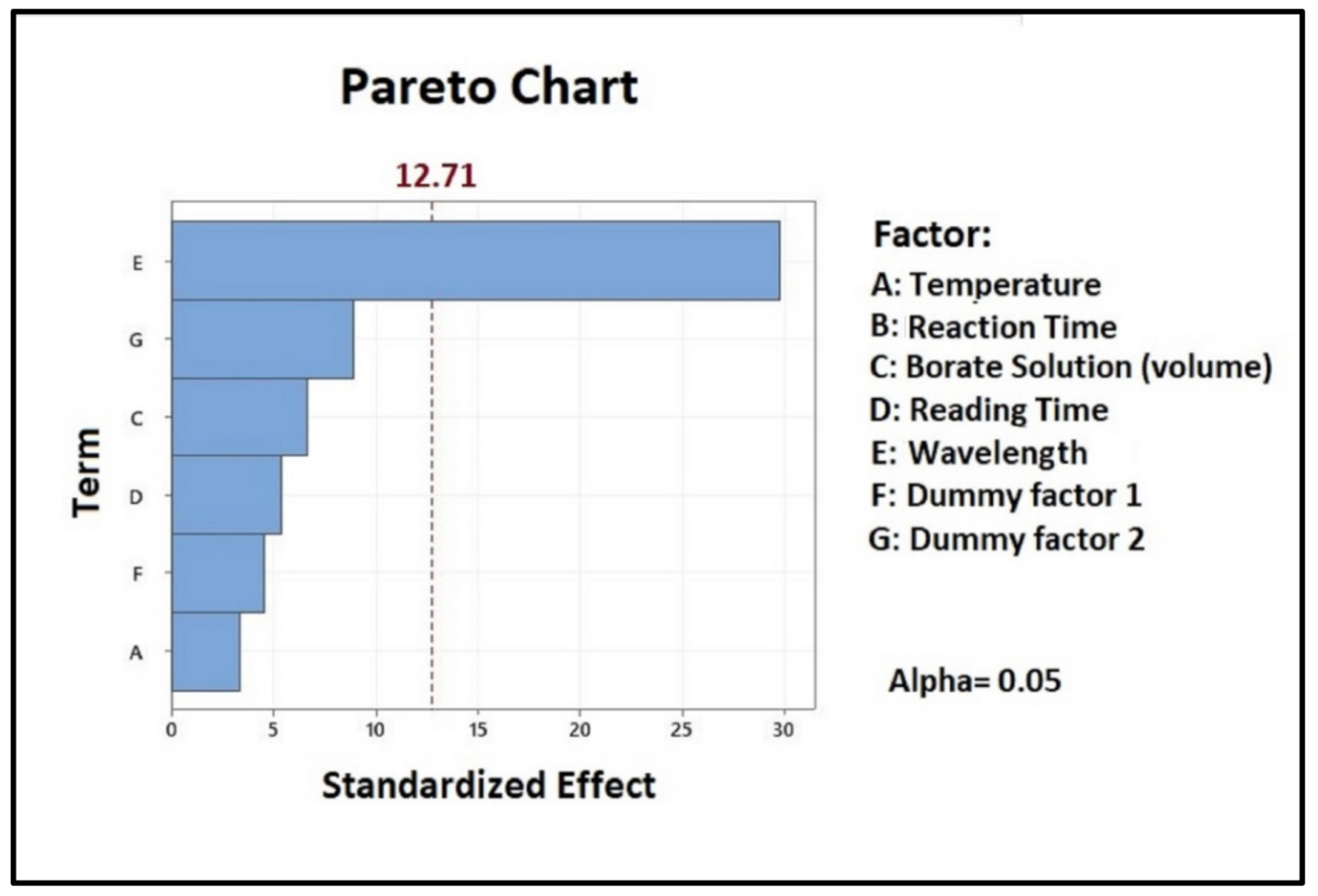
2.1. Plackett–Burman Test
2.2. Validation Results
2.2.1. Specificity
2.2.2. Linearity
2.2.3. System Precision, Repeatability and Intermediate Precision
2.2.4. Accuracy
2.3. Agreement between Methods
| Variable | Count | Mean | Standard Deviation | 95.0% LCL of the Mean | 95.0% UCL of the Mean |
|---|---|---|---|---|---|
| Method 1 | 12 | 1.09 | 0.02 | 1.07 | 1.10 |
| Method 2 | 12 | 1.24 | 0.14 | 1.14 | 1.33 |
| Difference | 12 | −0.15 | 0.15 | −0.25 | −0.05 |
| Parameter | Count | Value | Standard Deviation | 95.0% LCL of the Mean | 95.0% UCL of the Mean |
|---|---|---|---|---|---|
| Bias (Difference) | 12 | −0.15 | 0.15 | −0.25 | −0.05 |
| Lower Limit of Agreement (LL) | 12 | −0.45 | 0.08 | −0.62 | −0.28 |
| Upper Limit of Agreement (UL) | 12 | 0.15 | 0.08 | −0.02 | 0.32 |
| Test of the normality of differences assumption: | |||||
| Assumption | Value | Prob. level | Decision (α = 0.050). | ||
| Shapiro–Wilk | 0.937 | 0.4554 | Cannot reject normality | ||

3. Discussion
4. Materials and Methods
4.1. Commercial Supplement Composition
4.2. Design of Experiment: Strategy Planning
4.3. Spectrophotometry
4.3.1. Chemicals and Materials
4.3.2. Preparation of Standard Solutions
4.3.3. Procedure for Alpha-Galactosidase Quantification
| C | = | Concentration of the alpha-galactosidase (GalU/mL) in the sample solution obtained by interpolation of straight calibration |
| Pcp | = | Weight of sample (g) |
| 2g | = | Weight of the sachet (2 g) |
| VI | = | Volume of sample pour in the last dilution |
| Vf | = | Final volume dilution (mL) |
4.4. Fluorimetric Alpha-Galalactosidase Assay
4.5. Method Comparisons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| α-Gal | Alpha-galactosidase |
| cGMP | Current Good Manufacturing Practice |
| FDA | Food and Drug Administration |
| GalNAc | N-acetylgalactosamine |
| GalU | Galactosidase units |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals |
| RSD | Relative Standard Deviation |
| DS | Standard Deviation |
References
- Bishop, D.F.; Desnick, R.J. Affinity purification of α-galactosidase A from human spleen. placenta and plasma with elimination of pyrogen contamination. J. Biol. Chem. 1982, 256, 1307. [Google Scholar] [CrossRef]
- Katrolia, P.; Rajashekhara, E.; Yan, Q.; Jiang, Z. Biotechnological potential of microbial α-galactosidases. Crit. Rev. Biotechnol. 2013, 34, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, J.W.; Quirk, J.M.; Brady, R.O. Purification and properties of the two major isozymes of α-galactosidase from human placenta. J. Biol. Chem. 1978, 253, 184. [Google Scholar] [CrossRef]
- De Souza Vandenberghe, L.P.; Karp, S.G.; Pagnoncelli, M.G.B.; Rodrigues, C.; Medeiros, A.B.P.; Soccol, C.R. Digestive Enzymes: Industrial Applications in Food Products. In Green Bio-Processes; Springer: Singapore, 2019; pp. 267–291. [Google Scholar] [CrossRef]
- Kizhner, T.; Azulay, Y.; Hainrichson, M.; Tekoah, Y.; Arvatz, G.; Shulman, A.; Ruderfer, I.; Aviezer, D.; Shaaltiel, Y. Characterization of a chemically modified plant cell culture expressed human alpha-Galactosidase-A enzyme for treatment of Fabry disease. Mol. Genet. Metab. 2015, 114, 259–267. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Silvestroni, A.; Connes, C.; Juillard, V.; de Giori, G.S.; Piard, J.C.; Sesma, F. Reduction of non -digestible oligosaccharides in soymilk: Application of engineered lactic acid bacteria that produce α-galactosidase. Gen. Mol. Res. 2004, 3, 432–440. [Google Scholar]
- Solomons, N.W.; Guerrero, A.M.; Zepeda, E.; Grazioso, C. The efficacy of an oral α-galactosidase to promote oligosaccaride hydrolysis and to reduce intolerance symptom after ingestion of beans: A dose response trial. Am. J. Clin. Nutr. 1991, 53, P28. [Google Scholar]
- Irvine, E.J.; Tack, J.; Crowell, M.D.; Gwee, K.A.; Ke, M.; Schmulson, M.J.; Whitehead, W.E.; Spiegel, B. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006, 130, 1538–1551. [Google Scholar] [CrossRef]
- Di Nardo, G.; Oliva, S.; Ferrari, F.; Mallardo, S.; Barbara, G.; Cremon, C.; Aloi, M.; Cucchiara, S. Efficacy and tolerability of alpha-galactosidase in treating gas-related symptoms in children: A randomized. double-blind. placebo controlled trial. BMC Gastroenterol. 2013, 13, 142. [Google Scholar] [CrossRef]
- Hillilä, M.; Färkkilä, M.A.; Sipponen, T.; Rajala, J.; Koskenpato, J. Does oral α-galactosidase relieve irritable bowel symptoms? Scand. J. Gastroenterol. 2015, 51, 16–21. [Google Scholar] [CrossRef]
- Di Stefano, M.; Miceli, E.; Gotti, S.; Missanelli, A.; Mazzocchi, S.; Corazza, G.R. The Effect of Oral α-Galactosidase on Intestinal Gas Production and Gas-Related Symptoms. Dig. Dis. Sci. 2006, 52, 78–83. [Google Scholar] [CrossRef]
- Maconi, G.; Bolzacchini, E.; Radice, E.; Marzocchi, M.; Badini, M. Alpha-galactosidase versus active charcoal for improving sonographic visualization of abdominal organs in patients with excessive intestinal gas. J. Ultrasound 2012, 15, 232–238. [Google Scholar] [CrossRef][Green Version]
- Ganiats, T.G.; Norcross, W.A.; Halverson, A.L.; Burford, P.A.; Palinkas, L.A. Does beano prevent gas? A double blind crossover study of oral alfa-galactosidase to treat dietary oligosaccharide intolerance. J. Fam. Pract. 1994, 39, 441–445. [Google Scholar]
- Longstreth, G.F.; Drossman, D.A. Severe irritable bowel and functional abdominal pain syndromes: Managing the patient and health care costs. Clin. Gastroenterol. Hepatol. 2005, 3, 397–400. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Meng, K.; Bai, Y.; Shi, P.; Luo, H.; Yang, P.; Zhou, Z.; Zhang, Z.; Yao, B. A novel protease-resistant α-galactosidase with high hydrolytic activity from Gibberella sp. F75: Gene cloning. expression. and enzymatic characterization. Appl. Microbiol. Biotechnol. 2009, 83, 875–884. [Google Scholar] [CrossRef]
- Worthington, R.E.; Beuchat, L.R. Alpha-galactosidase activity of fungi on intestinal gas-forming peanut oligosaccharides. J. Agric. Food Chem. 1974, 22, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, Y.; Tashiro, M.; Katou, M.; Sakuma, C.; Hirano, T.; Hakamata, W.; Nishio, T. Enzymatic synthesis of novel oligosaccharides from N-acetylsucrosamine and melibiose using Aspergillus niger alpha-galactosidase. and properties of the products. Biosci. Biotechnol. Biochem. 2016, 80, 1836–1842. [Google Scholar] [CrossRef]
- Liao, J.; Okuyama, M.; Ishihara, K.; Yamori, Y.; Iki, S.; Tagami, T.; Mori, H.; Chiba, S.; Kimura, A. Kinetic properties and substrate inhibition of α-galactosidase from Aspergillus niger. Biosci. Biotechnol. Biochem. 2016, 80, 1747–1752. [Google Scholar] [CrossRef]
- Unzueta, U.; Vázquez, F.; Accardi, G.; Mendoza, R.; Toledo-Rubio, V.; Giuliani, M.; Sannino, F.; Parrilli, E.; Abasolo, I.; Schwartz, S., Jr.; et al. Strategies for the production of difficult-to-express full-length eukaryotic proteins using microbial cell factories: Pro-duction of human alpha-galactosidase A. Appl. Microbiol. Biotechnol. 2015, 99, 5863–5874. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Singh, A.; Batra, N.; Singh, J. Microbial production and biotechnological applications of α-galactosidase. Int. J. Biol. Macromol. 2020, 150, 1294–1313. [Google Scholar] [CrossRef]
- Jang, J.M.; Yang, Y.; Wang, R.; Bao, H.; Yuan, H.; Yang, J. Characterization of a high performance α-galactosidase from Irpex lacteus and its usage in removal of raffinose family oligosaccharides from soymilk. Int. J. Biol. Macromol. 2019, 131, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cao, M.-E.; Cerdán, M.-E.; González-Siso, M.-I.; Becerra, M. Optimization of Saccharomyces cerevisiae α-galactosidase production and application in the degradation of raffinose family oligosaccharides. Microb. Cell Factories 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Fei, Y.; Jiao, W.; Wang, Y.; Liang, J.; Liu, G.; Li, L. Cloning and expression of a novel α-galactosidase from Lactobacillus amylolyticus L6 with hydrolytic and transgalactosyl properties. PLoS ONE 2020, 15, e0235687. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.; Önal, S. Cross-linked α-galactosidase aggregates: Optimization. characterization and application in the hydrolysis of raffi-nose-type oligosaccharides in soymilk. J. Sci. Food Agric. 2019, 99, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Reis, A.P.; Guimarães, V.M.; Falkoski, D.L.; Fialho, L.D.S.; De Rezende, S.T. Purification and Characterization of Aspergillus terreus α-Galactosidases and Their Use for Hydrolysis of Soymilk Oligosaccharides. Appl. Biochem. Biotechnol. 2011, 164, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D. Regarding the Regulation of Dietary Supplements. Am. J. Public Health 2015, 105, e3. [Google Scholar] [CrossRef]
- Resnik, D.B. Proportionality in Public Health Regulation: The Case of Dietary Supplements. Food Ethic 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Starr, R.R. Too Little. Too Late: Ineffective Regulation of Dietary Supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef]
- Frankos, V.H.; Street, D.A.; O’Neill, R.K. FDA regulation of dietary supplements and requirements regarding adverse event re-porting. Clin. Pharmacol. Ther. 2010, 87, 239–244. [Google Scholar] [CrossRef]
- Joseph, J.; Kropp, D.; Madsen, D.; Yannicelli, S. Regulation of dietary supplements. JAMA 2004, 291, 560. [Google Scholar]
- Maughan, R.J. Quality Assurance Issues in the Use of Dietary Supplements. with Special Reference to Protein Supplements. J. Nutr. 2012, 143, 1843S–1847S. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Fong, H.H.; Farnsworth, N.R. The role of quality assurance and standardization in the safety of botanical die-tary supplements. Chem. Res. Toxicol. 2007, 20, 577–582. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Fu, P.P.; Chiang, H.-M.; Xia, Q.; Chen, T.; Chen, B.H.; Yin, J.-J.; Wen, K.-C.; Lin, G.; Yu, H. Quality Assurance and Safety of Herbal Dietary Supplements. J. Environ. Sci. Health Part C 2009, 27, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, B.; Senal, M.O.; Akgoz, M.; Gören, A.C. Development and validation of a generic method for quantification of collagen in food supplement tablets using liquid chromatography coupled with time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Mei, S.; Zhang, Q.; Xin, N.; Chen, H. Application of the Plackett-Burman design to determine the main factors affecting the anti-oxidative activity of goat’s milk casein hydrolyzed by Alcalase and papain. Acta Sci. Pol. Technol. Aliment. 2018, 17, 257–266. [Google Scholar]
- Ghaderi, H.; Arasteh, J.; Hesampour, A. Using response surface methodology in combination with Plackett–Burman design for optimization of culture media and extracellular expression of Trichoderma reesei synthetic endoglucanase II in Escherichia coli. Mol. Biol. Rep. 2018, 45, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, Y.R.; A Soliman, N.; A Gaballa, A.; A Sabry, S.; I El-Diwany, A. Lipase production from a novel thermophilic Bacillus sp.: Application of Plackett-Burman design for evaluating culture conditions affecting enzyme formation. Acta Microbiol. Pol. 2002, 51, 353–366. [Google Scholar]
- Plackett, R.L. Literature on Testing the Equality of Variances and Covariances in Normal Populations. J. R. Stat. Soc. 1946, 109, 457. [Google Scholar] [CrossRef]
- Shi, X.; He, C.; Meng, J.; Xin, N.; Ji, Z. The application of the Plackett-Burman design in investigating ACE inhibitory peptide-producing conditions and media for Lactobacillus bulgaricus LB6. Acta Sci. Pol. Technol. Aliment. 2018, 17, 125–132. [Google Scholar] [PubMed]
- Schmulson, M.; Chang, L. Review article: The treatment of functional abdominal bloating and distension. Aliment. Pharmacol. Ther. 2011, 33, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.L.; Berry, J.A. The Regulation of Dietary Supplements. J. Am. Acad. Nurse Pract. 2003, 15, 410–414. [Google Scholar] [CrossRef][Green Version]
- Smith, Z.D.; Keller, J.R.; Bello, M.; Cordes, N.L.; Welch, C.F.; Torres, J.A.; Goodwin, L.A.; Pacheco, R.M.; Sandoval, C.W. Plackett-Burman experimental design to facilitate syntactic foam development. J. Appl. Polym. Sci. 2015, 133, 42892. [Google Scholar] [CrossRef]
- Chan, N.P.T.; Tarrant, M.; Ngan, E.; So, H.K.; Lok, K.Y.W.; Nelson, E.A.S. Agreement between self-/home-measured and assessor-measured waist circumference at three sites in adolescents/children. PLoS ONE 2018, 13, e0193355. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
| Analyst 1 (First Day) | Analyst 2 (Second Day) | ||||
|---|---|---|---|---|---|
| SAMPLE | Sample Weight (g) | Content Found (GalU/sachet) | Sample | Sample Weight (g) | Content Found (GalU/sachet) |
| A1 | 2.098 | 181.93 | B1 | 2.149 | 185.95 |
| A2 | 1.974 | 195.52 | B2 | 2.068 | 181.53 |
| A3 | 2.098 | 182.63 | B3 | 2.056 | 189.01 |
| A4 | 2.094 | 185.41 | B4 | 2.036 | 186.35 |
| A5 | 2.091 | 184.14 | B5 | 2.120 | 180.94 |
| A6 | 2.117 | 185.79 | B6 | 2.029 | 183.83 |
| AVERAGE | 185.91 | AVERAGE | 186.40 | ||
| DS | 4.95 | DS | 3.089 | ||
| RSD% | 2.661% | RSD% | 1.673% | ||
| RSD% TOTAL | 2.154% | ||||
| F calculated | 2.57 | Ftabulate | 5.05 | ||
| t calculated | 0.550 | t tabulate | 2.228 | ||
| Spectrophotometer (Method 1) | Fluorimeter (Method 2) |
|---|---|
| g/100 g | |
| 1.1501 | 1.0500 |
| 1.0743 | 1.3790 |
| 1.0907 | 1.2700 |
| 1.0832 | 1.4670 |
| 1.0929 | 1.3750 |
| 1.0938 | 1.2700 |
| 1.0678 | 1.2456 |
| 1.1118 | 1.3082 |
| 1.0962 | 1.0749 |
| 1.0644 | 1.1298 |
| 1.0814 | 1.0057 |
| 1.0702 | 1.2920 |
| ID | X1 | X2 | X3 | X4 | X5 | X6 | X7 | Y |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | −1 | 1 | −1 | −1 | 60.50 |
| 2 | −1 | 1 | 1 | 1 | −1 | 1 | −1 | 149.10 |
| 3 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 14.84 |
| 4 | 1 | −1 | −1 | 1 | 1 | 1 | −1 | 44.30 |
| 5 | −1 | 1 | −1 | −1 | 1 | 1 | 1 | 47.20 |
| 6 | 1 | −1 | 1 | −1 | −1 | 1 | 1 | 118.74 |
| 7 | 1 | 1 | −1 | 1 | −1 | −1 | 1 | 144.86 |
| 8 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 208.50 |
| Time (Minutes) | Reagent | Blank | Sample | Standard | Reference |
|---|---|---|---|---|---|
| T = 0 | Substrate solution | 2 mL | 2 mL | 2 mL | 2 mL |
| T = 5 | Borate solution | / | / | / | 5 mL |
| α-Galactosidase | / | / | 1mL | / | |
| H2O | 1 mL | / | / | / | |
| Sample | / | 1 mL | / | / | |
| T = 20 | Borate solution | 3 mL | 3 mL | 3 mL | / |
| Sample/standard | / | / | / | 1 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, E.; Bulfoni, M.; Nencioni, A.; Nencioni, E. Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements. Molecules 2021, 26, 1566. https://doi.org/10.3390/molecules26061566
Fabris E, Bulfoni M, Nencioni A, Nencioni E. Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements. Molecules. 2021; 26(6):1566. https://doi.org/10.3390/molecules26061566
Chicago/Turabian StyleFabris, Elena, Michela Bulfoni, Alessandro Nencioni, and Emanuele Nencioni. 2021. "Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements" Molecules 26, no. 6: 1566. https://doi.org/10.3390/molecules26061566
APA StyleFabris, E., Bulfoni, M., Nencioni, A., & Nencioni, E. (2021). Intra-Laboratory Validation of Alpha-Galactosidase Activity Measurement in Dietary Supplements. Molecules, 26(6), 1566. https://doi.org/10.3390/molecules26061566






