Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity
Abstract
:1. Introduction
2. Results
2.1. General Composition and Component Sugar Analysis
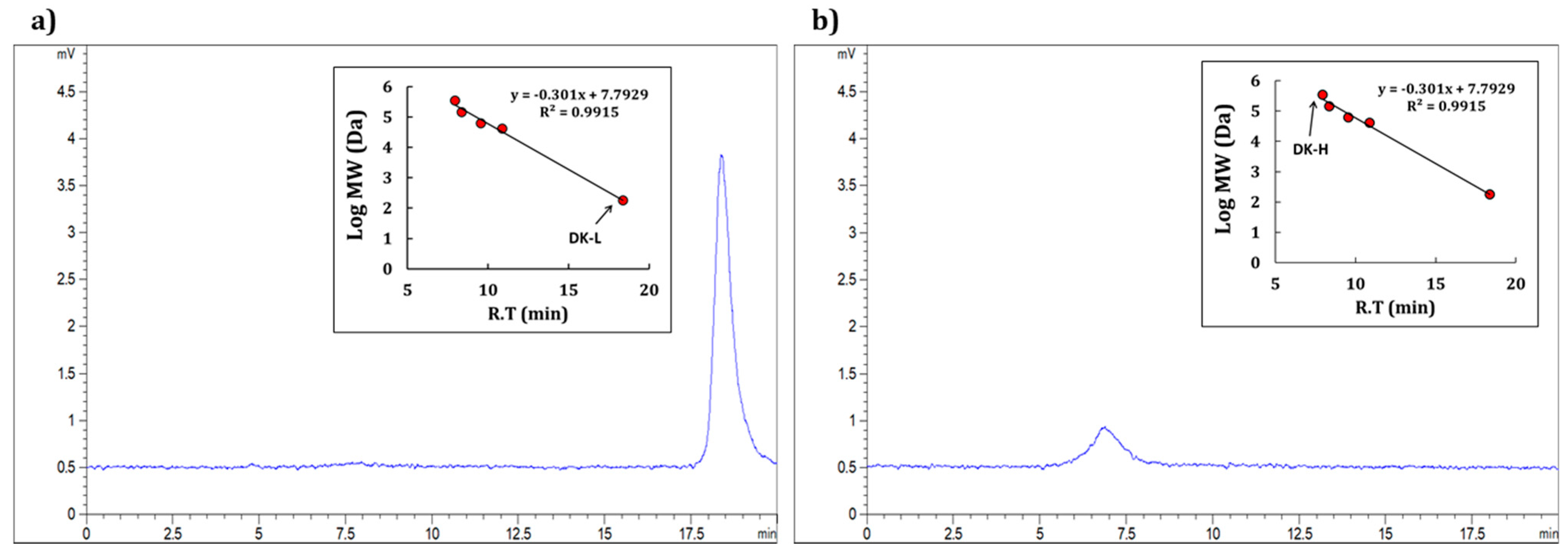
2.2. Component Sugars and Their Molecular Characterization
2.3. Antioxidant and Elastase Inhibitory Activities
2.4. Anti-inflammatory Activity Using RAW 264.7 Cells
2.5. Anti-Inflammatory Activity on the Skin Using HaCaT Cells
2.6. Antiwrinkle Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of Persimmon Extract and Its Fractionation
4.2. General Composition and Component Sugar Analysis
4.3. Antioxidant and Elastase Inhibitory Activity
4.4. Determination of Molecular Weight
4.5. Cytotoxicity and Anti-Inflammatory Activity Using RAW 264.7 Murine Macrophages
4.6. Anti-Inflammatory Activity on the Skin Using HaCaT Human Keratinocytes
4.7. Antiwrinkle Activity Using Human Dermal Fibroblasts (HDF)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dodon, J.; Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Gangwar, M.; Jana, S. The study of biofield energy treatment based herbomineral formulation in skin health and function. Am. J. Biosci. 2017, 5, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, W.J.; Park, S.Y.; Kim, H.; Chung, D.K. In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [Green Version]
- Garg, C.; Sharma, H.; Garg, M. Skin photo-protection with phytochemicals against photo-oxidative stress, photo-carcinogenesis, signal transduction pathways and extracellular matrix remodeling—An overview. Ageing Res. Rev. 2020, 62, 101127. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; de Melo, M.O.; de Camargo Junior, F.B. Effects of polysaccharide-based formulations on human skin. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and functionality of bioactive compounds present in persimmon. J. Chem. 2016, 2016, 3424025. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.-F.; Li, C.-M.; Xu, Y.-J.; Hu, W.-F.; Chen, M.-H.; Wan, Q.-h. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res. Int. 2008, 41, 208–217. [Google Scholar] [CrossRef]
- Jang, I.-C.; Jo, E.-K.; Bae, M.-S.; Lee, H.-J.; Jeon, G.-I.; Park, E.; Yuk, H.-G.; Ahn, G.-H.; Lee, S.-C. Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J. Med. Plants Res. 2010, 4, 155–160. [Google Scholar]
- Matsumoto, K.; Watanabe, Y.; Ohya, M.-a.; Yokoyama, S.-I. Young persimmon fruits prevent the rise in plasma lipids in a diet-induced murine obesity model. Biol. Pharm. Bull. 2006, 29, 2532–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Yokoyama, S.I.; Gato, N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother. Res. 2010, 24, 205–210. [Google Scholar] [CrossRef]
- Gato, N.; Kadowaki, A.; Hashimoto, N.; Yokoyama, S.-I.; Matsumoto, K. Persimmon fruit tannin-rich fiber reduces cholesterol levels in humans. Ann. Nutr. Metab. 2013, 62, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Li, C.; Chen, Z.; Jia, L. Extraction and in vitro antioxidant activity of mopan persimmon polysaccharide. J. Appl. Polym. Sci 2012, 124, 1751–1756. [Google Scholar] [CrossRef]
- Park, H.-R.; Hwang, D.; Hong, H.-D.; Shin, K.-S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct. Foods 2017, 37, 460–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Mo, X.; Lu, X.; Zhang, Y.; Qin, L. Modification, characterization and structure–anticoagulant activity relationships of persimmon polysaccharides. Carbohydr. Polym. 2010, 82, 515–520. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Ha, H.; Kim, R.; Cho, C.-W.; Song, Y.-R.; Hong, H.-D.; Kim, T. Anti-osteoporotic effects of polysaccharides isolated from persimmon leaves via osteoclastogenesis inhibition. Nutrients 2018, 10, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lu, X.; Zhang, Y.; Qin, L.; Zhang, J. Sulfated modification and immunomodulatory activity of water-soluble polysaccharides derived from fresh Chinese persimmon fruit. Int. J. Biol. Macromol. 2010, 46, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and totalcarbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction, 1st ed.; Tiwari, B.K., Brennan, C., Brunton, N.P., Eds.; Wiley-Blackwell: Chicester, UK, 2012. [Google Scholar] [CrossRef]
- Chen, J.; Miao, M.; Campanella, O.; Jiang, B.; Jin, Z. Biological macromolecule delivery system for improving functional performance of hydrophobic nutraceuticals. Curr. Opin. Food Sci. 2016, 9, 56–61. [Google Scholar] [CrossRef]
- Katekawa, E.; Caverzan, J.; Mussi, L.; Camargo-Junior, F.B.; Sufi, B.; Padovani, G.; Nazato, L.; Nogueira, C.; Magalhães, W.V.; Di Stasi, L.C. Novel topical skin hydration agent containing Anadenanthera colubrina polysaccharide-standardized herbal preparation. J. Cosmet. Dermatol. 2020, 19, 1691–1698. [Google Scholar] [CrossRef]
- Guo, L.; Qi, J.; Du, D.; Liu, Y.; Jiang, X. Current advances of Dendrobium officinale polysaccharides in dermatology: A literature review. Pharm. Biol. 2020, 58, 664–673. [Google Scholar] [CrossRef]
- Xu, J.; Yue, R.-Q.; Liu, J.; Ho, H.-M.; Yi, T.; Chen, H.-B.; Han, Q.-B. Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol. 2014, 67, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Dong, Q.; Ding, K.; Fang, J. Characterization of a pectic polysaccharide from the leaves of Diospyros kaki and its modulating activity on lymphocyte proliferation. Biopolymers 2010, 93, 649–656. [Google Scholar] [CrossRef]
- Song, Y.-R.; Han, A.-R.; Lim, T.-G.; Kang, J.-H.; Hong, H.-D. Discrimination of structural and immunological features of polysaccharides from persimmon leaves at different maturity stages. Molecules 2019, 24, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2009, 119, 477–483. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2011, 24, 64–73. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell Longev. 2015, 2016, 5692852. [Google Scholar] [CrossRef] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, E.; Lee, B.; Cho, W.K.; Ma, J.Y.; Park, K.I. Ethanolic extracts of Artemisia apiacea Hance improved atopic dermatitis-like skin lesions in vivo and suppressed TNF-Alpha/IFN-Gamma-induced proinflammatory chemokine production in vitro. Nutrients 2018, 10, 806. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.K.; Park, J.M.; Jeon, I.H.; Kim, H.S.; Jang, S.I. Effect of persimmon leaf extract on utraviolet B-induced inflammation in HaCaT keratinocytes and mice. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 583–590. [Google Scholar] [CrossRef]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Hong, Y.H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [Green Version]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.-W.; Zhu, S.; Yin, H.-P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.; Kim, W.J.; Chung, D.-K. Effect of paraprobiotic prepared from Kimchi-derived Lactobacillus plantarum K8 on skin moisturizing activity in human keratinocyte. J. Funct. Foods 2020, 75, 104244. [Google Scholar] [CrossRef]



| Sample | Total Sugar (mg Gal 1 Equivalent/g) | Uronic Acid (mg GalA 2 Equivalent/g) | Polyphenol (mg GA 3 Equivalent /g) |
|---|---|---|---|
| DK | 230.3 ± 4.9 b | 27.7 ± 0.2 b | 1.9 ± 0.6 b |
| DK-L | 210.1 ± 2.7 c | 16.8 ± 0.3 b | 0.7 ± 0.2 b |
| DK-H | 460.6 ± 7.6 a | 436.9 ± 13.2 a | 109.6 ± 5.0 a |
| Sample | DK-H (Mole%) |
|---|---|
| Mannose | 2.2 ± 0.8 |
| Rhamnose | 2.3 ± 0.0 |
| Glucuronic acid | ‒ |
| Galacturonic acid | 73.2 ± 2.1 |
| Glucose | 5.0 ± 0.3 |
| Galactose | 7.1 ± 0.3 |
| Xylose | 2.9 ± 0.3 |
| Arabinose | 7.4 ± 0.4 |
| Fucose | ‒ |
| Sample | ABTS 1 (IC50 4, μg/mL) | DPPH 2 (IC50, μg/mL) | FRAP 3 (mmol/g) | Elastase Inhibition (IC50, μg/mL) |
|---|---|---|---|---|
| DK | 10.3 ± 0.2 b | 17.1 ± 2.7 b | 65.6 ± 1.6 b | 647.1 ± 68.6 a |
| DK-L | 24 ± 0.4 a | 32.8 ± 3.1 a | 19.2 ± 2.6 b | n.d. |
| DK-H | 0.5 ± 0.0 c | 1.5 ± 1.6 c | 2203.8 ± 70.8 a | 8.1 ± 1.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity. Molecules 2021, 26, 1600. https://doi.org/10.3390/molecules26061600
Hwang KC, Shin HY, Kim WJ, Seo MS, Kim H. Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity. Molecules. 2021; 26(6):1600. https://doi.org/10.3390/molecules26061600
Chicago/Turabian StyleHwang, Ki Cheol, Hyun Young Shin, Woo Jung Kim, Mi Suk Seo, and Hoon Kim. 2021. "Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity" Molecules 26, no. 6: 1600. https://doi.org/10.3390/molecules26061600
APA StyleHwang, K. C., Shin, H. Y., Kim, W. J., Seo, M. S., & Kim, H. (2021). Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity. Molecules, 26(6), 1600. https://doi.org/10.3390/molecules26061600





