Progress in Molecular Nanoarchitectonics and Materials Nanoarchitectonics
Abstract
1. Introduction
2. Basic Nanoarchitectonics
2.1. Atom/Molecular-Level Nanoarchitectonics, Observation
2.2. Atom/Molecular-Level Nanoarchitectonics, Synthesis
2.3. Nanoarchitectonics toward Materials
2.4. Langmuir-Blodgett Nanoarchitectonics
3. Advanced Nanoarchitectonics Applications
3.1. Energy-Oriented Applications
3.2. Bio-Related Applications
4. Perspectives
Funding
Conflicts of Interest
References
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rhodium-Catalyzed C(sp2)- or C(sp3)-H bond functionalization assisted by removable directing groups. Angew. Chem. Int. Ed. 2019, 58, 8304–8329. [Google Scholar] [CrossRef]
- Muramatsu, W.; Hattori, T.; Yamamoto, H. Game change from reagent- to substrate-controlled peptide synthesis. Bull. Chem. Soc. Jpn. 2020, 93, 759–767. [Google Scholar] [CrossRef]
- Okamoto, T.; Yu, C.P.; Mitsui, C.; Yamagishi, M.; Ishii, H.; Takeya, J. Bent-shaped p-type small-molecule organic semiconductors: A molecular design strategy for next-generation practical applications. J. Am. Chem. Soc. 2020, 142, 9083–9096. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuhara, D.; Suzuki, M.; Hayashi, H.; Aratani, N. Synthesis and morphological control of organic semiconducting materials using the precursor approach. Bull. Chem. Soc. Jpn. 2020, 93, 1234–1267. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent development of pH-responsive polymers for cancer nanomedicine. Molecules 2019, 24, 4. [Google Scholar] [CrossRef]
- Jablonský, M.; Andrea Škulcová, A.; Šima, J. Use of deep eutectic solvents in polymer chemistry—A review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Yamago, S. Photoactivation of organotellurium Compounds in precision polymer synthesis: Controlled radical polymerization and radical coupling reactions. Bull. Chem. Soc. Jpn. 2020, 93, 287–298. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Pramoda, K. Borocarbonitrides, BxCyNz, 2D nanocomposites with novel properties. Bull. Chem. Soc. Jpn. 2019, 92, 441–468. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (nano)materials for novel biomedical applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Yajima, T.; Tsujimoto, Y.; Yamamoto, T.; Tassel, C.; Kobayashi, Y. Exploring structures and properties through anion chemistry. Bull. Chem. Soc. Jpn. 2019, 92, 1349–1357. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on carbon/polyaniline hybrids: Design and synthesis for supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, C.-M.; Liao, W.-S. A special connection between nanofabrication and analytical devices: Chemical lift-off lithography. Bull. Chem. Soc. Jpn. 2019, 92, 600–607. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Kirthi, A.V.; Akksadha, M.; Indu, S.; Dharshini, U.D.; Pushpamalar, J.; Karthik, L. Recent advancements in the applications of carbon nanodots: Exploring the rising star of nanotechnology. Nanoscale Adv. 2020, 2, 1760–1773. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating artificial intelligence and nanotechnology for precision cancer medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Roukes, M. Plenty of room, indeed. Sci. Am. 2001, 285, 48–51. [Google Scholar] [CrossRef]
- Ariga, K. From nanotechnology to nanoarchitectonics. J. Inorg. Organomet. Polym. 2015, 25, 177–178. [Google Scholar] [CrossRef][Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef]
- Liu, R.; Hudalla, G.A. Using self-assembling peptides to integrate biomolecules into functional supramolecular biomaterials. Molecules 2019, 24, 1450. [Google Scholar] [CrossRef] [PubMed]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.K.; Bergueiro, J.; Meijer, E.W.; Aida, T. Supramolecular polymerization: A conceptual expansion for innovative materials. Prog. Polym. Sci. 2020, 105, 101250. [Google Scholar] [CrossRef]
- Datta, S.; Kato, Y.; Higashiharaguchi, S.; Aratsu, K.; Isobe, A.; Saito, T.; Prabhu, D.D.; Kitamoto, Y.; Hollamby, M.J.; Smith, A.J.; et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 2020, 583, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. The concept of an ideal antibiotic: Implications for drug design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-based drug-delivery systems in biotechnological applications: Recent advances and perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y. Design of fluorescent peptide nucleic acid probes carrying cyanine dyes for targeting double-stranded RNAs for analytical applications. Bull. Chem. Soc. Jpn. 2020, 93, 406–413. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics revolution and evolution: From small science to big technology. Small Sci. 2021, 1, 2000032. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Li, M.; Richards, G.J.; Hill, J.P. Nanoarchitectonics: A conceptual paradigm for design and synthesis of dimension-controlled functional nanomaterials. J. Nanosci. Nanotechnol. 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Govindaraju, T.; Avinash, M.B. Two-dimensional nanoarchitectonics: Organic and hybrid materials. Nanoscale 2012, 4, 6102–6117. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Liu, W.; Liu, Z. Graphene nanoarchitectonics: A new material horizon for reinforcement of sustainable polymers. Front. Mater. 2020, 7, 276. [Google Scholar] [CrossRef]
- Ariga, K.; Shionoya, M. Nanoarchitectonics for coordination asymmetry and related chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef]
- Wang, H.; Yin, S.; Eid, K.; Li, Y.; Xu, Y.; Li, X.; Xue, H.; Wang, L. Fabrication of mesoporous cage-bell Pt nanoarchitectonics as efficient catalyst for oxygen reduction reaction. ACS Sustain. Chem. Eng. 2018, 6, 11768–11774. [Google Scholar] [CrossRef]
- Giese, M.; Spengler, M. Cellulose nanocrystals in nanoarchitectonics—towards photonic functional materials. Mol. Syst. Des. Eng. 2019, 4, 29–48. [Google Scholar] [CrossRef]
- Abe, H.; Liu, J.; Ariga, K. Catalytic nanoarchitectonics for environmentally compatible energy generation. Mater. Today 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Ariga, K.; Ishihara, S.; Abe, H. Atomic architectonics, nanoarchitectonics and microarchitectonics for strategies to make junk materials work as precious catalysts. CrystEngComm 2016, 18, 6770–6778. [Google Scholar] [CrossRef]
- Chen, G.; Sciortino, F.; Ariga, K. Atomic nanoarchitectonics for catalysis. Adv. Mater. Interfaces 2021, 8, 2001395. [Google Scholar]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Makita, T.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Review of advanced sensor devices employing nanoarchitectonics concepts. Beilstein J. Nanotechnol. 2019, 10, 2014–2030. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Yang, W.; Ariga, K. Soft nanoarchitectonics for enantioselective biosensing. Acc. Chem. Res. 2020, 53, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Watanabe, S.; Mori, T.; Takeya, J. Soft 2D nanoarchitectonics. NPG Asia Mater. 2018, 10, 90–106. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Supramolecular nanoarchitectonics for functional materials. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Pham, T.-A.; Qamar, A.; Dinh, T.; Masud, M.K.; Rais-Zadeh, M.; Senesky, D.G.; Yamauchi, Y.; Nguyen, N.-T.; Phan, H.-P. Nanoarchitectonics for wide bandgap semiconductor nanowires: Toward the next generation of nanoelectromechanical systems for environmental monitoring. Adv. Sci. 2020, 7, 2001294. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-Active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Giussi, J.M.; Cortez, M.I.; Marmisollé, W.A.; Azzaroni, O. Practical use of polymer brushes in sustainable energy applications: Interfacial nanoarchitectonics for high-efficiency devices. Chem. Soc. Rev. 2019, 48, 814–849. [Google Scholar] [CrossRef]
- Huang, H.J.; Yan, M.M.; Yang, C.Z.; He, H.Y.; Jiang, Q.G.; Yang, L.; Lu, Z.Y.; Sun, Z.Q.; Xu, X.T.; Bando, Y.; et al. Graphene nanoarchitectonics: Recent advances ingraphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef]
- Ariga, K.; Ishihara, S.; Abe, H.; Li, M.; Hill, J.P. Materials nanoarchitectonics for environmental remediation and sensing. J. Mater. Chem. 2012, 22, 2369–2377. [Google Scholar] [CrossRef]
- Pandeeswar, M.; Senanayak, S.P.; Govindaraju, T. Nanoarchitectonics of small molecule and DNA for ultrasensitive detection of mercury. ACS Appl. Mater. Interfaces 2016, 8, 30362–30371. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Jackman, J.A.; Cho, N.-J.; Hsu, S.-h.; Shrestha, L.K.; Mori, T.; Takeya, J. Nanoarchitectonic-based material platforms for environmental and bioprocessing applications. Chem. Rec. 2019, 19, 1891–1912. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef]
- Stulz, E. Nanoarchitectonics with porphyrin functionalized DNA. Acc. Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J.; Nishikawa, M.; Yoshikawa, G.; Mori, T.; Shrestha, L.K.; Ariga, K. Materials nanoarchitectonics for mechanical tools in chemical and biological sensing. Chem. Asian J. 2018, 13, 3366–3377. [Google Scholar] [CrossRef]
- Avinash, M.B.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Hsieh, P.-H.; Hsu, Y.-S.; Kaneti, Y.V.; Shieh, F.-K.; Yamauchi, Y.; Wu, K.C.-W. Nanoarchitectonics of biofunctionalized metal–organic frameworks with biological macromolecules and living cells. Small Methods 2019, 3, 1900213. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, J. Solid lipid matrix mediated nanoarchitectonics for improved oral bioavailability of drugs. Expert Opin. Drug Metab. Toxicol. 2019, 15, 499–515. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond self-assembly: Challenges to create bio-like hierarchic organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef]
- Ariga, K. Atomic and organic nanoarchitectonics. Trends Chem. 2020, 2, 779–782. [Google Scholar] [CrossRef]
- Nakamura, E. Atomic-resolution transmission electron microscopic movies for study of organic molecules, assemblies, and reactions: The first 10 years of development. Acc. Chem. Res. 2017, 50, 1281–1292. [Google Scholar] [CrossRef]
- Harano, K. Self-assembly mechanism in nucleation processes of molecular crystalline materials. Bull. Chem. Soc. Jpn. 2021, 94, 463–472. [Google Scholar] [CrossRef]
- Shimizu, T.; Lungerich, D.; Stuckner, J.; Murayama, M.; Harano, K.; Nakamura, E. Real-time video imaging of mechanical motions of a single molecular shuttle with sub-millisecond sub-angstrom precision. Bull. Chem. Soc. Jpn. 2020, 93, 1079–1085. [Google Scholar] [CrossRef]
- Kamei, K.; Shimizu, T.; Harano, K.; Nakamura, E. Aryl radical addition to curvatures of carbon nanohorns for single-molecule-level molecular imaging. Bull. Chem. Soc. Jpn. 2020, 93, 1603–1608. [Google Scholar] [CrossRef]
- Xing, J.; Schweighauser, L.; Okada, S.; Harano, K.; Nakamura, E. Atomistic structures and dynamics of prenucleation clusters in MOF-2 and MOF-5 syntheses. Nat. Commun. 2019, 10, 3608. [Google Scholar] [CrossRef]
- Toyota, S.; Yamamoto, Y.; Wakamatsu, K.; Tsurumaki, E.; Muñoz-Castro, A. Nano-Saturn with an ellipsoidal body: Anthracene macrocyclic ring-C70 complex. Bull. Chem. Soc. Jpn. 2019, 92, 1721–1728. [Google Scholar] [CrossRef]
- Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Synthesis of a carbon nanobelt. Science 2017, 356, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ikemoto, K.; Fukunaga, T.M.; Koretsune, T.; Arita, R.; Sato, S.; Isobe, H. Finite phenine nanotubes with periodic vacancy defects. Science 2019, 363, 151–155. [Google Scholar] [CrossRef]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and characterizations of functional polycyclic aromatic hydrocarbons and graphene nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Müllen, K. Evolution of graphene molecules: Structural and functional complexity as driving forces behind nanoscience. ACS Nano 2014, 8, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Gröning, O.; Wang, S.; Yao, X.; Pignedoli, C.A.; Borin, G.; Daniels, B.C.; Cupo, A.; Meunier, V.; Feng, X.; Narita, A.; et al. Engineering of robust topological quantum phases in graphene nanoribbons. Nature 2018, 560, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Krejči, O.; Foster, A.S.; Okuda, Y.; Orita, A.; Kawai, S. Synthesis of regioisomeric graphene nanoribbon junctions via heteroprecursors. J. Phys. Chem. C 2019, 123, 17632–17638. [Google Scholar] [CrossRef]
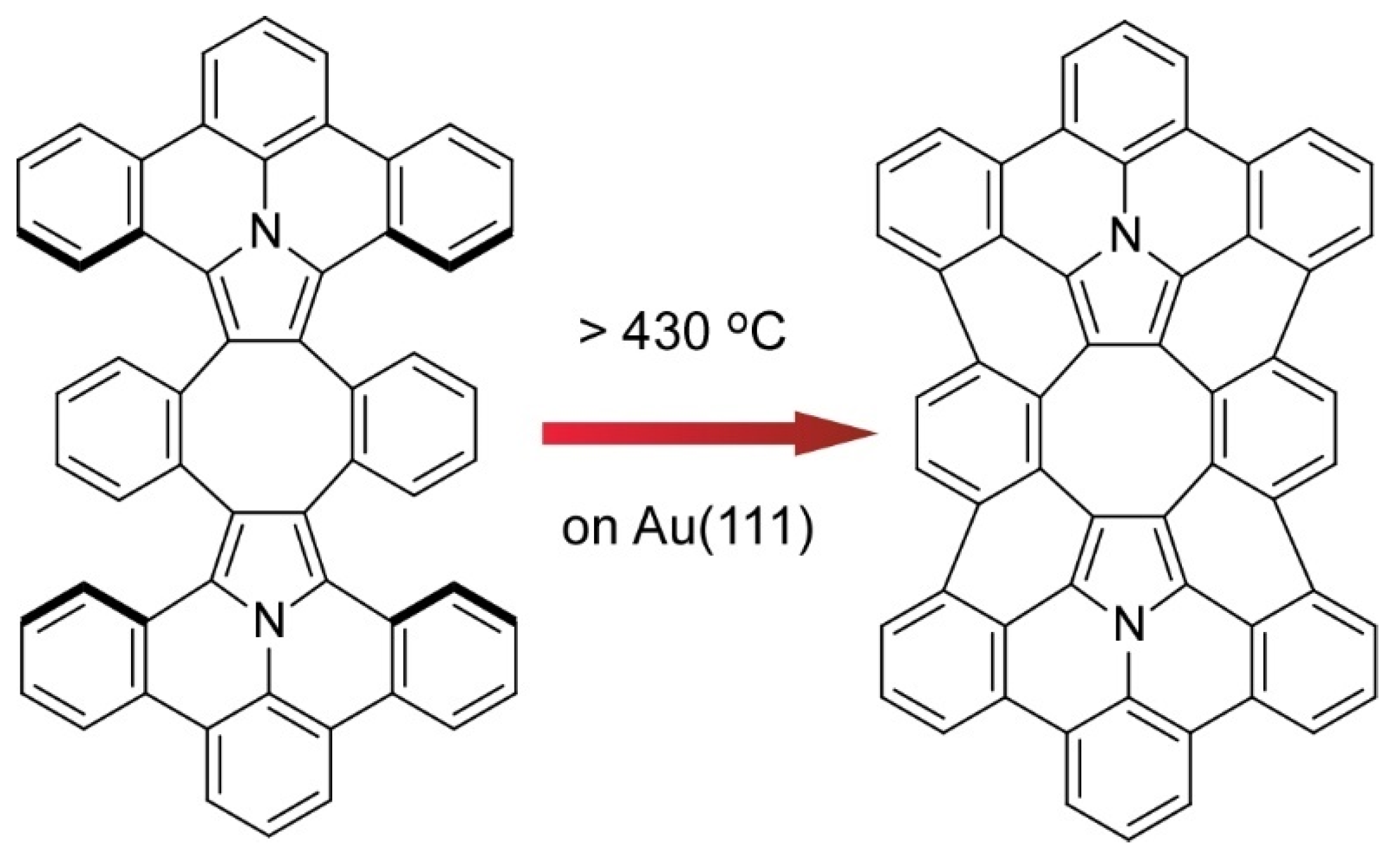
- Nakamura, K.; Li, Q.-Q.; Krejčí, O.; Foster, A.S.; Sun, K.; Kawai, S.; Ito, S. On-surface synthesis of a π-extended diaza[8]circulene. J. Am. Chem. Soc. 2020, 142, 11363–11369. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Krejčí, O.; Nishiuchi, T.; Sahara, K.; Kodama, T.; Pawlak, R.; Meyer, E.; Kubo, T.; Foster, A.S. Three-dimensional graphene nanoribbons as a framework for molecular assembly and local probe chemistry. Sci. Adv. 2020, 6, eaay8913. [Google Scholar] [CrossRef]
- Nayak, A.; Unayama, S.; Tai, S.; Tsuruoka, T.; Waser, R.; Aono, M.; Valov, I.; Hasegawa, T. Nanoarchitectonics for controlling the number of dopant atoms in solid electrolyte nanodots. Adv. Mater. 2018, 30, 1703261. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, T.; Yamamoto, K. Wet-chemical strategy for atom-precise metal cluster catalysts. Bull. Chem. Soc. Jpn. 2019, 92, 941–948. [Google Scholar] [CrossRef]
- Yamamoto, K.; Higuchi, M.; Shiki, S.; Tsuruta, M.; Chiba, H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature 2002, 415, 509–511. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T. Dendrimer Complexes Based on Fine-Controlled Metal Assembling. Bull. Chem. Soc. Jpn. 2006, 79, 511. [Google Scholar] [CrossRef]
- Cortez, M.L.; Lorenzo, A.; Marmisollé, W.A.; Von Bilderling, C.; Maza, E.; Pietrasanta, L.I.; Battaglini, F.; Ceolín, M.; Azzaroni, O. Azzaroni, Highly-organized stacked multilayers via layer-by-layer assembly of lipid-like surfactants and polyelectrolytes. Stratified supramolecular structures for (bio)electrochemical nanoarchitectonics. Soft Matter 2018, 14, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Shrestha, L.K. Supramolecular nanoarchitectonics for functional materials. APL Mater. 2019, 7, 120903. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Nanoarchitectonics through supramolecular gelation: Formation and switching of diverse nanostructures. Mol. Syst. Des. Eng. 2019, 4, 11–28. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular chiral nanoarchitectonics. Adv. Mater. 2020, 32, 1905657. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Mori, T.; Ariga, K. Molecular imprinting: Materials nanoarchitectonics with molecular information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Sangian, D.; Ide, Y.; Bando, Y.; Rowan, A.E.; Yamauchi, Y. Materials nanoarchitectonics using 2D layered materials: Recent developments in the intercalation process. Small 2018, 14, 1800551. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Li, Y.; Cai, Z.; Zakaria, M.B.; Masud, M.K.; Hossain, M.S.A.; Kim, J.; Zhang, W.; Na, J.; Yamauchi, Y.; et al. Nanoarchitectonics: A new materials horizon for Prussian blue and its analogues. Bull. Chem. Soc. Jpn. 2019, 92, 875–904. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Shrestha, L.K. Soft material nanoarchitectonics at interfaces: Molecular assembly, nanomaterial synthesis, and life control. Mol. Syst. Des. Eng. 2019, 4, 49–64. [Google Scholar] [CrossRef]
- Miyajima, N.; Wang, Y.-C.; Nakagawa, M.; Kurata, H.; Imura, Y.; Wang, K.-H.; Kawai, T. Water-phase synthesis of ultrathin Au nanowires with a two-dimensional parallel array structure. Bull. Chem. Soc. Jpn. 2020, 93, 1372–1377. [Google Scholar] [CrossRef]
- Akagi, K. Interdisciplinary chemistry based on integration of liquid crystals and conjugated polymers: Development and progress. Bull. Chem. Soc. Jpn. 2019, 92, 1509–1655. [Google Scholar] [CrossRef]
- Akagi, K.; Piao, G.; Kaneko, S.; Sakamaki, K.; Shirakawa, H.; Kyotani, M. Helical polyacetylene synthesized with a chiral nematic reaction field. Science 1998, 282, 1683–1686. [Google Scholar] [CrossRef]
- Ariga, K.; Ishii, M.; Mori, T. 2D nanoarchitectonics: Soft interfacial media as playgrounds for microobjects, molecular machines, and living cells. Chem. Eur. J. 2020, 26, 6461–6472. [Google Scholar] [CrossRef]
- Ariga, K. Molecular tuning nanoarchitectonics for molecular recognition and molecular manipulation. ChemNanoMat 2020, 6, 870–880. [Google Scholar] [CrossRef]
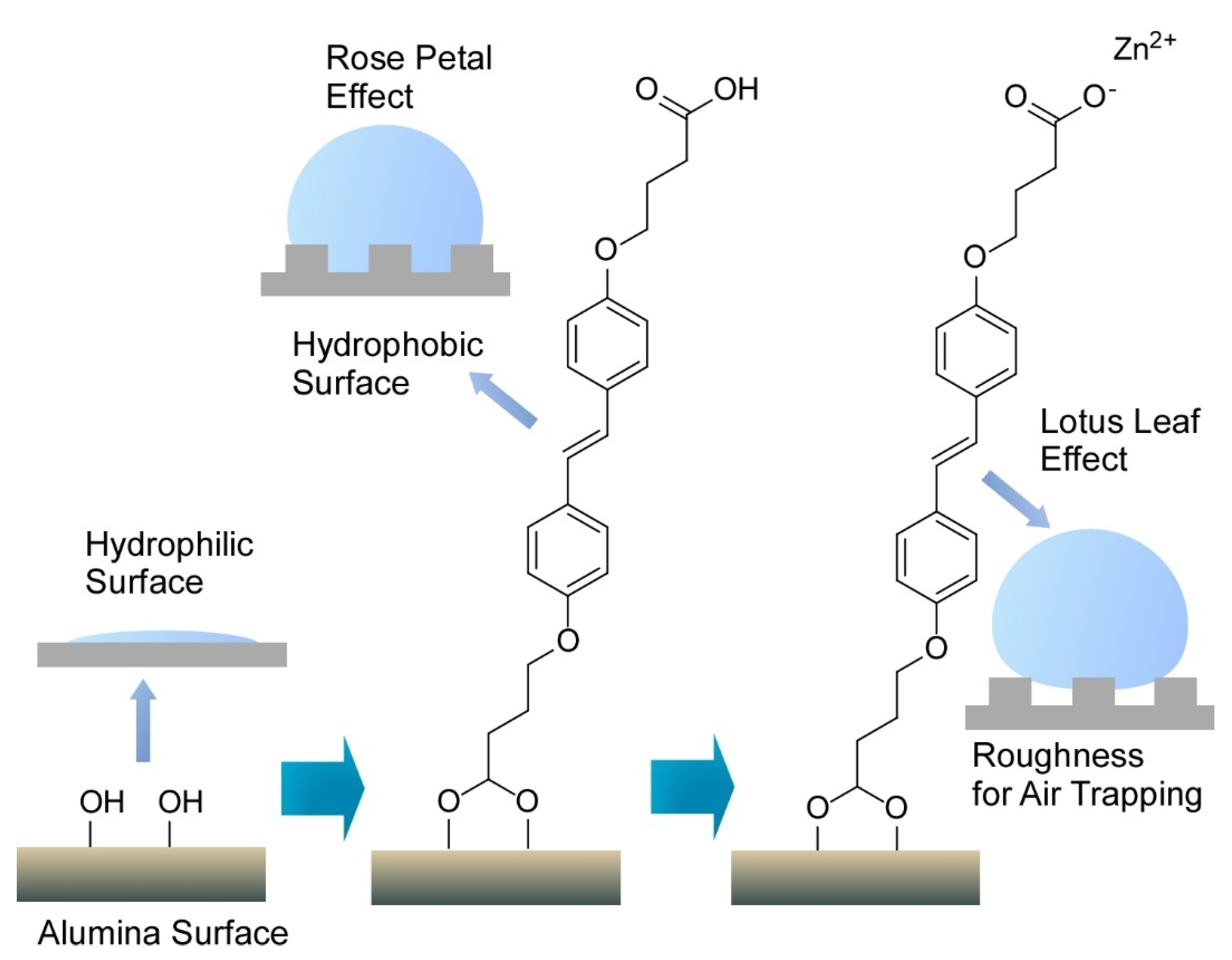
- Mukhopadhyay, R.D.; Vedhanarayanan, B.; Ajayaghosh, A. Creation of “rose petal” and “lotus leaf” effects on alumina by surface functionalization and metal-ion coordination. Angew. Chem. Int. Ed. 2017, 56, 16018–16022. [Google Scholar] [CrossRef]
- Makiura, R.; Motoyama, S.; Umemura, Y.; Yamanaka, H.; Sakata, O.; Kitagawa, H. Surface nano-architecture of a metal-organic framework. Nat. Mater. 2020, 9, 565–571. [Google Scholar] [CrossRef]
- Sakamoto, R.; Takada, K.; Sun, X.; Pal, T.; Tsukamoto, T.; Phua, E.J.H.; Rapakousiou, A.; Hoshiko, K.; Nishihara, H. The coordination nanosheet (CONASH). Coord. Chem. Rev. 2016, 320, 118–128. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Pan, Y.; Behera, N.; Jin, W. Metal-organic framework nanosheets: An emerging family of multifunctional 2D materials. Coord. Chem. Rev. 2019, 395, 25–45. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Ariga, K.; Matsumoto, M.; Mori, T.; Shrestha, L.K. Materials nanoarchitectonics at two-dimensional liquid interfaces. Beilstein J. Nanotechnol. 2019, 10, 1559–1587. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Ji, Q.; Mori, T.; Miyazawa, K.; Yamauchi, Y.; Hill, J.P.; Ariga, K. Fullerene nanoarchitectonics: From zero to higher dimensions. Chem. Asian J. 2013, 8, 1662–1679. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Fullerene nanoarchitectonics with shape-shifting. Materials 2020, 13, 2280. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Zero-to-one (or more) nanoarchitectonics: How to produce functional materials from zero-dimensional single-element unit, fullerene. Mater. Adv. 2021, 2, 582–597. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous carbon tubes from fullerene crystals as the π-electron carbon source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Highly ordered 1D fullerene crystals for concurrent control of macroscopic cellular orientation and differentiation toward large-scale tissue engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.; Hill, J.P.; Ariga, K. Solvent engineering for shape-shifter pure fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Yamauchi, Y.; Hill, J.P.; Miyazawa, K.I.; Ariga, K. Fullerene Crystals with Bimodal Pore Architectures Consisting of Macropores and Mesopores. J. Am. Chem. Soc. 2013, 135, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Tsuruoka, T.; Acharya, S.; Ji, Q.; Hill, J.P.; Ariga, K.; Yamauchi, Y.; Shrestha, L.K. Mesoporous fullerene C70 cubes with highly crystalline frameworks and unusually enhanced photoluminescence properties. Mater. Horiz. 2018, 5, 285–290. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Sathish, M.; Hill, J.P.; Miyazawa, K.; Tsuruoka, T.; Sanchez-Ballester, N.M.; Honma, I.; Ji, Q.; Ariga, K. Alcohol-induced decomposition of Olmstead’s crystalline Ag(I)–fullerene heteronanostructure yields ‘bucky cubes’. J. Mater. Chem. C 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Hierarchically structured fullerene C70 cube for sensing volatile aromatic solvent vapors. ACS Nano 2016, 10, 6631–6637. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Intentional closing/opening of “hole-in-cube” fullerene crystals with microscopic recognition properties. ACS Nano 2017, 11, 7790–7796. [Google Scholar] [CrossRef]
- Bairi, P.; Minami, K.; Hill, J.P.; Nakanishi, W.; Shrestha, L.K.; Liu, C.; Harano, K.; Nakamura, E.; Ariga, K. Supramolecular differentiation for construction of anisotropic fullerene nanostructures by time-programmed control of interfacial growth. ACS Nano 2016, 10, 8796–8802. [Google Scholar] [CrossRef]
- Tang, Q.; Maji, S.; Jiang, B.; Sun, J.; Zhao, W.; Hill, J.P.; Ariga, K.; Fuchs, H.; Ji, Q.; Shrestha, L.K. Manipulating the structural transformation of fullerene microtubes to fullerene microhorns having microscopic recognition properties. ACS Nano 2019, 13, 14005–14012. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsu, S.-h.; Maji, S.; Chahal, M.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post-assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2020, 7, 787–795. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Li, J. Langmuir nanoarchitectonics from basic to frontier. Langmuir 2019, 35, 3585–3599. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takimoto, K.; Kato, M.; Nagaoka, S.; Tamura, K.; Yamagishi, A. Real-time monitoring of low pressure oxygen molecules over wide temperature range: Feasibility of ultrathin hybrid films of iridium(III) complexes and clay nanosheets. Bull. Chem. Soc. Jpn. 2020, 93, 194–199. [Google Scholar] [CrossRef]
- Onda, M.; Yoshihara, K.; Koyano, H.; Ariga, K.; Kunitake, T. Molecular recognition of nucleotides by the guanidinium unit at the surface of aqueous micelles and bilayers. A comparison of microscopic and macroscopic interfaces. J. Am. Chem. Soc. 1996, 118, 8524–8530. [Google Scholar] [CrossRef]
- Ariga, K. Molecular recognition at the air–water interface: Nanoarchitectonic design and physicochemical understanding. Phys. Chem. Chem. Phys. 2020, 22, 24856–24869. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Ohto, K.; Tanaka, Y.; Aoyama, Y.; Kunitake, T. Molecular recognition of sugars by monolayers of resorcinol-dodecanal cyclotetramer. J. Am. Chem. Soc. 1991, 113, 444–450. [Google Scholar] [CrossRef]
- Kurihara, K.; Ohto, K.; Honda, Y.; Kunitake, T. Efficient, complementary binding of nucleic acid bases to diaminotriazine-functionalized monolayers on water. J. Am. Chem. Soc. 1991, 113, 5077–5079. [Google Scholar] [CrossRef]
- Ariga, K.; Kunitake, T. Molecular recognition at air−water and related interfaces: complementary hydrogen bonding and multisite interaction. Acc. Chem. Res. 1998, 31, 371–378. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.P.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Russell, K.C.; Leblanc, R.M. Effect of complementary hydrogen bonding additives in subphase on the structure and properties of the 2-amino-4,6-dioctadecylamino-1,3,5-triazine amphiphile at the air-water interface: Studies by ultraviolet-visible absorption spectroscopy and brewster angle microscopy. Langmuir 1998, 14, 2174–2186. [Google Scholar]
- Neal, J.F.; Zhao, W.; Grooms, A.J.; Smeltzer, M.A.; Shook, B.M.; Flood, A.H.; Allen, H.C. Interfacial supramolecular structures of amphiphilic receptors drive aqueous phosphate recognition. J. Am. Chem. Soc. 2019, 141, 7876–7886. [Google Scholar] [CrossRef]
- Okuno, M.; Yamada, S.; Ohto, T.; Tada, H.; Nakanishi, W.; Ariga, K.; Ishibashi, T. Hydrogen bonds and molecular orientations of supramolecular structure between barbituric acid and melamine derivative at the air/water interface revealed by heterodyne-detected vibrational sum frequency generation spectroscopy. J. Phys. Chem. Lett. 2020, 11, 2422–2429. [Google Scholar] [CrossRef]
- Grooms, A.J.; Neal, J.F.; Ng, K.C.; Zhao, W.; Flood, A.H.; Allen, H.C. Thermodynamic signatures of the origin of anti-Hofmeister selectivity for phosphate at aqueous interfaces. J. Phys. Chem. A 2020, 124, 5621–5630. [Google Scholar] [CrossRef]
- Sakurai, M.; Tamagawa, H.; Inoue, Y.; Ariga, K.; Kunitake, T. Theoretical study of intermolecular interaction at the lipid−water Interface. 1. Quantum chemical analysis using a reaction field theory. J. Phys. Chem. B 1997, 101, 4810–4816. [Google Scholar] [CrossRef]
- Tamagawa, H.; Sakurai, M.; Inoue, Y.; Ariga, K.; Kunitake, T. Theoretical study of intermolecular interaction at the lipid−water interface. 2. Analysis based on the Poisson−Boltzmann equation. J. Phys. Chem. B 1997, 101, 4817–4825. [Google Scholar] [CrossRef]
- Oishi, Y.; Torii, Y.; Kato, T.; Kuramori, M.; Suehiro, K.; Ariga, K.; Taguchi, K.; Kamino, A.; Koyano, A.H.; Kunitake, T. Molecular patterning of a guanidinium/orotate mixed monolayer through molecular recognition with flavin adenine dinucleotide. Langmuir 1997, 13, 519–524. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Hill, J.P. Mechanical control of nanomaterials and nanosystems. Adv. Mater. 2012, 24, 158–176. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. What can be done with the Langmuir-Blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. The evolution of molecular machines through interfacial nanoarchitectonics: From toys to tools. Chem. Sci. 2020, 11, 10594–10604. [Google Scholar] [CrossRef]
- Ariga, K.; Terasaka, Y.; Sakai, D.; Tsuji, H.; Kikuchi, J. Piezoluminescence based on molecular recognition by dynamic cavity array of steroid cyclophanes at the air-water interface. J. Am. Chem. Soc. 2000, 122, 7835–7836. [Google Scholar] [CrossRef]
- Ariga, K.; Nakanishi, T.; Terasaka, Y.; Tsuji, H.; Sakai, D.; Kikuchi, J. Piezoluminescence at the air-water interface through dynamic molecular recognition driven by lateral pressure application. Langmuir 2005, 21, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Michinobu, T.; Shinoda, S.; Nakanishi, T.; Hill, J.P.; Fujii, K.; Player, T.N.; Tsukube, H.; Ariga, K. Mechanical control of enantioselectivity of amino acid recognition by cholesterol-armed cyclen monolayer at the air-water interface. J. Am. Chem. Soc. 2006, 128, 14478–14479. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Okamoto, K.; Endo, H.; Hill, J.P.; Shinoda, S.; Matsukura, M.; Tsukube, H.; Suzuki, Y.; Kanekiyo, Y.; Ariga, K. Mechanical tuning of molecular recognition to discriminate the single-methyl-group difference between thymine and uracil. J. Am. Chem. Soc. 2010, 132, 12868–12870. [Google Scholar] [CrossRef]
- Sakakibara, K.; Joyce, L.A.; Mori, T.; Fujisawa, T.; Shabbir, S.H.; Hill, J.P.; Anslyn, E.V.; Ariga, K. A mechanically controlled indicator displacement assay. Angew. Chem. Int. Ed. 2012, 51, 9643–9646. [Google Scholar] [CrossRef]
- Mori, T.; Komatsu, H.; Sakamoto, N.; Suzuki, K.; Hill, J.P.; Matsumoto, M.; Sakai, H.; Ariga, K.; Nakanishi, W. Molecular rotors confined at an ordered 2D interface. Phys. Chem. Chem. Phys. 2018, 20, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Chin, H.; Kawashima, K.; Ngo, H.T.; Cho, N.-J.; Nakanishi, W.; Hill, J.P.; Ariga, K. Dynamic control of intramolecular rotation by tuning the surrounding two-dimensional matrix field. ACS Nano 2019, 13, 2410–2419. [Google Scholar] [CrossRef]
- Ishikawa, D.; Mori, T.; Yonamine, Y.; Nakanishi, W.; Cheung, D.J.; Hill, J.P.; Ariga, K. Mechanochemical tuning of the binaphthyl conformation at the air-water Interface. Angew. Chem. Int. Ed. 2015, 54, 8988–8991. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ishikawa, D.; Yonamine, Y.; Fujii, Y.; Hill, J.P.; Ichinose, I.; Ariga, K.; Nakanishi, W. Mechanically induced opening-closing action of binaphthyl molecular pliers: Digital phase transition versus continuous conformational change. ChemPhysChem 2017, 18, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, W.; Saito, S.; Sakamoto, N.; Kashiwagi, A.; Yamaguchi, S.; Sakai, H.; Ariga, K. Monitoring fluorescence response of amphiphilic flapping molecules in compressed monolayers at the air-water interface. Chem. Asian J. 2019, 14, 2869–2876. [Google Scholar] [CrossRef]
- Ishii, M.; Mori, T.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Helicity manipulation of a double-paddled binaphthyl in a two-dimensional matrix field at the air-water interface. ACS Nano 2020, 14, 13294–13303. [Google Scholar] [CrossRef] [PubMed]
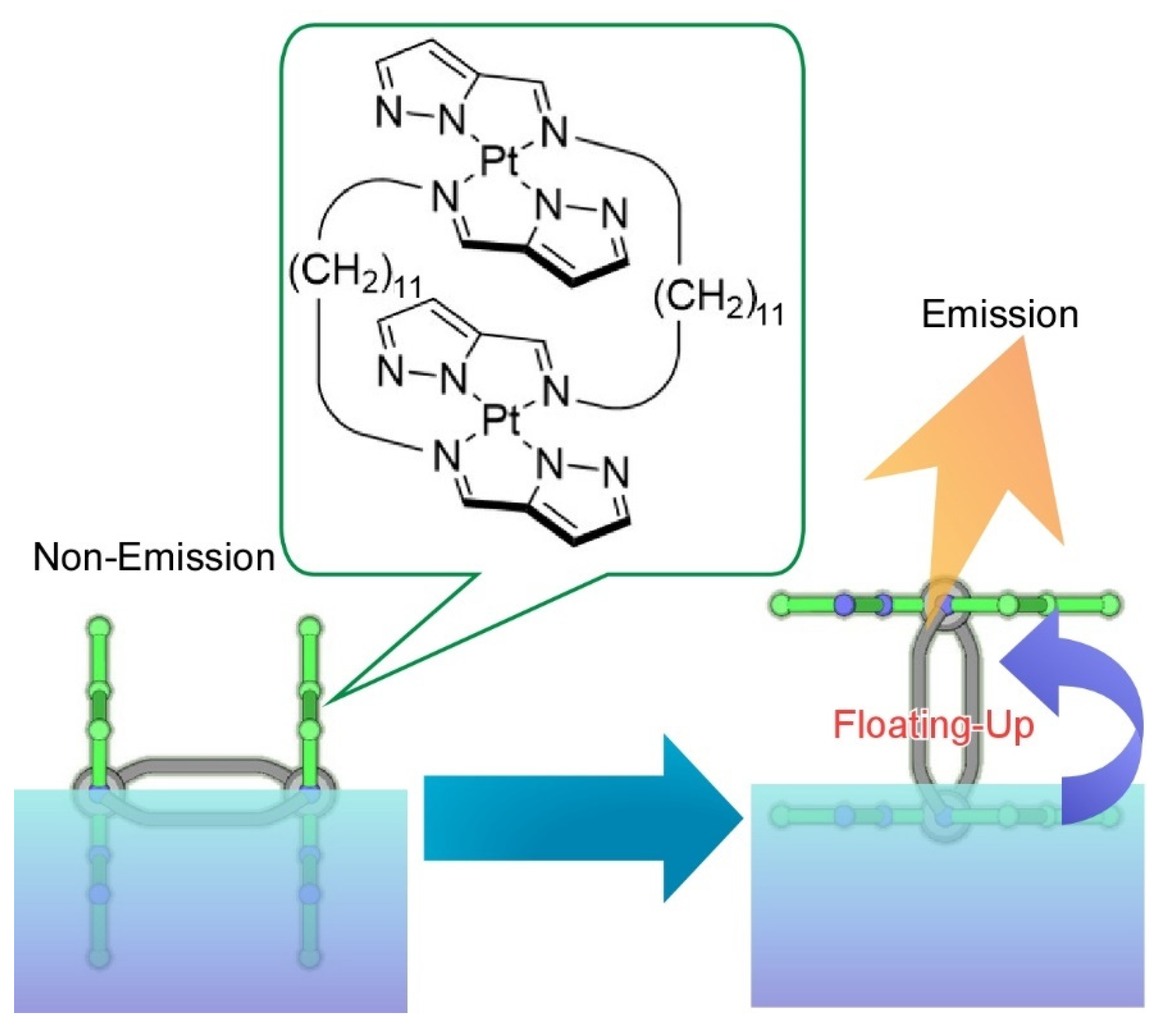
- Adachi, J.; Mori, T.; Inoue, R.; Naito, M.; Le, N.H.-T.; Kawamorita, S.; Hill, J.P.; Naota, T.; Ariga, K. Emission control by molecular manipulation of double-paddled binuclear PtII complexes at the air-water interface. Chem. Asian J. 2020, 15, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tanaka, H.; Dalui, A.; Mitoma, N.; Suzuki, K.; Matsumoto, M.; Aggarwal, N.; Patnaik, A.; Acharya, S.; Shrestha, L.K.; et al. Carbon nanosheets by morphology-retained carbonization of two-dimensional assembled anisotropic carbon nanorings. Angew. Chem. Int. Ed. 2018, 57, 9679–9683. [Google Scholar] [CrossRef]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-aligned fullerene nanowhiskers as a scaffold for orienting cell growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sasabe, H.; Kido, J. Review of molecular engineering for horizontal molecular orientation in organic light-emitting devices. Bull. Chem. Soc. Jpn. 2019, 92, 716–728. [Google Scholar] [CrossRef]
- Yokoyama, D.; Sasabe, H.; Furukawa, Y.; Adachi, C.; Kido, J. Molecular stacking induced by intermolecular C–H···N hydrogen bonds leading to high carrier mobility in vacuum-deposited organic films. Adv. Funct. Mater. 2011, 21, 1375–1382. [Google Scholar] [CrossRef]
- Yokoyama, D. Molecular orientation in small-molecule organic light-emitting diodes. J. Mater. Chem. 2011, 21, 19187–19202. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir–Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, T.; Kimura, Y.; Fujimori, A. Maintenance properties of enzyme molecule stereostructure at high temperature by adsorption on organo-modified magnetic nanoparticle layer template. Bull. Chem. Soc. Jpn. 2019, 92, 1662–1671. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B.S. Layer-by-layer assembly: Recent progress from layered assemblies to layered nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Ito, M.; Yamashita, Y.; Tsuneda, Y.; Mori, T.; Takeya, J.; Watanabe, S.; Ariga, K. 100 °C-Langmuir-Blodgett method for fabricating highly oriented, ultrathin films of polymeric semiconductors. ACS Appl. Mater. Interfaces 2020, 12, 56522–56529. [Google Scholar] [CrossRef]
- Jackman, J.A.; Ferhan, A.R.; Cho, N.-J. Surface-based nanoplasmonic sensors for biointerfacial science applications. Bull. Chem. Soc. Jpn. 2019, 92, 1404–1412. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; McShane, M.J.; Lvov, Y.M.; Vinu, A.; Hill, J.P. Inorganic nanoarchitectonics for biological applications. Chem. Mater. 2012, 24, 728–737. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Jiang, X.-F.; Bando, Y. Blowing route towards advanced inorganic foams. Bull. Chem. Soc. Jpn. 2019, 92, 245–263. [Google Scholar] [CrossRef]
- Yamada, Y. Concentrated battery electrolytes: Developing new functions by manipulating the coordination states. Bull. Chem. Soc. Jpn. 2020, 93, 109–118. [Google Scholar] [CrossRef]
- Kishimoto, K.; Nomura, S.; Tanaka, K. Chemical sensing of acrolein-amine conjugates for food quality control: A case study of milk products. Bull. Chem. Soc. Jpn. 2019, 92, 1018–1023. [Google Scholar] [CrossRef]
- Sikder, T.; Rahman, M.; Jakariya; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste-A review. J. Clean Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, L.; Li, L.; Wang, S.; Song, G.; Gao, Z.; Zhang, X.; Wang, T.; Li, Y.; Zhang, L.; et al. Novel zinc-based infinite coordination polymer for highly selective ammonia gas sensing at room temperature. Bull. Chem. Soc. Jpn. 2020, 93, 1070–1073. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, C.; Saito, M.; Espulgar, W.V.; Dou, X.; Terada, Y.; Obara, A.; Uchiyama, S.; Tamiya, E. Cauliflower-like nanostructured localized surface plasmon resonance biosensor chip for cytokine detection. Bull. Chem. Soc. Jpn. 2020, 93, 1121–1126. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Wen, M.; Li, G.; Liu, H.; Chen, J.; An, T.; Yamashita, H. Metal–organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: Recent progress and challenges. Environ. Sci. Nano 2019, 6, 1006–1025. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Mashima, M. Redox-active α-diimine complexes of early transition metals: From bonding to catalysis. Bull. Chem. Soc. Jpn. 2020, 93, 799–820. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional mesoporous silica nanomaterials for catalysis and environmental applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Eguchi, M.; Nozawa, S.; Maeda, K. Improved electrochemical water oxidation over chromium-substituted cobalt aluminate spinels. Bull. Chem. Soc. Jpn. 2020, 93, 13–19. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent improvements in the production of solar fuels: From CO2 reduction to water splitting and artificial photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef]
- Leow, W.R.; Chen, X. Surface complexation for photocatalytic organic transformations. Bull. Chem. Soc. Jpn. 2019, 92, 505–510. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous metal catalysts templated on clay nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Zhao, Y.; Carné-Sánchez, A.; Malgras, V.; Kim, J.; Kim, J.H.; Wang, S.; Liu, J.; Jiang, J.-S.; et al. Hollow carbon nanobubbles: Monocrystalline MOF nanobubbles and their pyrolysis. Chem. Sci. 2017, 8, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of flexible microsupercapacitors with binder-free ZIF-8 derived carbon films via electrophoretic deposition. Bull. Chem. Soc. Jpn. 2020, 93, 176–181. [Google Scholar] [CrossRef]
- Mártire, A.P.; Segovia, G.M.; Azzaroni, O.; Rafti, M.; Marmisollé, W. Layer-by-layer integration of conducting polymers and metal organic frameworks onto electrode surfaces: Enhancement of the oxygen reduction reaction through electrocatalytic nanoarchitectonics. Mol. Syst. Des. Eng. 2019, 4, 893–900. [Google Scholar] [CrossRef]
- Nakano, M.; Nagai, T. Thermometers for monitoring cellular temperature. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 2–9. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent progress on the sensing of pathogenic bacteria using advanced nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, J.; Nguyen, N.; Walton, A.; Flewitt, A.; Zu, X.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Pang, P.; Lai, Y.; Zhang, Y.; Wang, H.; Conlan, X.A.; Barrow, C.J.; Yang, W. Recent advancement of biosensor technology for the detection of microcystin-LR. Bull. Chem. Soc. Jpn. 2020, 93, 637–646. [Google Scholar] [CrossRef]
- Yamamura, S.; Amachi, S. Microbiology of inorganic arsenic: From metabolism to bioremediation. J. Biosci. Bioeng. 2014, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. App. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Li, B.L.; Setyawati, M.I.; Chen, L.; Xie, J.; Ariga, K.; Lim, C.-T.; Garaj, S.; Leong, D.T. Directing assembly and disassembly of 2D MoS2 nanosheets with DNA for drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 15286–15296. [Google Scholar] [CrossRef]
- Castanheira, E.J.; Correia, T.R.; Rodrigues, J.M.M.; Mano, J.F. Novel biodegradable laminarin microparticles for biomedical applications. Bull. Chem. Soc. Jpn. 2020, 93, 713–719. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Ultrasound-activated nanomaterials for therapeutics. Bull. Chem. Soc. Jpn. 2020, 93, 220–229. [Google Scholar] [CrossRef]
- Eom, S.; Choi, G.; Nakamura, H.; Choy, J.-H. 2-Dimensional nanomaterials with imaging and diagnostic functions for nanomedicine; a review. Bull. Chem. Soc. Jpn. 2020, 93, 1–12. [Google Scholar] [CrossRef]
- Arora, H.; Ramesh, M.; Rajasekhar, K.; Govindaraju, T. Molecular tools to detect alloforms of Aβ and Tau: Implications for multiplexing and multimodal diagnosis of Alzheimer’s disease. Bull. Chem. Soc. Jpn. 2020, 93, 507–546. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef]
- Muraoka, T. Biofunctional molecules inspired by protein mimicry and manipulation. Bull. Chem. Soc. Jpn. 2020, 93, 138–153. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Tang, J.; Komiyama, M.; Ariga, K. Dynamism of supramolecular DNA/RNA nanoarchitectonics: From interlocked structures to molecular machines. Bull. Chem. Soc. Jpn. 2020, 93, 581–603. [Google Scholar] [CrossRef]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Dynamism of supramolecular DNA/RNA nanoarchitectonics: From interlocked structures to molecular machines. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for hybrid and related materials for bio-oriented applications. Adv. Funct. Mater. 2018, 28, 1702905. [Google Scholar] [CrossRef]
- Bando, T.; Sugiyama, H. Sequence-specific PI polyamides make it possible to regulate DNA structure and function. Bull. Chem. Soc. Jpn. 2020, 93, 205–215. [Google Scholar] [CrossRef]
- Roy, B.; Govindaraju, T. Amino acids and peptides as functional components in arylenediimide-based molecular architectonics. Bull. Chem. Soc. Jpn. 2019, 92, 1883–1901. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Q.; Yan, X. Self-assembling peptide-based nanoarchitectonics. Bull. Chem. Soc. Jpn. 2019, 92, 70–79. [Google Scholar] [CrossRef]
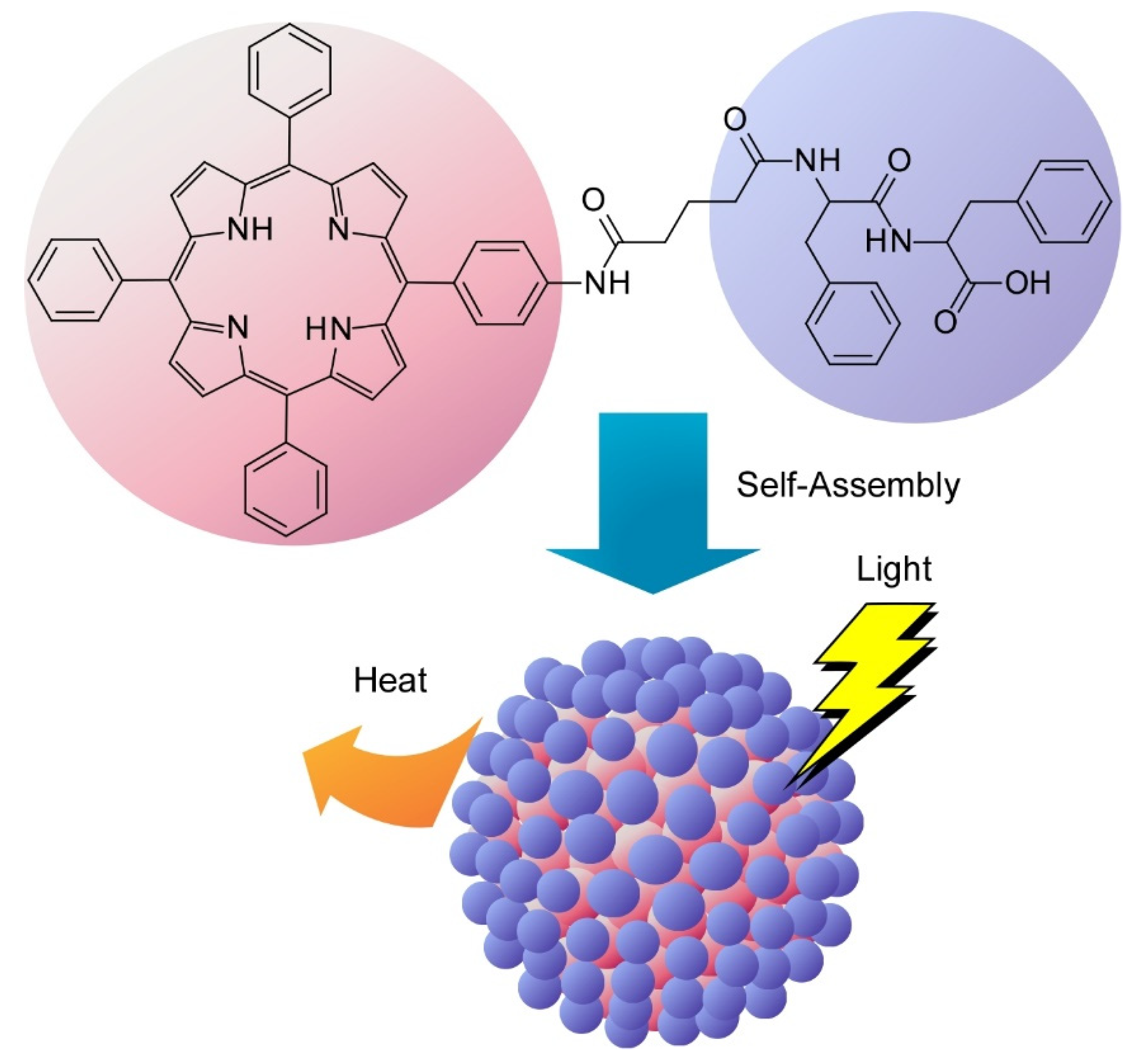
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Q.; Yuan, C.; Li, S.; Xing, R.; Yan, X. Amino acid coordination driven self-assembly for enhancing both the biological stability and tumor accumulation of curcumin. Angew. Chem. Int. Ed. 2018, 57, 17084–17088. [Google Scholar] [CrossRef]
- Song, J.; Yuan, C.; Jiao, T.; Xing, R.; Yang, M.; Adams, D.J.; Yan, X. Multifunctional antimicrobial biometallohydrogels based on amino Acid coordinated self-assembly. Small 2020, 16, 1907309. [Google Scholar] [CrossRef]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Self-assembling peptides as building blocks of functional materials for biomedical applications. Bull. Chem. Soc. Jpn. 2019, 92, 391–399. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Aiba, Y.; Ariyasu, S.; Abe, S. Enzyme nanoarchitectonics: Organization and device application. Bull. Chem. Soc. Jpn. 2020, 93, 379–392. [Google Scholar] [CrossRef]
- Komiyama, M.; Ariga, K. Nanoarchitectonics to prepare practically useful artificial enzymes. Mol. Catal. 2019, 475, 110492. [Google Scholar] [CrossRef]
- Oohora, K.; Onoda, A.; Hayashi, T. Hemoproteins reconstituted with artificial metal complexes as biohybrid catalysts. Acc. Chem. Res. 2019, 52, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Vong, K. The journey to in vivo synthetic chemistry: From azaelectrocyclization to artificial metalloenzymes. Bull. Chem. Soc. Jpn. 2020, 93, 1275–1286. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hsieh, C.-T.; Hsu, S.-h. Materials nanoarchitectonics as cell regulators. ChemNanoMat 2019, 5, 692–702. [Google Scholar] [CrossRef]
- Song, J.; Jia, X.; Ariga, K. Interfacial nanoarchitectonics for responsive cellular biosystems. Mater. Today Bio 2020, 8, 100075. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jia, X.; Ariga, K. Methods with nanoarchitectonics for small molecules and nanostructures to regulate living cells. Small Methods 2020, 4, 2000500. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, K.; Abbas, M.; Yan, X. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Mallouk, T.E. Two-dimensional metal oxide nanosheets as building blocks for artificial photosynthetic assemblies. Bull. Chem. Soc. Jpn. 2019, 92, 38–54. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Functional design of ionic liquids: Unprecedented liquids that contribute to energy technology, bioscience, and materials sciences. Bull. Chem. Soc. Jpn. 2019, 92, 852–868. [Google Scholar] [CrossRef]
- Lu, T.; Xu, X.; Zhang, S.; Pan, L.; Wang, Y.; Alshehri, S.M.; Ahamad, T.; Kim, M.; Na, J.; Hossain, M.S.A.; et al. High-performance capacitive deionization by lignocellulose-derived eco-friendly porous carbon materials. Bull. Chem. Soc. Jpn. 2020, 93, 1014–1019. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in low microbial biomass microbiome studies: Issues and recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Design of temperature-responsive polymer-grafted surfaces for cell sheet preparation and manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef]
- Cabral, H.; Kinoh, H.; Kataoka, K. Tumor-targeted nanomedicine for immunotherapy. Acc. Chem. Res. 2020, 53, 2765–2776. [Google Scholar] [CrossRef]
- Ariga, K. Interfaces working for biology: Solving biological mysteries and opening up future nanoarchitectonics. ChemNanoMat 2016, 2, 333–343. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2017, 1, 208–211. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Shrestha, L.K. Nanoarchitectonics from molecular units to living-creature-like motifs. Chem. Rec. 2018, 18, 676–695. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Yamauchi, Y. Nanoarchitectonics from atom to life. Chem. Asian J. 2020, 15, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Shrestha, L.K. Intelligent nanoarchitectonics for self-assembling systems. Adv. Intell. Syst. 2020, 2, 1900157. [Google Scholar] [CrossRef]
- Toyao, T.; Maeno, Z.; Takakusagi, S.; Kamachi, T.; Takigawa, I.; Shimizu, K. Machine learning for catalysis informatics: Recent applications and prospects. ACS Catal. 2020, 10, 2260–2297. [Google Scholar] [CrossRef]
- Fujinami, M.; Seino, J.; Nakai, H. Quantum chemical reaction prediction method based on machine learning. Bull. Chem. Soc. Jpn. 2020, 93, 685–693. [Google Scholar] [CrossRef]
- Noé, F.; Tkatchenko, A.; Müller, K.-R.; Clementi, C. Machine learning for molecular simulation. Ann. Rev. Phys. Chem. 2020, 71, 361–390. [Google Scholar] [CrossRef]
- Fujinami, F.; Maekawara, H.; Isshiki, R.; Seino, J.; Yamaguchi, J.; Nakai, H. Solvent selection scheme using machine learning based on physicochemical description of solvent molecules: Application to cyclic organometallic reaction. Bull. Chem. Soc. Jpn. 2020, 93, 841–845. [Google Scholar] [CrossRef]
- Nair, R.V.; Jayasree, R.S. Role of advanced nanomaterials in biosensing. In Drug Delivery Nanosystems for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–227. [Google Scholar]
- Afify, A.S.; Elsayed, A.; Hassan, M.; Ataalla, M.; Mohamed, A.; Hussain, A.; Abu-Khadra, A.S.; Tulliani, J.-M. Synthesis and characterization of nano-tungsten oxide precipitated onto natural inorganic clay for humidity-sensing applications. Ceramics 2018, 1, 120–127. [Google Scholar] [CrossRef]






















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariga, K. Progress in Molecular Nanoarchitectonics and Materials Nanoarchitectonics. Molecules 2021, 26, 1621. https://doi.org/10.3390/molecules26061621
Ariga K. Progress in Molecular Nanoarchitectonics and Materials Nanoarchitectonics. Molecules. 2021; 26(6):1621. https://doi.org/10.3390/molecules26061621
Chicago/Turabian StyleAriga, Katsuhiko. 2021. "Progress in Molecular Nanoarchitectonics and Materials Nanoarchitectonics" Molecules 26, no. 6: 1621. https://doi.org/10.3390/molecules26061621
APA StyleAriga, K. (2021). Progress in Molecular Nanoarchitectonics and Materials Nanoarchitectonics. Molecules, 26(6), 1621. https://doi.org/10.3390/molecules26061621





