Dialkylboryl-Substituted Cyclic Disilenes Synthesized by Desilylation-Borylation of Trimethylsilyl-Substituted Disilenes
Abstract
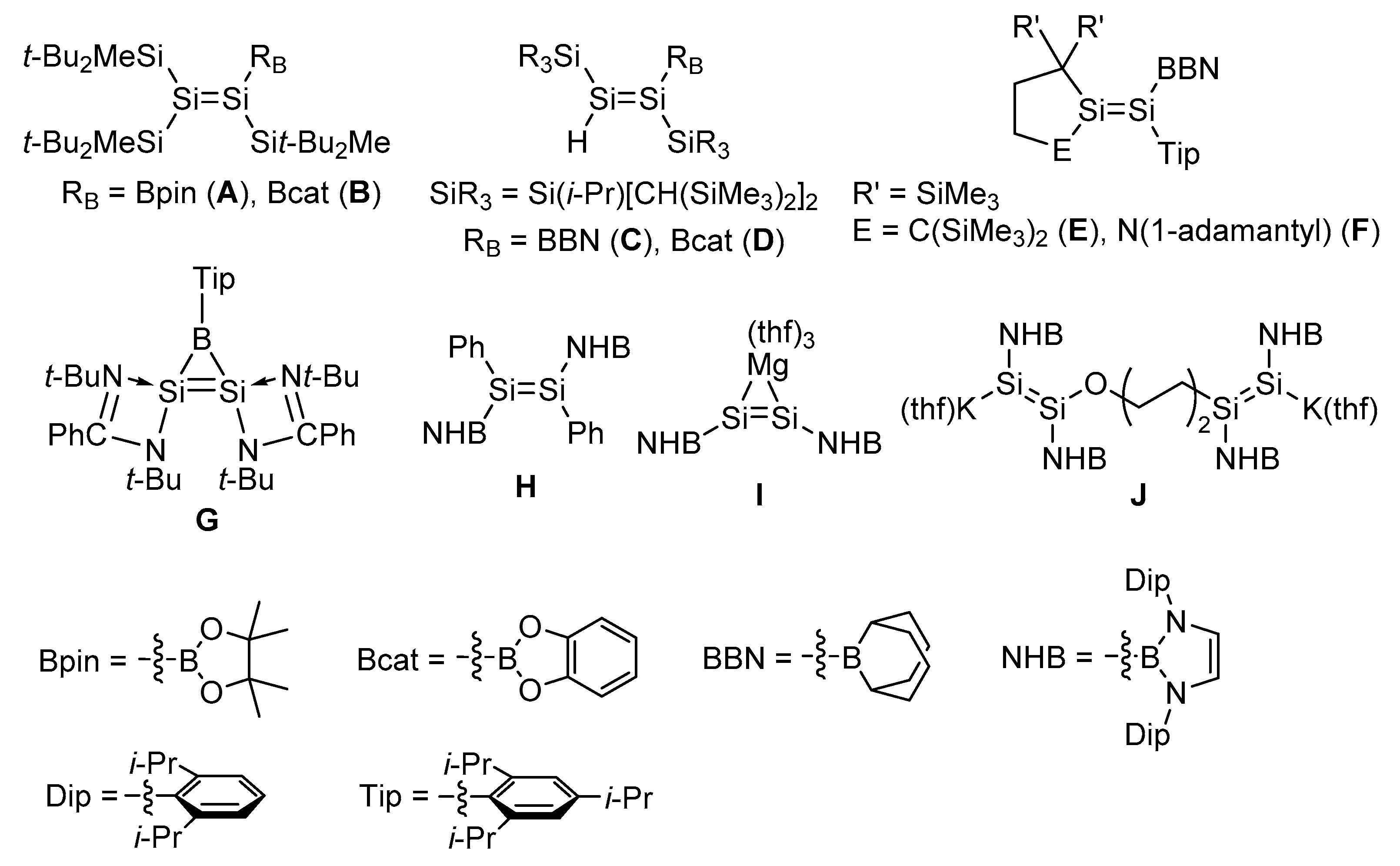
1. Introduction
2. Results and Discussion
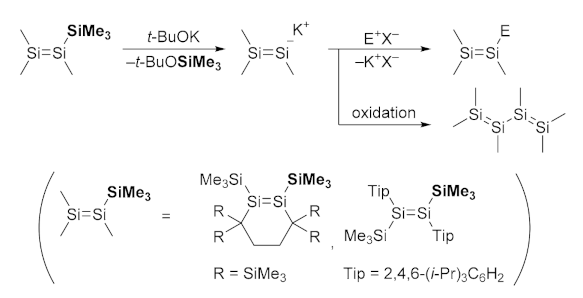
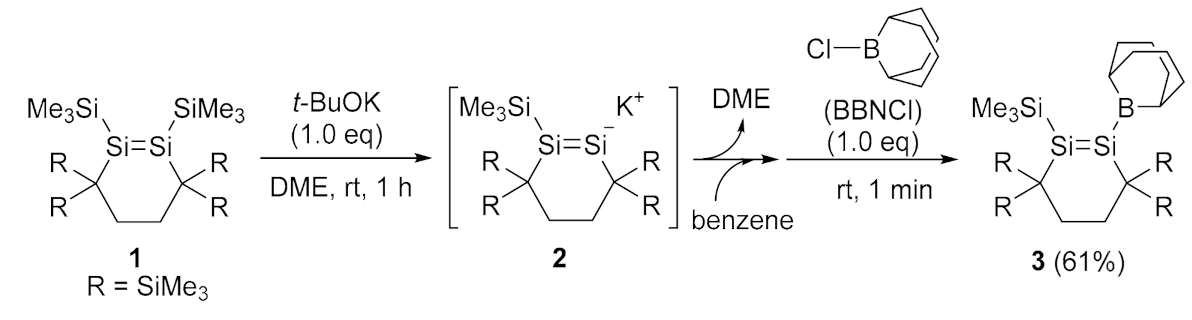
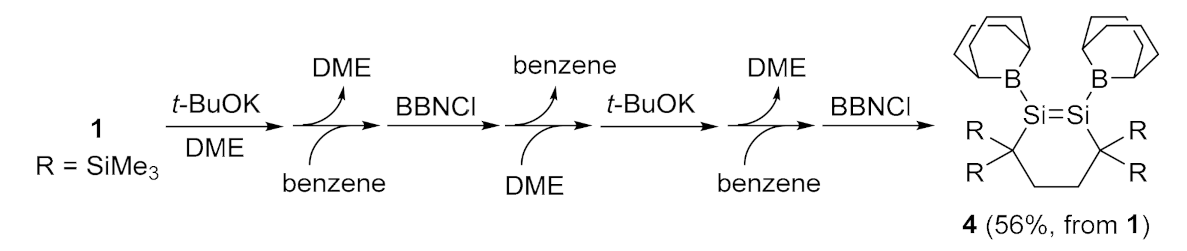
2.1. Desilylation-Borylation of Silyl-Substituted Disilenes
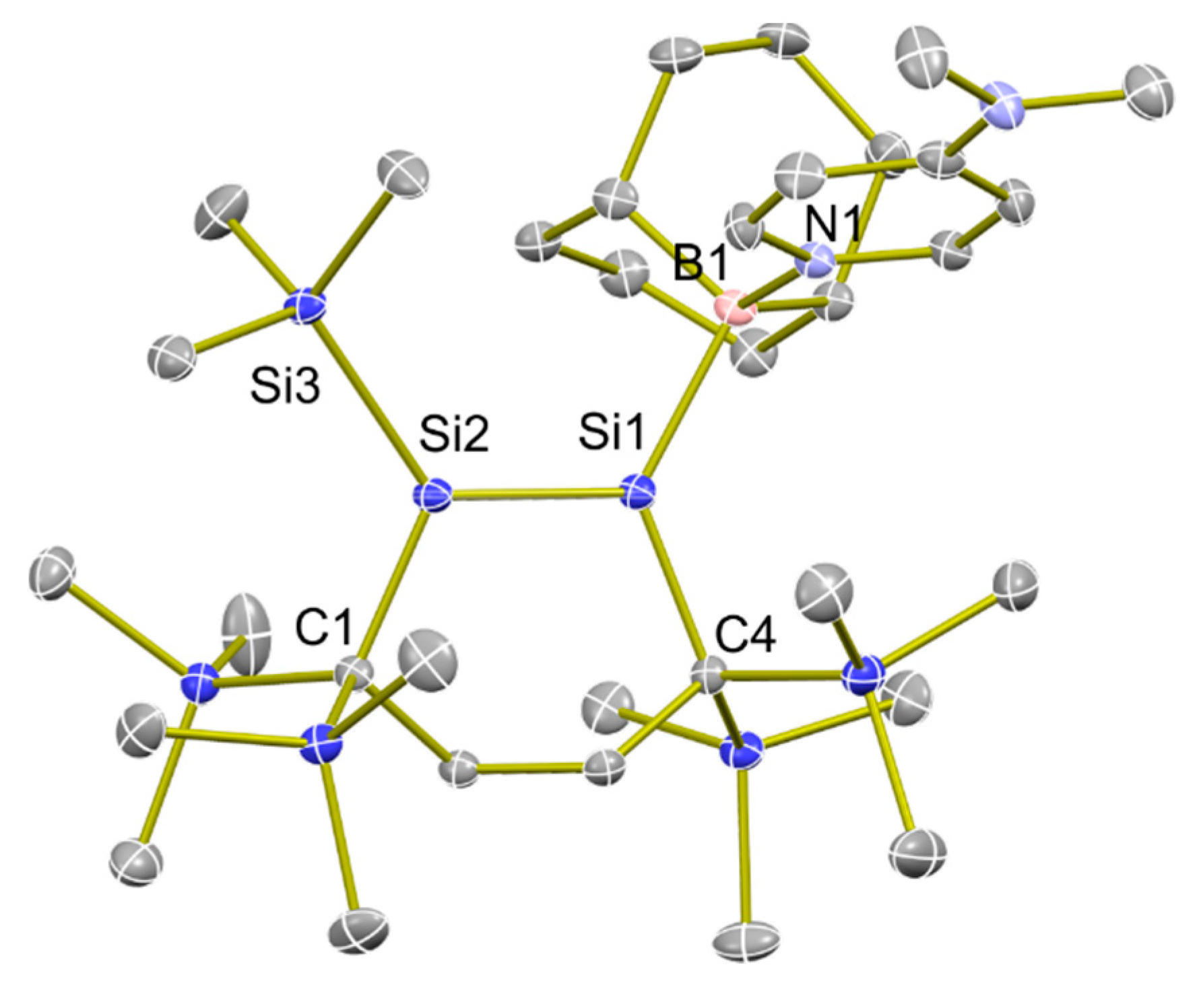
2.2. X-ray Analysis of 3 and 4
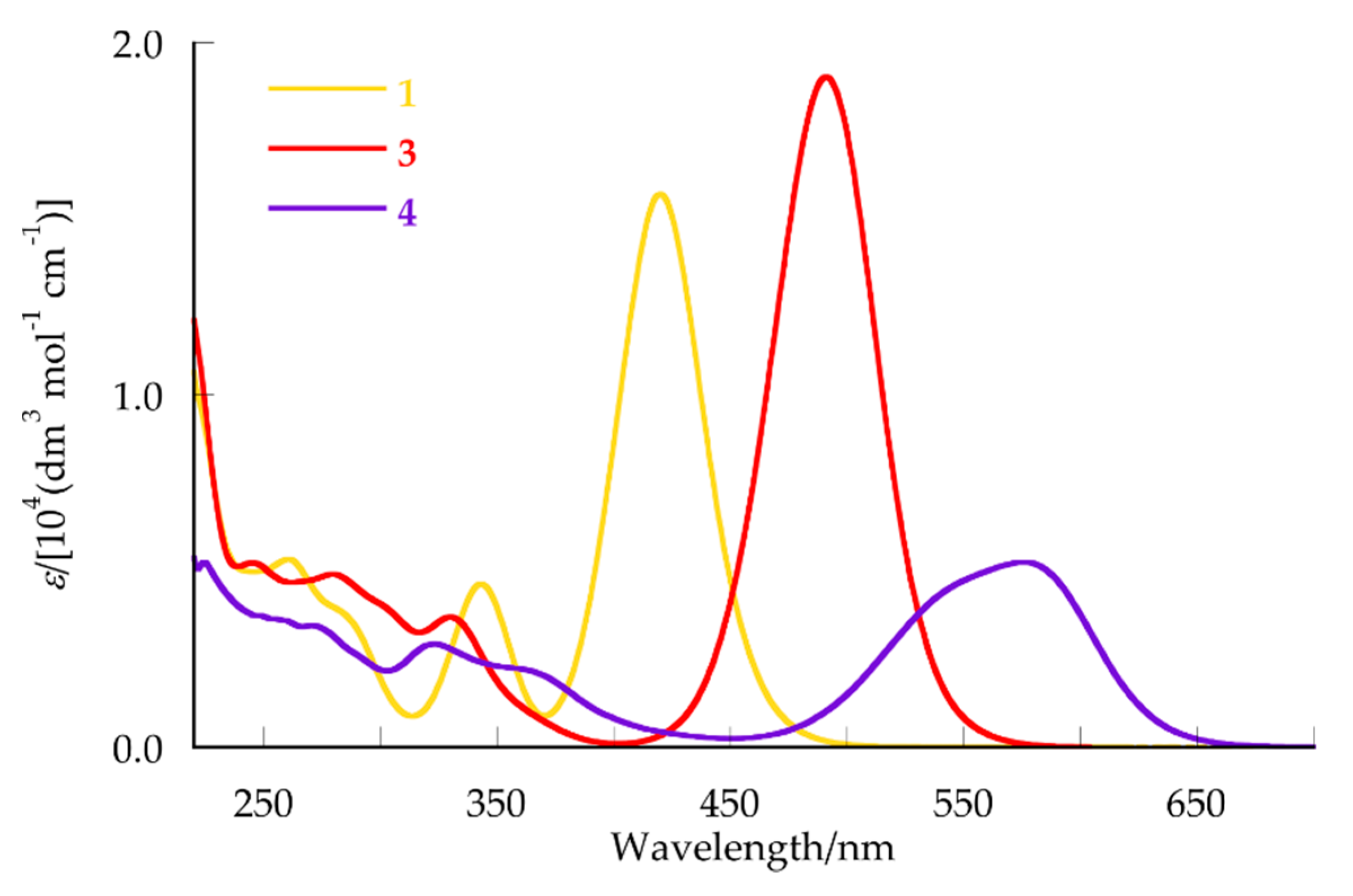
2.3. UV-Vis Spectra of 3 and 4
2.4. Reactions of Boryldisilenes with 4-(N,N-Dimethylamino)pyridine (DMAP)
3. Conclusions
4. Materials and Methods
4.1. General Procedure
4.2. Materials
4.3. Synthesis of (Dialkylboryl)disilene 3
4.4. Synthesis of 1,2-Di(dialkylboryl)disilene 4
4.5. Synthesis of Boryldisilene-DMAP Complex 5
4.6. Reaction of 5 with Triphenylborane
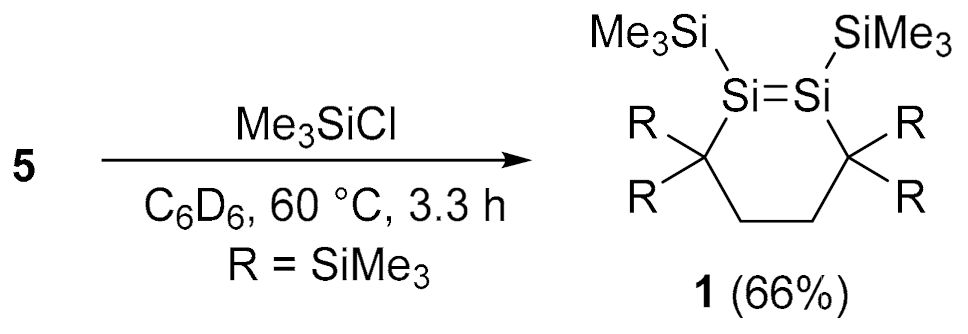
4.7. Reaction of 5 with Me3SiCl
4.8. Reaction of Diboryldislene 4 with DMAP Followed by Me3SiCl
4.9. Single-Crystal X-ray Diffraction Analysis
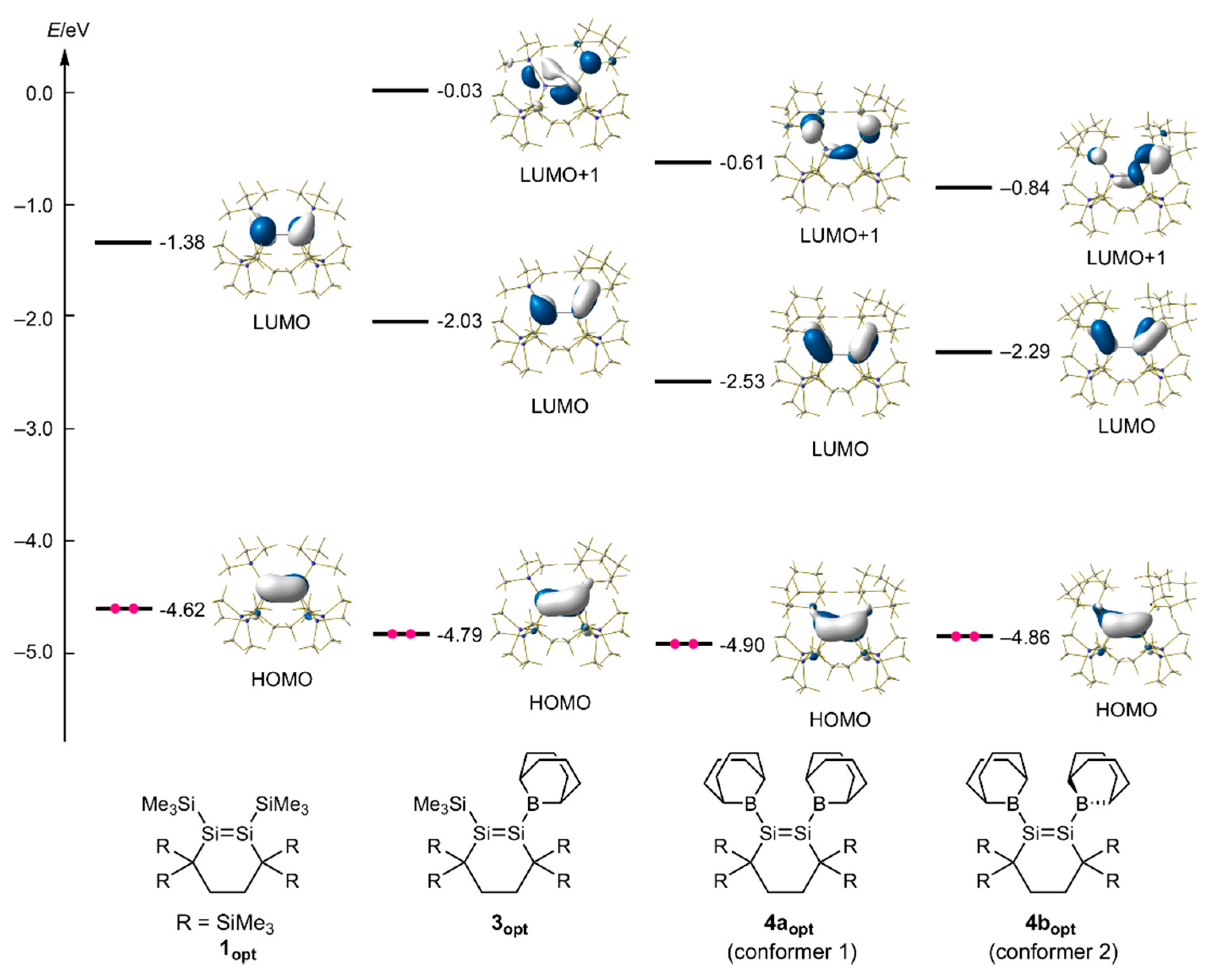
4.10. Computational Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availibility:
References
- Okazaki, R.; West, R. Chemistry of Stable Disilenes. Adv. Organomet. Chem. 1996, 39, 231–273. [Google Scholar]
- Kira, M.; Iwamoto, T. Progress in the Chemistry of Stable Disilenes. Adv. Organomet. Chem. 2006, 54, 73–148. [Google Scholar]
- Scheschkewitz, D. Anionic Reagents with Silicon-Containing Double Bonds. Chem. Eur. J. 2009, 15, 2476–2485. [Google Scholar] [CrossRef]
- Abersfelder, K.; Scheschkewitz, D. Synthesis of Homo- and Heterocyclic Silanes via Intermediates with Si=Si Bonds. Pure Appl. Chem. 2010, 82, 595–602. [Google Scholar] [CrossRef]
- Scheschkewitz, D. The Versatile Chemistry of Disilenides: Disila Analogues of Vinyl Anions as Synthons in Low-Valent Silicon Chemistry. Chem. Lett. 2011, 40, 2–11. [Google Scholar] [CrossRef]
- Iwamoto, T.; Ishida, S. Multiple Bonds with Silicon: Recent Advances in Synthesis, Structure, and Functions of Stable Disilenes. In Structure and Bonding (Berlin, Germany); Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 156, pp. 125–202. [Google Scholar]
- Präsang, C.; Scheschkewitz, D. Reactivity in the Periphery of Functionalised Multiple Bonds of Heavier Group 14 Elements. Chem. Soc. Rev. 2016, 45, 900–921. [Google Scholar] [CrossRef] [PubMed]
- Rammo, A.; Scheschkewitz, D. Functional Disilenes in Synthesis. Chem. Eur. J. 2018, 24, 6866–6885. [Google Scholar] [CrossRef] [PubMed]
- Hanusch, F.; Groll, L.; Inoue, S. Recent Advances of Group 14 Dimetallenes and Dimetallynes in Bond Activation and Catalysis. Chem. Sci. 2021, 12, 2001–2015. [Google Scholar] [CrossRef]
- Inoue, S.; Ichinohe, M.; Sekiguchi, A. An Isolable Boryl-substituted Disilene from the Reaction of an sp2-type Silyl Anion with Haloboranes: Synthesis and Characterization. Chem. Lett. 2008, 37, 1044–1045. [Google Scholar] [CrossRef]
- Takeuchi, K.; Ikoshi, M.; Ichinohe, M.; Sekiguchi, A. Addition of Amines and Hydroborane to the Disilyne RSi≡SiR (R = SiiPr[CH(SiMe3)2]2) Giving Amino- and Boryl-substituted Disilenes. J. Am. Chem. Soc. 2010, 132, 930–931. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Ichinohe, M.; Sekiguchi, A. Hydroboration of Disilyne RSi≡SiR (R = SiiPr[CH(SiMe3)2]2), Giving Boryl-Substituted Disilenes. Organometallics 2011, 30, 2044–2050. [Google Scholar] [CrossRef]
- Kosai, T.; Iwamoto, T. Stable Push-Pull Disilene: Substantial Donor-Acceptor Interactions through the Si=Si Double Bond. J. Am. Chem. Soc. 2017, 139, 18146–18149. [Google Scholar] [CrossRef] [PubMed]
- Kosai, T.; Iwamoto, T. Cleavage of Two Hydrogen Molecules by Boryldisilenes. Chem. Eur. J. 2018, 24, 7774–7780. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Chaliha, R.; Siddiqui, M.M.; Banerjee, S.; Munch, A.; Herbst-Irmer, R.; Stalke, D.; Jemmis, E.D.; Roesky, H.W. A Neutral Three-Membered 2π Aromatic Disilaborirane and the Unique Conversion into a Four-Membered BSi2N-Ring. Angew. Chem. Int. Ed. 2020, 59, 23015–23019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Yang, H.; Cui, C. Synthesis of Boryl-Substituted Disilane, Disilene, and Silyl Cation. Organometallics 2020, 39, 4164–4168. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, J.; Yang, H.; Cui, C. Isolation of a 1-Magnesium-2,3-disilacyclopropene and a Related Bis(disilenide). J. Am. Chem. Soc. 2020, 142, 4131–4135. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Fujieda, K.; Garoni, E.; Kamada, K.; Matsui, H.; Nakano, M.; Iwamoto, T. Synthesis and Functionalization of a 1,4-Bis(trimethylsilyl)tetrasila-1,3-diene through the Selective Cleavage of Si(sp2)–Si(sp3) Bonds under Mild Reaction Conditions. Organometallics 2018, 37, 172–175. [Google Scholar] [CrossRef]
- Akasaka, N.; Tanaka, K.; Ishida, S.; Iwamoto, T. Synthesis and Functionalization of a 1,2-Bis(trimethylsilyl)-1,2-disilacyclohexene That Can Serve as a Unit of cis-1,2-Dialkyldisilene. Inorganics 2018, 6, 21. [Google Scholar] [CrossRef]
- Kawachi, A.; Minamimoto, T.; Tamao, K. Boron–Metal Exchange Reaction of Silylboranes with Organometallic Reagents: A New Route to Arylsilyl Anions. Chem. Lett. 2001, 30, 1216–1217. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Hoveyda, A.H. Metal-free Catalytic C-Si Bond Formation in an Aqueous Medium. Enantioselective NHC-catalyzed Silyl Conjugate Additions to Cyclic and Acyclic α,β-Unsaturated Carbonyls. J. Am. Chem. Soc. 2011, 133, 7712–7715. [Google Scholar]
- Ito, H.; Horita, Y.; Yamamoto, E. Potassium tert-Butoxide-mediated Regioselective Silaboration of Aromatic Alkenes. Chem. Commun. 2012, 48, 8006–8008. [Google Scholar] [CrossRef]
- Cui, B.; Jia, S.; Tokunaga, E.; Shibata, N. Defluorosilylation of Fluoroarenes and Fluoroalkanes. Nat. Commun. 2018, 9, 4393. [Google Scholar] [CrossRef] [PubMed]
- Shishido, R.; Uesugi, M.; Takahashi, R.; Mita, T.; Ishiyama, T.; Kubota, K.; Ito, H. General Synthesis of Trialkyl- and Dialkylarylsilylboranes: Versatile Silicon Nucleophiles in Organic Synthesis. J. Am. Chem. Soc. 2020, 142, 14125–14133. [Google Scholar] [CrossRef] [PubMed]
- Zirngast, M.; Flock, M.; Baumgartner, J.; Marschner, C. Formation of Formal Disilene Fluoride Adducts. J. Am. Chem. Soc. 2008, 130, 17460–17470. [Google Scholar] [CrossRef]
- Bursch, M.; Gasevic, T.; Stuckrath, J.B.; Grimme, S. Comprehensive Benchmark Study on the Calculation of 29Si NMR Chemical Shifts. Inorg. Chem. 2021, 60, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Auer, D.; Strohmann, C.; Arbuznikov, A.V.; Kaupp, M. Understanding Substituent Effects on 29Si Chemical Shifts and Bonding in Disilenes. A Quantum Chemical Analysis. Organometallics 2003, 22, 2442–2449. [Google Scholar]
- Brown, H.C.; Kulkarni, S.U. Organoboranes. J. Organomet. Chem. 1979, 168, 281–293. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71 Pt 1, 3–8. [Google Scholar] [CrossRef]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Maeda, S.; Harabuchi, Y.; Osada, Y.; Taketsugu, T.; Morokuma, K.; Ohno, K. Available online: https://iqce.jp/GRRM/ (accessed on 2 February 2021).
- Maeda, S.; Ohno, K.; Morokuma, K. Systematic Exploration of the Mechanism of Chemical Reactions: The Global Reaction Route Mapping (GRRM) Strategy Using the ADDF and AFIR Methods. Phys. Chem. Chem. Phys. 2013, 15, 3683–3701. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0. Theoretical Chemistry Institute; University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]




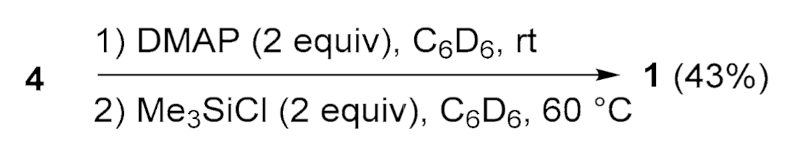
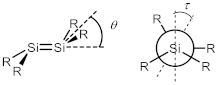
| Compound | Distance/Å | Bent Angle θ/° (Angle Sum/°) | Twist Angle τ/° | |||
|---|---|---|---|---|---|---|
| Si=Si | Si–B | Si1 | Si2 | Si=Si | Si–B | |
| 3 | 2.1990(8) | 1.994(3) | 9.8 (SiBBN) (359.15(8)) | 9.6 (SiSiMe3) (359.16(6)) | 19.3 | 5.0 |
| 4 | 2.2114(5) | 1.9851(14) (Si1) 2.0156(15) (Si2) | 4.4 (359.83(5)) | 8.0 (359.36(5)) | 16.7 | 15.5 (Si1) 41.2 (Si2) |
| 1 | 2.1762(5) | – | 12.9 (358.52(3)) | 6.5 (359.61(3)) | 17.7 | – |
| Compound | Distance/Å | Bent Angle θ/° (Angle Sum/°) | Twist Angle τ/° | |||
|---|---|---|---|---|---|---|
| Si=Si | Si–B | Si1 | Si2 | Si=Si | Si–B | |
| 3opt | 2.19225 | 1.97039 | 11.8 (358.80) | 10.3 (359.10) | 20.2 | 2.5 |
| 4aopt | 2.20152 | 1.98758 (Si1) 1.99036 (Si2) | 11.7 (358.80) | 15.0 (358.04) | 27.7. | 8.1 (Si1) 8.3 (Si2) |
| 4bopt | 2.20285 | 1.98078(Si1) 2.00371 (Si2) | 1.8 (359.97) | 7.1 (359.50) | 18.9 | 13.2 (Si1) 42.6 (Si2) |
| 5opt | 2.19009 | 2.10907 | 10.1 (359.14) | 5.4 (359.69) | 8.1 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Akasaka, N.; Kosai, T.; Honda, S.; Ushijima, Y.; Ishida, S.; Iwamoto, T. Dialkylboryl-Substituted Cyclic Disilenes Synthesized by Desilylation-Borylation of Trimethylsilyl-Substituted Disilenes. Molecules 2021, 26, 1632. https://doi.org/10.3390/molecules26061632
Tanaka K, Akasaka N, Kosai T, Honda S, Ushijima Y, Ishida S, Iwamoto T. Dialkylboryl-Substituted Cyclic Disilenes Synthesized by Desilylation-Borylation of Trimethylsilyl-Substituted Disilenes. Molecules. 2021; 26(6):1632. https://doi.org/10.3390/molecules26061632
Chicago/Turabian StyleTanaka, Kaho, Naohiko Akasaka, Tomoyuki Kosai, Shunya Honda, Yuya Ushijima, Shintaro Ishida, and Takeaki Iwamoto. 2021. "Dialkylboryl-Substituted Cyclic Disilenes Synthesized by Desilylation-Borylation of Trimethylsilyl-Substituted Disilenes" Molecules 26, no. 6: 1632. https://doi.org/10.3390/molecules26061632
APA StyleTanaka, K., Akasaka, N., Kosai, T., Honda, S., Ushijima, Y., Ishida, S., & Iwamoto, T. (2021). Dialkylboryl-Substituted Cyclic Disilenes Synthesized by Desilylation-Borylation of Trimethylsilyl-Substituted Disilenes. Molecules, 26(6), 1632. https://doi.org/10.3390/molecules26061632





