Obesity and Energy Substrate Transporters in Ovarian Cancer—Review
Abstract
:1. Introduction
2. From Diagnosis to Setting a Proper Treatment Plan in OC
2.1. Signs and Symptoms
2.2. Diagnosis
2.3. Surgical Treatment and Chemotherapy
2.4. Maintenance Treatment
3. Obesity and Ovarian Cancer
4. Glucose Metabolism in Cancer Cells
4.1. A Broad Role of GLUT 1 in Cancer Development
4.2. GLUT 1 in OC
4.3. A Potential Role of GLUT 3 in OC and Other Cancers
4.4. A Role of GLUT 4 in OC and Other Cancers
Potential Inhibitors
4.5. A Role of MCT in OC and Other Cancers
5. Adipocytes and the Role of Lipid Transporters
5.1. Fatty Acid Binding Protein 4
5.2. CD36
5.3. Fatty Acid-Binding Protein 6 (FABP 6)
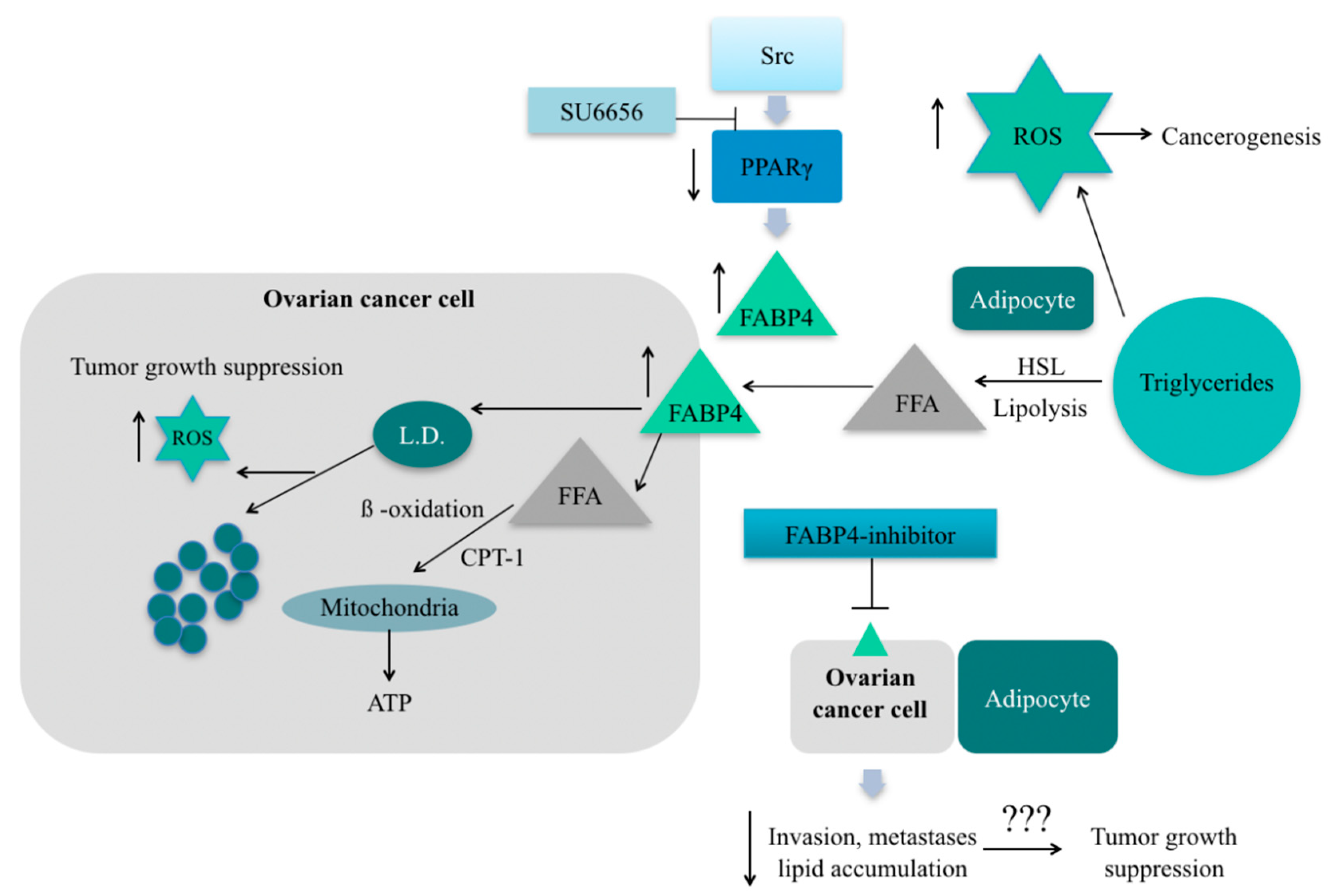
5.4. Role of FFA Oxidation in Ovarian Cancer Progression
6. Role of Adipokines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| OC/OvCa | Ovarian cancer |
| EOC | Epithelial ovarian cancer |
| HGSC | High-grade serous carcinoma |
| FABP | Fatty acid-binding protein |
| GLUT | Glucose transporter |
| MCTs | Monocarboxylate transporters |
| MRI | Magnetic resonance imagining |
| CT | Computed tomography |
| VEGF | Vascular epithelial growth factor |
| PARP-inhibitor | Poly (ADP-ribose) polymerase inhibitor |
| FFA | Free fatty acids |
| ROS | Reactive oxygen species |
| TSP-1 | Thrombospondin-1 |
| DNMT1 | DNA methyltransferase 1 |
| SUSD2 | Sushi domain containing 2 |
| SRC | Proto-oncogene protein tyrosine kinase |
| MAPK | Mitogen-activated protein kinase |
| CPT | Carnitine palmitoyltransferase |
References
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [Green Version]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. Available online: https://pubmed.ncbi.nlm.nih.gov/29809280 (accessed on 29 May 2018).
- Bosetti, C.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Chatenoud, L.; Negri, E.; La Vecchia, C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann. Oncol. 2013, 24, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef] [Green Version]
- Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [CrossRef]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Phys. 2016, 93, 937–944. [Google Scholar]
- Rooth, C. Ovarian cancer: Risk factors, treatment and management. Br. J. Nurs. 2013, 22, S23–S30. [Google Scholar] [CrossRef]
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Phys. 2009, 80, 609–616. [Google Scholar]
- Moyer, V.A. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 271–281. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. Available online: http://www.sciencedirect.com/science/article/pii/S0889858818307603 (accessed on 16 March 2021). [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis Prim. 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Du, X.; Hidayat, K.; Shi, B.-M. Abdominal obesity and gastroesophageal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, 37. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, T.-T.; Zhao, J.-J.; Qi, S.-F.; Du, P.; Liu, D.-W.; Tian, Q.-B. The association between overweight, obesity and ovarian cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2015, 45, 1107–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, H.S.; Kim, H.J.; Hong, J.H.; Lee, J.K.; Lee, N.W.; Song, J.Y. Obesity and epithelial ovarian cancer survival: A systematic review and meta-analysis. J. Ovarian Res. 2014, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Foong, K.W.; Bolton, H. Obesity and ovarian cancer risk: A systematic review. Post Reprod. Health 2017, 23, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Direk, K.; Cecelja, M.; Astle, W.; Chowienczyk, P.; Spector, T.D.; Falchi, M.; Andrew, T. The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc. Disord. 2013, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Tworoger, S.S.; Huang, T. Obesity and Ovarian Cancer BT—Obesity and Cancer; Pischon, T., Nimptsch, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 155–176. [Google Scholar] [CrossRef]
- de Andrade Barreto, E.; de Souza Santos, P.T.; Bergmann, A.; de Oliveira, I.M.; Wernersbach Pinto, L.; Blanco, T.; Rossini, A.; Pinto Kruel, C.D.; Mattos Albano, R.; Ribeiro Pinto, L.F. Alterations in glucose metabolism proteins responsible for the Warburg effect in esophageal squamous cell carcinoma. Exp. Mol. Pathol. 2016, 101, 66–73. Available online: http://www.sciencedirect.com/science/article/pii/S0014480016300971 (accessed on 16 March 2021). [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [Green Version]
- Guaita-Esteruelas, S.; Gumà, J.; Masana, L.; Borràs, J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol. Cell Endocrinol. 2018, 462, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Macheda, M.L.; Rogers, S.; Best, J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 2005, 202, 654–662. [Google Scholar] [CrossRef]
- Suganuma, N.; Segade, F.; Matsuzu, K.; Bowden, D.W. Differential expression of facilitative glucose transporters in normal and tumour kidney tissues. BJU Int. 2007, 99, 1143–1149. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; He, J.; Lv, J.; Pan, R.; Lv, T. A high serum-free fatty acid level is associated with cancer. J. Cancer Res. Clin Oncol. 2020, 146, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Floresta, G.; Pistarà, V.; Amata, E.; Dichiara, M.; Marrazzo, A.; Prezzavento, O.; Rescifina, A. Adipocyte fatty acid binding protein 4 (FABP4) inhibitors. A comprehensive systematic review. Eur. J. Med. Chem. 2017, 138, 854–873. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. Available online: http://science.sciencemag.org/content/123/3191/309.abstract. (accessed on 16 March 2021). [CrossRef]
- Tilekar, K.; Upadhyay, N.; Iancu, C.V.; Pokrovsky, V.; Choe, J.-Y.; Ramaa, C.S. Power of two: Combination of therapeutic approaches involving glucose transporter (GLUT) inhibitors to combat cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188457. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Queckborner, S.; Turnbull, A.; Michie, C.O.; Williams, A.R.W.; Rye, T.; Gourley, C.; Langdon, S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer 2018, 18, 636. [Google Scholar] [CrossRef]
- Schlößer, H.A.; Drebber, U.; Urbanski, A.; Haase, S.; Baltin, C.; Berlth, F.; Neiß, S.; von Bergwelt-Baildon, M.; Fetzner, U.K.; Warnecke-Eberz, U.; et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer 2017, 20, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Cantuaria, G.; Magalhaes, A.; Penalver, M.; Angioli, R.; Braunschweiger, P.; Gomez-Marin, O.; Kanhoush, R.; Gomez-Fernandez, C.; Nadji, M. Expression of GLUT-1 Glucose Transporter in Borderline and Malignant Epithelial Tumors of the Ovary. Gynecol. Oncol. 2000, 79, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ancey, P.-B.; Contat, C.; Meylan, E. Glucose transporters in cancer—from tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef] [PubMed]
- Joost, H.G.; Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members (review). Mol. Membr. Biol. 2001, 18, 247–256. [Google Scholar] [CrossRef]
- Onetti, R.; Baulida, J.; Bassols, A. Increased glucose transport in ras-transformed fibroblasts: A possible role for N-glycosylation of GLUT1. FEBS Lett. 1997, 407, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Airley, R.E.; Mobasheri, A. Hypoxic Regulation of Glucose Transport, Anaerobic Metabolism and Angiogenesis in Cancer: Novel Pathways and Targets for Anticancer Therapeutics. Chemotherapy 2007, 53, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.C.; Bilan, P.J.; Tsakiridis, T.; Tsiani, E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism 2016, 65, 124–139. [Google Scholar] [CrossRef]
- Younes, M.; Brown, R.W.; Stephenson, M.; Gondo, M.; Cagle, P.T. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer 1997, 80, 1046–1051. [Google Scholar] [CrossRef]
- Semaan, A.; Munkarah, A.R.; Arabi, H.; Bandyopadhyay, S.; Seward, S.; Kumar, S.; Qazi, A.; Hussein, Y.; Morris, R.T.; Ali-Fehmi, R. Expression of GLUT-1 in epithelial ovarian carcinoma: Correlation with tumor cell proliferation, angiogenesis, survival and ability to predict optimal cytoreduction. Gynecol. Oncol. 2011, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Saito, A.; Miyagi, Y.; Yamanaka, S.; Marat, D.; Doi, C.; Yoshikawa, T.; Tsuburaya, A.; Ito, T.; Satoh, S. Suppression of facilitative glucose transporter 1 mRNA can suppress tumor growth. Cancer Lett. 2000, 154, 175–182. Available online: http://www.sciencedirect.com/science/article/pii/S030438350000392X. (accessed on 16 March 2021). [CrossRef]
- Tsukioka, M.; Matsumoto, Y.; Noriyuki, M.; Yoshida, C.; Nobeyama, H.; Yoshida, H. Expression of glucose transporters in epithelial ovarian carcinoma: Correlation with clinical characteristics and tumor angiogenesis. Oncol. Rep. 2007, 18, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Konishi, I.; Mandai, M.; Kuroda, H.; Komatsu, T.; Nanbu, K.; Sakahara, H.; Mori, T. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: Correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br. J. Cancer 1997, 76, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-M.; Deng, S.-H.; Liu, T.; Han, R.; Zhang, T.; Xu, Y. TGF-β-mediated exosomal lnc-MMP2-2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Med. 2018, 7, 5118–5129. [Google Scholar] [CrossRef] [PubMed]
- Henriet, P.; Emonard, H. Matrix metalloproteinase-2: Not (just) a “hero” of the past. Biochimie 2019, 166, 223–232. [Google Scholar] [CrossRef]
- Ito, S.; Fukusato, T.; Nemoto, T.; Sekihara, H.; Seyama, Y.; Kubota, S. Coexpression of Glucose Transporter 1 and Matrix Metalloproteinase-2 in Human Cancers. JNCI J. Natl. Cancer Inst. 2002, 94, 1080–1091. [Google Scholar] [CrossRef]
- Kalir, T.; Wang, B.Y.; Goldfischer, M.; Haber, R.S.; Reder, I.; Demopoulos, R.; Cohen, C.J.; Burstein, D.E. Immunohistochemical staining of GLUT1 in benign, borderline, and malignant ovarian epithelia. Cancer 2002, 94, 1078–1082. [Google Scholar] [CrossRef]
- Alakus, H.; Batur, M.; Schmidt, M.; Drebber, U.; Baldus, S.E.; Vallböhmer, D.; Prenzel, K.L.; Metzger, R.; Bollschweiler, E.; Hölscher, A.H.; et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl. Med. Commun. 2010, 31, 532–538. [Google Scholar] [CrossRef]
- Cho, H.; Lee, Y.S.; Kim, J.; Chung, J.-Y.; Kim, J.-H. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Investig. 2013, 31, 607–615. [Google Scholar] [CrossRef]
- Meijer, T.W.H.; Schuurbiers, O.C.J.; Kaanders, J.H.A.M.; Looijen-Salamon, M.G.; de Geus-Oei, L.-F.; Verhagen, A.F.T.M.; Lok, J.; van der Heijden, H.F.M.; Rademakers, S.E.; Span, P.N.; et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: Spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer 2012, 76, 316–323. [Google Scholar] [CrossRef]
- Rudlowski, C.; Moser, M.; Becker, A.J.; Rath, W.; Buttner, R.; Schroder, W.; Schurmann, A. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology 2004, 66, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 glucose transporter expression in colorectal carcinoma: A marker for poor prognosis. Cancer 1998, 83, 34–40. [Google Scholar] [CrossRef]
- Yin, C.; Gao, B.; Yang, J.; Wu, J. Glucose Transporter-1 (GLUT-1) Expression is Associated with Tumor Size and Poor Prognosis in Locally Advanced Gastric Cancer. Med. Sci. Monit. Basic Res. 2020, 26, e920778. Available online: https://pubmed.ncbi.nlm.nih.gov/32201432 (accessed on 23 March 2020). [CrossRef]
- Lidgren, A.; Bergh, A.; Grankvist, K.; Rasmuson, T.; Ljungberg, B. Glucose transporter-1 expression in renal cell carcinoma and its correlation with hypoxia inducible factor-1 alpha. BJU Int. 2008, 101, 480–484. [Google Scholar]
- Shin, S.J.; Kim, J.Y.; Kwon, S.Y.; Mun, K.-C.; Cho, C.H.; Ha, E. Ciglitazone enhances ovarian cancer cell death via inhibition of glucose transporter-1. Eur. J. Pharmacol. 2014, 743, 17–23. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Idowu, M.O.; Oh, U.; Wang, X.-Y.; Temkin, S.M.; Fang, X. Ovarian Cancer Relies on Glucose Transporter 1 to Fuel Glycolysis and Growth: Anti-Tumor Activity of BAY-876. Cancers 2018, 11, 33. Available online: https://pubmed.ncbi.nlm.nih.gov/30602670 (accessed on 21 December 2018).
- Kocdor, M.A.; Kocdor, H.; Pereira, J.S.; Vanegas, J.E.; Russo, I.H.; Russo, J. Progressive increase of glucose transporter-3 (GLUT-3) expression in estrogen-induced breast carcinogenesis. Clin. Transl. Oncol. 2013, 15, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-Q.; Keating, A.F. Functional properties and genomics of glucose transporters. Curr. Genom. 2007, 8, 113–128. [Google Scholar] [CrossRef]
- Noguchi, Y.; Marat, D.; Saito, A.; Yoshikawa, T.; Doi, C.; Fukuzawa, K.; Tsuburaya, A.; Satoh, S.; Ito, T. Expression of facilitative glucose transporters in gastric tumors. Hepatogastroenterology 1999, 46, 2683–2689. [Google Scholar]
- Godoy, A.; Ulloa, V.; Rodríguez, F.; Reinicke, K.; Yañez, A.J.; García, M.D.; Medina, R.A.; Carrasco, M.; Barberis, S.; Castro, T.; et al. Differential subcellular distribution of glucose transporters GLUT1–6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J. Cell Physiol. 2006, 207, 614–627. [Google Scholar] [CrossRef]
- Higashi, T.; Tamaki, N.; Honda, T.; Torizuka, T.; Kimura, T.; Inokuma, T.; Ohshio, G.; Hosotani, R.; Imamura, M.; Konishi, J. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J. Nucl. Med. 1997, 38, 1337–1344. [Google Scholar] [PubMed]
- McBrayer, S.K.; Cheng, J.C.; Singhal, S.; Krett, N.L.; Rosen, S.T.; Shanmugam, M. Multiple myeloma exhibits novel dependence on GLUT4, GLUT8, and GLUT11: Implications for glucose transporter-directed therapy. Blood 2012, 119, 4686–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, K.; Kajiyama, H.; Mizokami, Y.; Ino, K.; Nomura, S.; Mizutani, S.; Terauchi, M.; Kikkawa, F. Placental leucine aminopeptidase (P-LAP) and glucose transporter 4 (GLUT4) expression in benign, borderline, and malignant ovarian epithelia. Gynecol. Oncol. 2005, 98, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, X.; Tu, W.; Qi, Z.; Li, H.; Liu, F.; Yang, Y.; Zhang, Z.; Wang, Z. Apatinib inhibits glycolysis by suppressing the VEGFR2/AKT1/SOX5/GLUT4 signaling pathway in ovarian cancer cells. Cell Oncol. 2019, 42, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, C.; Reis, R.M.; Ricardo, S.; Longatto-Filho, A.; Schmitt, F.; Baltazar, F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010, 2010, 427694. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Nunes, A.; Afonso, J.; Granja, S.; Baltazar, F. Lactate and Lactate Transporters as Key Players in the Maintenance of the Warburg Effect. Adv. Exp. Med. Biol. 2020, 1219, 51–74. [Google Scholar]
- Yang, H.; Zou, W.; Chen, B. Overexpression of CD147 in ovarian cancer is initiated by the hypoxic microenvironment. Cell Biol. Int. 2013, 37, 1139–1142. [Google Scholar] [CrossRef]
- Pinheiro, C.; Longatto-Filho, A.; Ferreira, L.; Pereira, S.M.M.; Etlinger, D.; Moreira, M.A.R.; Jubé, L.F.; Queiroz, G.S.; Schmitt, F.; Baltazar, F. Increasing Expression of Monocarboxylate Transporters 1 and 4 Along Progression to Invasive Cervical Carcinoma. Int. J. Gynecol. Pathol. 2008, 27, 568–574. Available online: https://journals.lww.com/intjgynpathology/Fulltext/2008/10000/Increasing_Expression_of_Monocarboxylate.16.aspx (accessed on 1 October 2013). [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 2481–2497. Available online: https://www.sciencedirect.com/science/article/pii/S0167488916300660 (accessed on 1 January 2016). [CrossRef] [Green Version]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. Available online: https://www.sciencedirect.com/science/article/pii/S221287781930403X (accessed on 1 January 2020). [CrossRef]
- Pinheiro, C.; Granja, S.; Longatto-Filho, A.; Faria, A.M.; Fragoso, M.C.B.V.; Lovisolo, S.M.; Lerário, A.M.; Almeida, M.Q.; Baltazar, F.; Zerbini, M.C.N. Metabolic reprogramming: A new relevant pathway in adult adrenocortical tumors. Oncotarget 2015, 6, 42. Available online: https://www.oncotarget.com/article/5623/text/ (accessed on 1 January 2015). [CrossRef] [PubMed]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [Green Version]
- Gharpure, K.M.; Pradeep, S.; Sans, M.; Rupaimoole, R.; Ivan, C.; Wu, S.Y.; Bayraktar, E.; Nagaraja, A.S.; Mangala, L.S.; Zhang, X. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 2018, 9, 2923. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Niu, X.; Du, Y.; Chen, Y.; Liu, X.; Xu, L.; Iwakura, Y.; Ma, X.; Li, Y.; Yao, Z. IL-17A promotes fatty acid uptake through the IL-17A/IL-17RA/p-STAT3/FABP4 axis to fuel ovarian cancer growth in an adipocyte-rich microenvironment. Cancer Immunol. Immunother. 2020, 69, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.N.M.; Kim, M.-K.; Vo, V.T.A.; Choi, J.-W.; Choi, J.H.; Kim, H.-W.; Cha, S.-K.; Park, K.-S.; Jeong, Y. Inhibition of oncogenic Src induces FABP4-mediated lipolysis via PPARγ activation exerting cancer growth suppression. EBioMedicine 2019, 41, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Chiang, C.-Y.; Daifotis, H.A.; Nieman, K.M.; Fahrmann, J.F.; Lastra, R.R.; Romero, I.L.; Fiehn, O.; Lengyel, E. Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance. Cancer Res. 2020, 80, 1748–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in chronic kidney disease: Novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, Y.; Mao, Y.; Liu, Y.; Yao, D.; Yang, L.; Garson, K.; Vanderhyden, B.C.; Wang, Q. PAX2 promotes epithelial ovarian cancer progression involving fatty acid metabolic reprogramming. Int. J. Oncol. 2020, 56, 697–708. Available online: https://pubmed.ncbi.nlm.nih.gov/31922217 (accessed on 10 January 2020).
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse roles of fatty acid binding proteins (FABPs) in development and pathogenesis of cancers. Gene 2018, 676, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Venturi, M.; Hambly, R.J.; Glinghammar, B.; Rafter, J.J.; Rowland, I.R. Genotoxic activity in human faecal water and the role of bile acids: A study using the alkaline comet assay. Carcinogenesis 1997, 18, 2353–2359. [Google Scholar] [CrossRef] [Green Version]
- Ohmachi, T.; Inoue, H.; Mimori, K.; Tanaka, F.; Sasaki, A.; Kanda, T.; Fujii, H.; Yanaga, K.; Mori, M. Fatty acid binding protein 6 is overexpressed in colorectal cancer. Clin. Cancer Res. 2006, 12, 5090–5095. [Google Scholar] [CrossRef] [Green Version]
- Narisawa, T.; Reddy, B.S.; Weisburger, J.H. Effect of bile acids and dietary fat on large bowel carcinogenesis in animal models. Gastroenterol. Jpn. 1978, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Deng, L.; Li, X.; Wang, G.; Li, Y.; Chen, M. High expression of FABP4 and FABP6 in patients with colorectal cancer. World J. Surg. Oncol. 2019, 17, 171. [Google Scholar] [CrossRef]
- Sawyer, B.T.; Qamar, L.; Yamamoto, T.M.; McMellen, A.; Watson, Z.L.; Richer, J.K.; Behbakht, K.; Schlaepfer, I.R.; Bitler, B.G. Targeting Fatty Acid Oxidation to Promote Anoikis and Inhibit Ovarian Cancer Progression. Mol. Cancer Res. 2020, 18, 1088–1098. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Mohamed, E.M.; Xu, G.G.; Waters, M.; Jing, K.; Ma, Y.; Zhang, Y.; Spiegel, S.; Idowu, M.O.; Fang, X. Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget 2016, 7, 3832–3846. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Z.; Liu, S.; Li, J.; Wu, L.; Lv, X.; Xu, J.; Chen, B.; Zhao, S.; Yang, H. CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer. Transl. Oncol. 2021, 14, 101023. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P. The role of adipokines in chronic inflammation. Immunol. Targets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.H.; Kim, H.-J.; Kim, C.Y.; Kim, Y.H.; Ju, W.; Kim, S.C. Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet. Gynecol. Sci. 2016, 59, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. 2004, 68, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.U.; Ha, K.H.; Han, S.J.; Kim, H.J.; Kim, D.J. The Association of Adiponectin and Visceral Fat with Insulin Resistance and β-Cell Dysfunction. J. Korean Med. Sci. 2019, 34, e7. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. Available online: https://pubmed.ncbi.nlm.nih.gov/31121868 (accessed on 22 May 2019).
- Hoffmann, M.; Gogola, J.; Ptak, A. Adiponectin Reverses the Proliferative Effects of Estradiol and IGF-1 in Human Epithelial Ovarian Cancer Cells by Downregulating the Expression of Their Receptors. Horm. Cancer 2018, 9, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Ouh, Y.-T.; Cho, H.W.; Lee, J.K.; Choi, S.H.; Choi, H.J.; Hong, J.H. CXC chemokine ligand 1 mediates adiponectin-induced angiogenesis in ovarian cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2019, 42, 1010428319842699. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Ocon-Grove, O.M.; Hadley, J.A.; Giles, J.R.; Johnson, P.A.; Ramachandran, R. Expression of adiponectin and its receptors is altered in epithelial ovarian tumors and ascites-derived ovarian cancer cell lines. Int. J. Gynecol. Cancer 2015, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Pulliam, N.; Özeş, A.; Buechlein, A.; Ding, N.; Keer, H.; Rusch, D.; O’Hagan, H.; Stack, M.S.; Nephew, K.P. Epigenetic Targeting of Adipocytes Inhibits High-Grade Serous Ovarian Cancer Cell Migration and Invasion. Mol. Cancer Res. 2018, 16, 1226–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163. [Google Scholar] [PubMed]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef]
- Crean-Tate, K.K.; Reizes, O. Leptin Regulation of Cancer Stem Cells in Breast and Gynecologic Cancer. Endocrinology 2018, 159, 3069–3080. Available online: https://pubmed.ncbi.nlm.nih.gov/29955847 (accessed on 1 August 2018).
- Diaz, E.S.; Karlan, B.Y.; Li, A.J. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol. Oncol. 2013, 129, 353–357. [Google Scholar] [CrossRef]
- Choi, J.-H.; Park, S.-H.; Leung, P.C.K.; Choi, K.-C. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J. Clin. Endocrinol. Metab. 2005, 90, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, A.; Hashemy, S.I.; Aghaei, M.; Panjehpour, M. Leptin induces matrix metalloproteinase 7 expression to promote ovarian cancer cell invasion by activating ERK and JNK pathways. J. Cell Biochem. 2018, 119, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Saeidi, J.; Mohtashami, M.; Hashemy, I. Estrogen-independent role of ERα in ovarian cancer progression induced by leptin/Ob-Rb axis. Mol. Cell Biochem. 2019, 458, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Y.; Gong, C.; Ji, T.; Zhou, X.; Zhang, T. Targeting Leptin as a Therapeutic Strategy against Ovarian Cancer Peritoneal Metastasis. Anticancer Agents Med. Chem. 2017, 17, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhang, H.; Yao, L.; Jiang, S.; Lu, H.; Xing, X. Leptin contributes to the taxol chemoresistance in epithelial ovarian cancer. Oncol. Lett. 2019, 18, 561–570. Available online: https://pubmed.ncbi.nlm.nih.gov/31289528 (accessed on 21 May 2019).

| Type of GLUT | Percent of OC with Overexpressed GLUT | Major Subtype of OC with Higher Expression of GLUT | Proposed Role in Dif-ferent Cancer (Not Only in OC) | Potential Inhibitors |
|---|---|---|---|---|
| GLUT 1 | 96% [49] 98.7% [44] | - Serous adenocarcinoma [35,42,44,51,52] - Advanced stages of OC (detected only in serous adenocarcinoma) [33,44,51,53] | - Adaptation to hypoxia [49] - Basal influx of glucose, which is a source of energy [28,40] - Possibly involved in mechanism—metastasis formation [46,47,48] - Possibly involved in new vessel formation via VEGF [44] | - Ciglitazone—changing amount of GLUT1 in plasma membrane [57] - BAY-876—reduction of glucose utilization rate [58] - STF31 and metformin—inhibition GLUT1 |
| GLUT 3 | 92.8% [44] | The current research does not prove any correlations | - Glucose reuptake [44] - Factor of neovascularization via VEGF [44] | not detected |
| GLUT 4 | 84.4% [44] | Mucinous and clear cells adenocarcinoma | Angiogenesis, metastasis formation via VEGFR2/AKT1/GSK3β/SOX5/GLUT4 pathway [66] | Apatinib–modulatory factor in VEGFR2/AKT1/GSK3β/SOX5/GLUT4 pathway [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baczewska, M.; Bojczuk, K.; Kołakowski, A.; Dobroch, J.; Guzik, P.; Knapp, P. Obesity and Energy Substrate Transporters in Ovarian Cancer—Review. Molecules 2021, 26, 1659. https://doi.org/10.3390/molecules26061659
Baczewska M, Bojczuk K, Kołakowski A, Dobroch J, Guzik P, Knapp P. Obesity and Energy Substrate Transporters in Ovarian Cancer—Review. Molecules. 2021; 26(6):1659. https://doi.org/10.3390/molecules26061659
Chicago/Turabian StyleBaczewska, Marta, Klaudia Bojczuk, Adrian Kołakowski, Jakub Dobroch, Paweł Guzik, and Paweł Knapp. 2021. "Obesity and Energy Substrate Transporters in Ovarian Cancer—Review" Molecules 26, no. 6: 1659. https://doi.org/10.3390/molecules26061659








