Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo
Abstract
:1. Introduction
2. Results
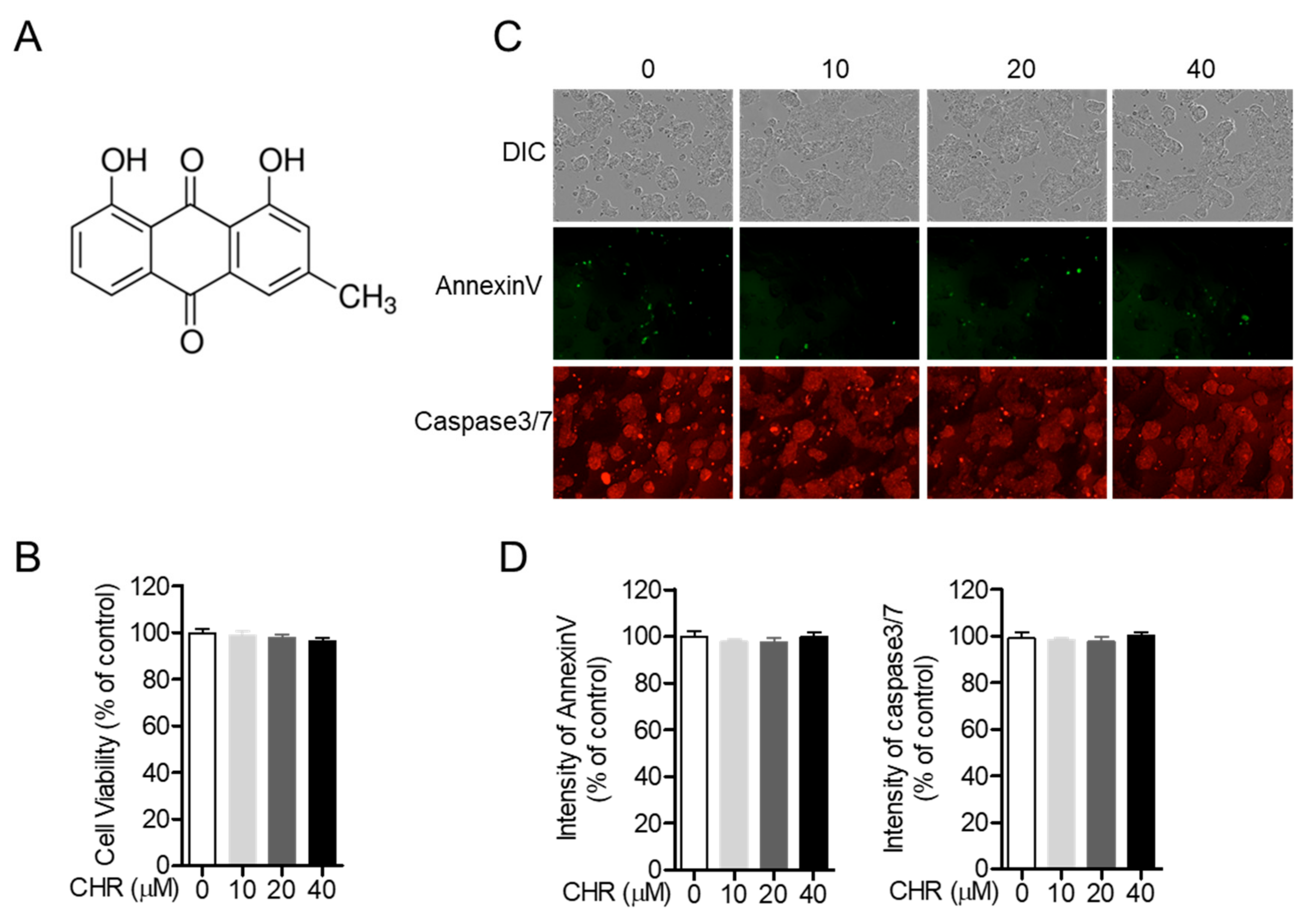
2.1. Chrysophanol Does Not Show Cytotoxicity on HT-29 Colorectal Cells
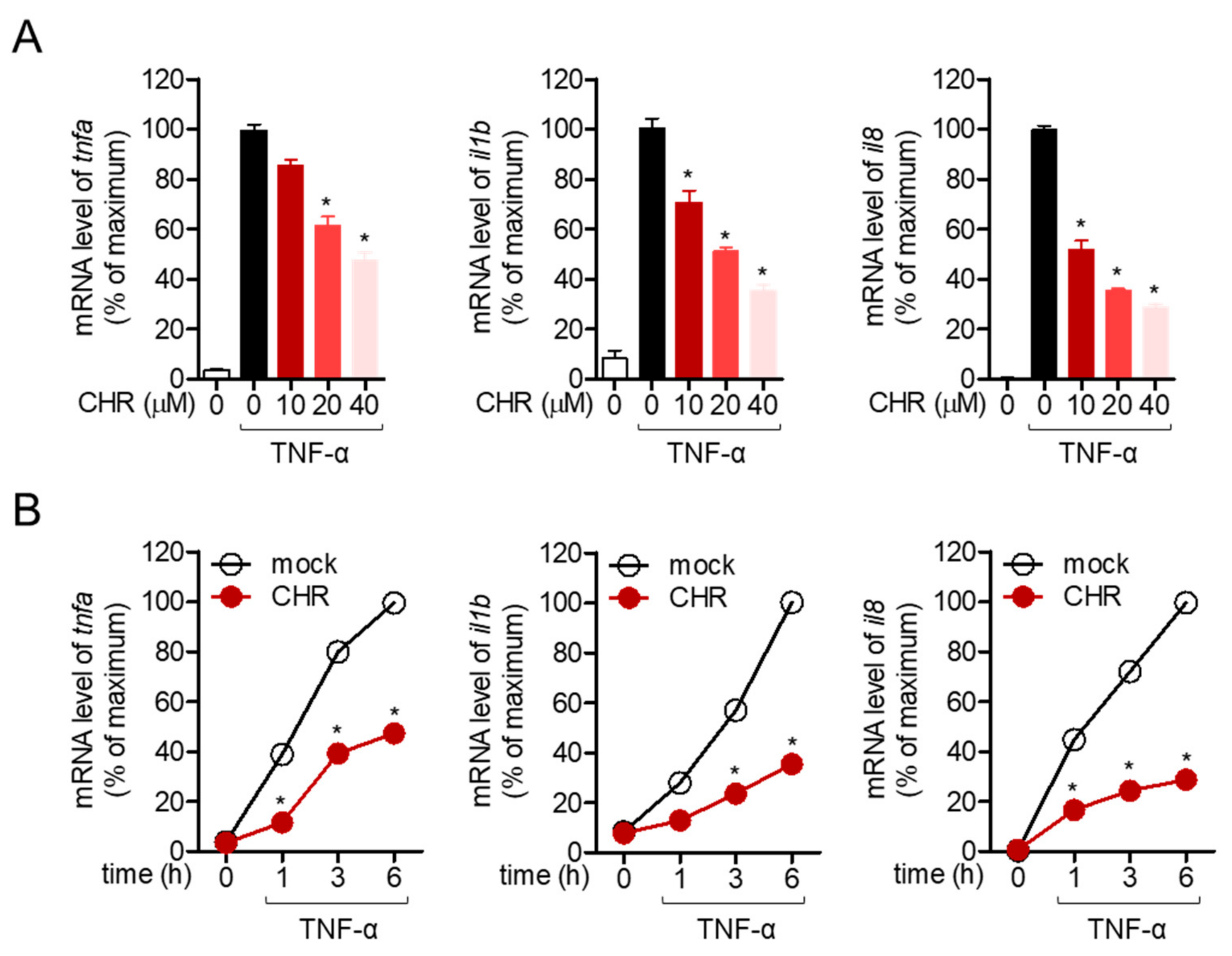
2.2. Pre-Treatment with Chrysophanol Inhibits the Expression of Pro-Inflammatory Cytokines in Stimulated HT-29 Cells
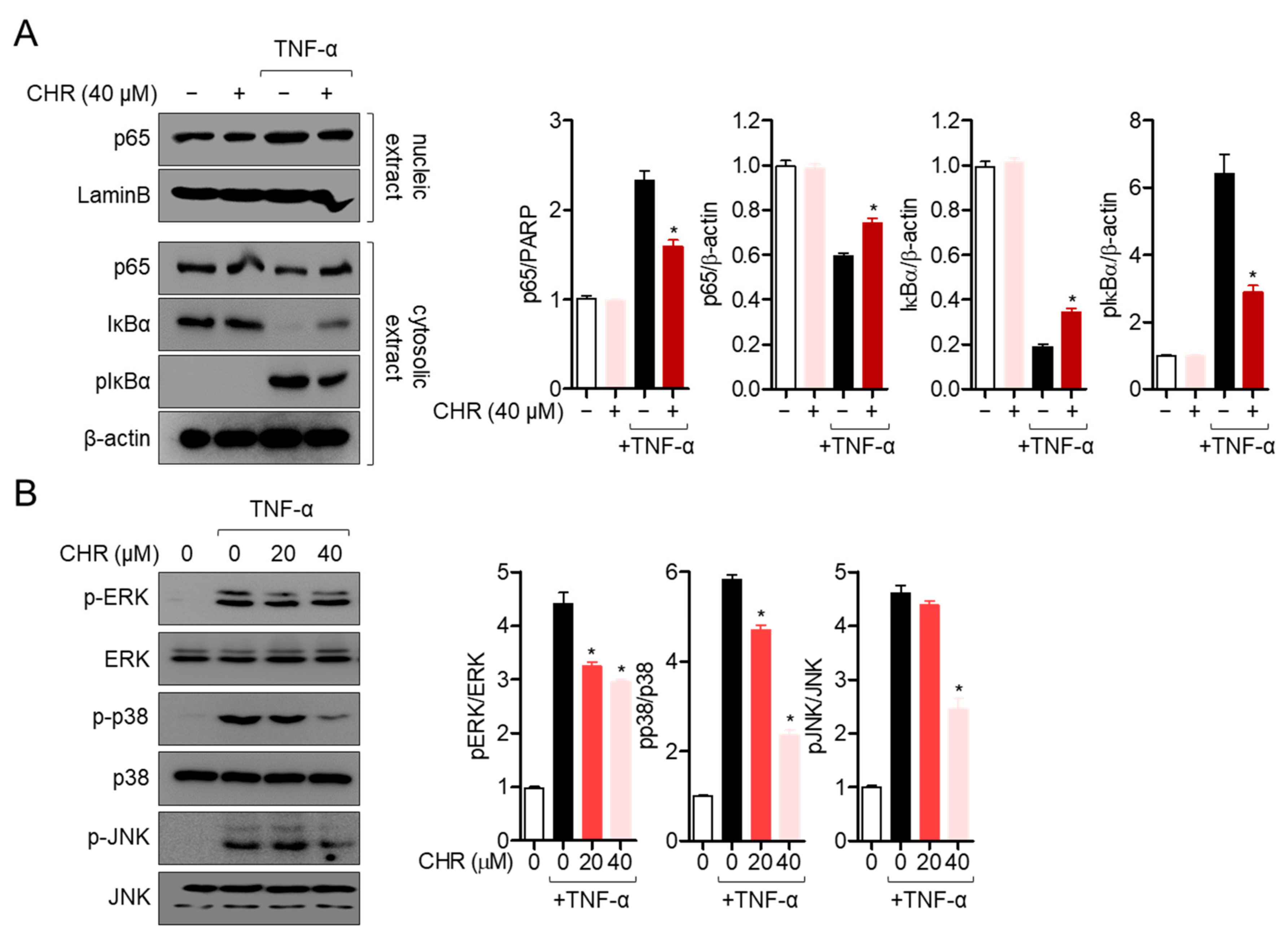
2.3. Pre-Treatment with Chrysophanol Mitigates p65 Translocation and MAPK Pathway in Activated HT-29 Cells
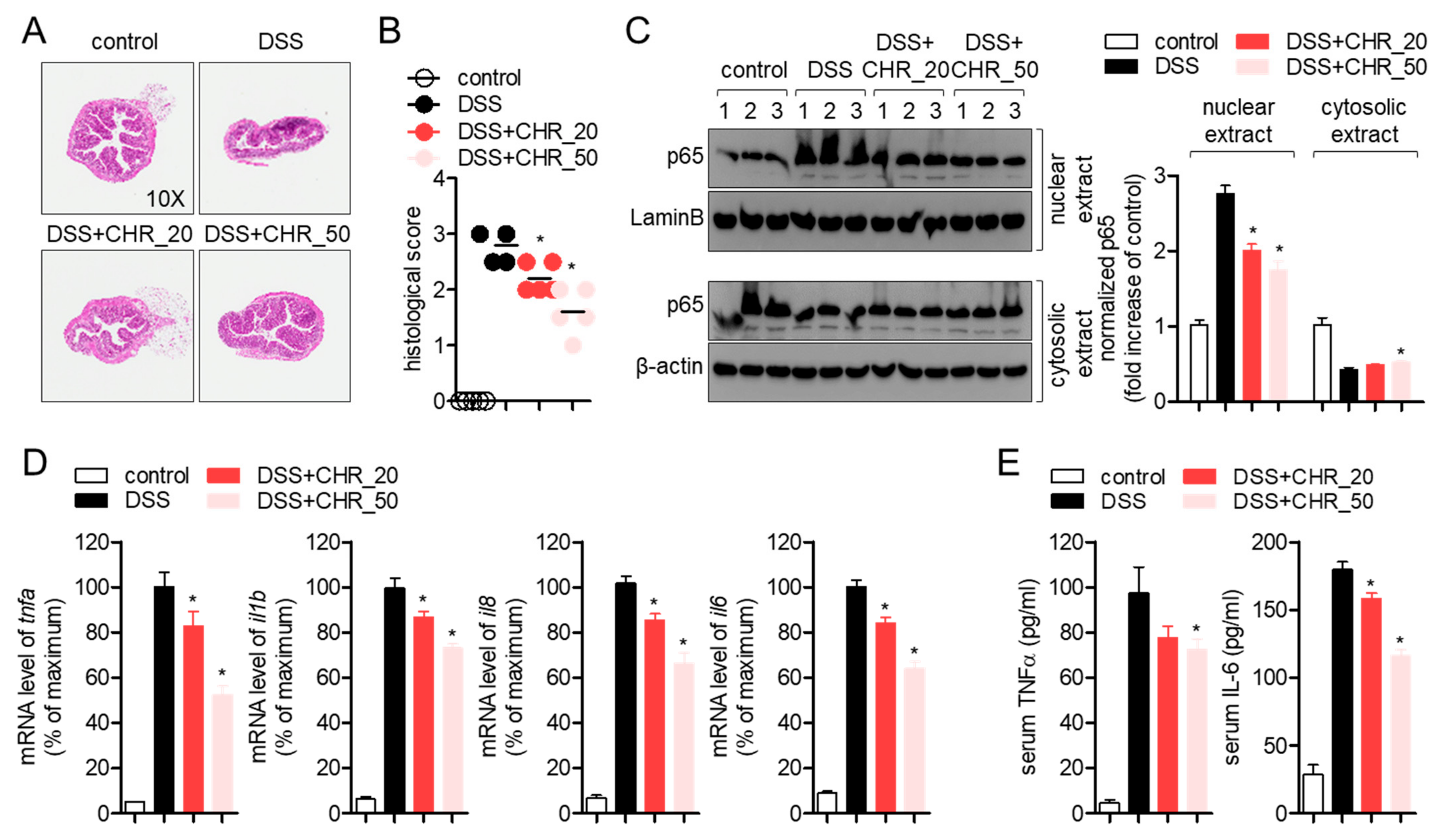
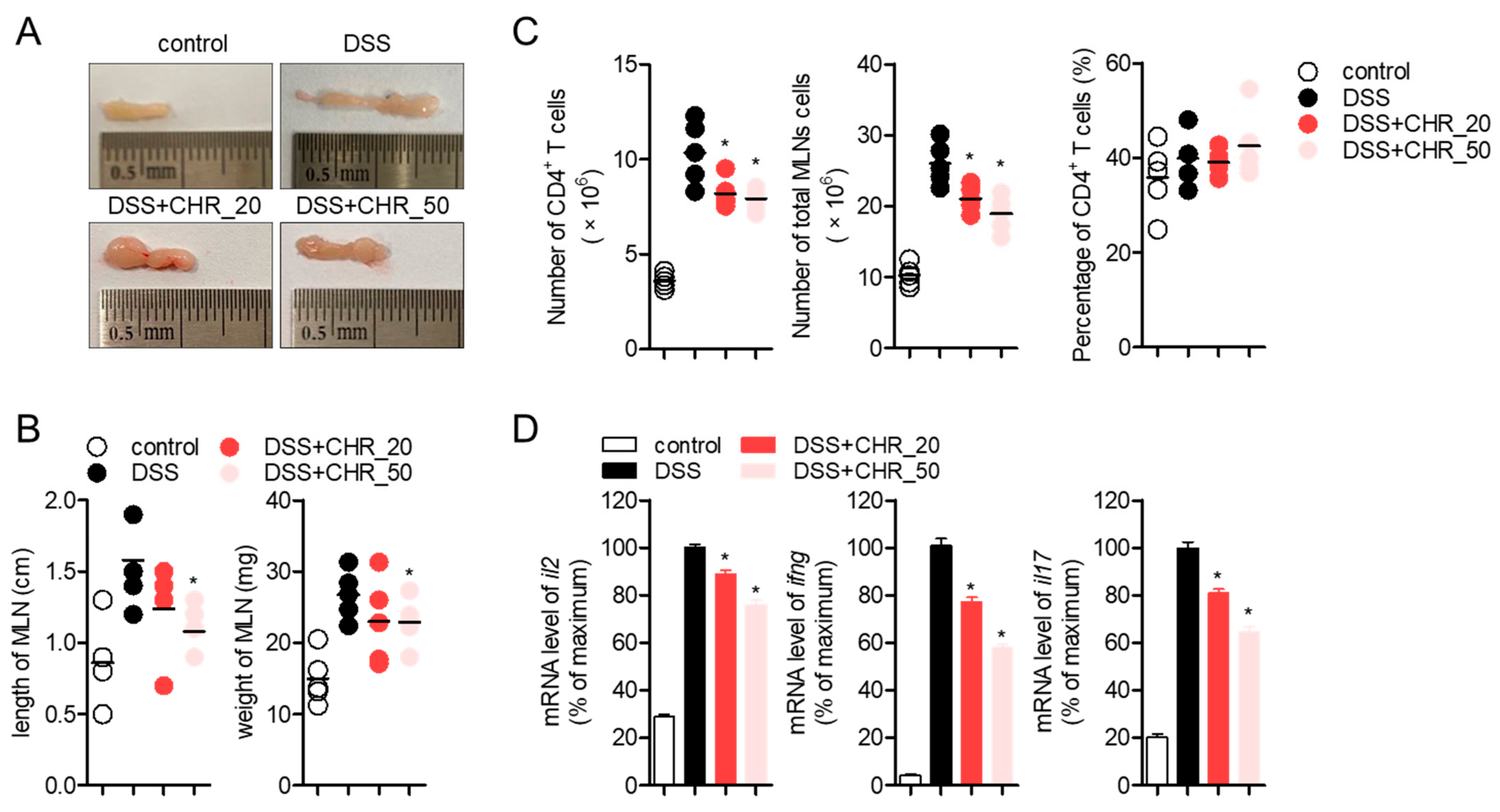
2.4. Oral Administration of Chrysophanol Protects Mice from DSS-Induced IBD In Vivo
2.5. Oral Administration of Chrysophanol Attenuates the Expression of Pro-Inflammatory Cytokines on Colon Tissues of DSS-Induced IBD Model
2.6. Oral Administration of Chrysophanol Decreases the Expression of Effector Cytokines from Mesenteric Lymph Nodes
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Mice
4.3. Isolation of Chrysophanol from Rumex crispus L.
4.4. Reagents and Antibodies
4.5. MTT Viability Assay
4.6. Determination of AnnexinV and Caspase3/7 Expression
4.7. Quantitative Realtime PCR Analysis
4.8. Western Blot Analysis
4.9. Induction of IBD Model Using DSS
4.10. Determination of Disease Activity Index
4.11. Histological Analysis with H&E Staining
4.12. Purification of CD4+ T Cells from Mesenteric Lymph Nodes
4.13. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wen, Z.; Fiocchi, C. Inflammatory bowel disease: Autoimmune or immune-mediated pathogenesis? Clin. Dev. Immunol. 2004, 11, 195–204. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Shehab, K.W.; Ghishan, F.K.; Kiela, P.R. T Lymphocyte Dynamics in Inflammatory Bowel Diseases: Role of the Microbiome. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Lee, H.J.; Kim, W.J.; Han, K.I.; Iwasa, M.; Kobayashi, K.; Debnath, T.; Tang, Y.; Kwak, Y.S.; Yoon, J.H.; et al. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE 2019, 14, e0210854. [Google Scholar] [CrossRef] [Green Version]
- Prelipcean, C.C.; Mihai, C.; Gogalniceanu, P.; Mihai, B. What is the impact of age on adult patients with inflammatory bowel disease? Clujul. Med. 2013, 86, 3–9. [Google Scholar] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.S.; Wichary, J.; Zadnik, M.; Reinisch, W. Competition for Clinical Trials in Inflammatory Bowel Diseases. Gastroenterol. 2019, 157, 1457–1461.e2. [Google Scholar] [CrossRef] [Green Version]
- Horsthuis, K.; Stokkers, P.C.F.; Stoker, J. Detection of inflammatory bowel disease: Diagnostic performance of cross-sectional imaging modalities. Abdom. Imaging 2008, 33, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Muzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmrich, J.; Seyfarth, M.; Fleig, W.E.; Emmrich, F. Treatment of inflammatory bowel disease with anti-CD4 monoclonal antibody. Lancet 1991, 338, 570–571. [Google Scholar] [CrossRef]
- Im, N.K.; Jung, Y.S.; Choi, J.H.; Yu, M.H.; Jeong, G.S. Inhibitory effect of the leaves of Rumex crispus L. on LPS-induced nitric oxide production and the expression of iNOS and COX-2 in macrophages. Nat. Prod. Sci. 2014, 20, 51–57. [Google Scholar]
- Tutin, F.; Clewer, H.W.B. CCXXXII.-The constituents of Cluytia similis. J. Chem. Soc. Trans. 1912, 101, 2221–2234. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Yu, S.; Lv, W.; Guan, Z.; Sun, M.; Wang, J. Chrysophanol attenuates airway inflammation and remodeling through nuclear factor-kappa B signaling pathway in asthma. Phyther. Res. 2019, 33, 2702–2713. [Google Scholar] [CrossRef] [PubMed]
- Coopoosamy, R.M.; Magwa, M.L. Antibacterial activity of chrysophanol isolated from Aloe excelsa (Berger). Afr. J. Biotechnol. 2006, 5, 1508–1510. [Google Scholar]
- Kuo, Y.H.; Lee, P.H.; Wein, Y.S. Four new compounds from the seeds of Cassia fistula. J. Nat. Prod. 2002, 65, 1165–1167. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.H.; Chen, P.Y.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Wu, S.H.; Ip, S.W.; Wu, C.T.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced necrotic-like cell death through an impaired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch. Pharm. Res. 2012, 35, 887–895. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, S.; Sun, R.; Wang, L.; Xing, C.; Liang, R.; Kong, Q. Chrysophanol Relieves Cognition Deficits and Neuronal Loss Through Inhibition of Inflammation in Diabetic Mice. Neurochem. Res. 2018, 43, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Mei, L.; Ye, S.; Liu, X.; Xu, Q.; Miao, J.; Du, S.; Chen, D.; Li, C.; Li, H. Chrysophanol demonstrates anti-inflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. Int. Immunopharmacol. 2018, 56, 90–97. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, H.Y.; Kim, H.M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 2018, 54, 238–244. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.S. Chrysophanol mitigates T cell activation by regulating the expression of CD40 ligand in activated T cells. Int. J. Mol. Sci. 2020, 21, 6122. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Erben, U.; Kühl, A.A. The Role of T-Cell Subsets in Chronic Inflammation in Celiac Disease and Inflammatory Bowel Disease Patients: More Common Mechanisms or More Differences? Inflamm. Intest. Dis. 2016, 1, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.C.; Lee, B.J.; Park, D.H.; Hong, S.H.; Um, J.Y. Anti-inflammatory activity of chrysophanol through the suppression of NF-κB/caspase-1 activation in vitro and in vivo. Molecules 2010, 15, 6436–6451. [Google Scholar] [CrossRef] [Green Version]
- Yuenyongsawad, S.; Bunluepuech, K.; Wattanapiromsakul, C.; Tewtrakul, S. Anti-cancer activity of compounds from Cassia garrettiana heartwood. Songklanakarin J. Sci. Technol. 2014, 36, 189–194. [Google Scholar]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar] [CrossRef] [Green Version]
- Roy, U.; Gálvez, E.J.C.; Iljazovic, A.; Lesker, T.R.; Błażejewski, A.J.; Pils, M.C.; Heise, U.; Huber, S.; Flavell, R.A.; Strowig, T. Distinct Microbial Communities Trigger Colitis Development upon Intestinal Barrier Damage via Innate or Adaptive Immune Cells. Cell Rep. 2017, 21, 994–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Han, W.; Liang, J.; Ji, J.; Wang, J.; Cantor, H.; Lu, L. Glatiramer acetate ameliorates inflammatory bowel disease in mice through the induction of Qa-1-restricted CD8+ regulatory cells. Eur. J. Immunol. 2013, 43, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Chyuan, I.T.; Tsai, H.F.; Wu, C.S.; Hsu, P.N. TRAIL suppresses gut inflammation and inhibits colitogeic T-cell activation in experimental colitis via an apoptosis-independent pathway. Mucosal Immunol. 2019, 12, 980–989. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: New York, NY, USA, 2015; pp. 113–124. ISBN 9783319161044. [Google Scholar]
- Erdman, S.E.; Rao, V.P.; Poutahidis, T.; Rogers, A.B.; Taylor, C.L.; Jackson, E.A.; Ge, Z.; Lee, C.W.; Schauer, D.B.; Wogan, G.N.; et al. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Kolios, G.; Robertson, D.A.F.; Jordan, N.J.; Minty, A.; Caput, D.; Ferrara, P.; Westwick, J. Interleukin-8 production by the human colon epithelial cell line HT-29: Modulation by interleukin-13. Br. J. Pharmacol. 1996, 119, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Mazlam, M.Z.; Hodgson, H.J.F. Interrelation between interleukin-6, interleukin-1β, plasma C-reactive protein values, and in vitro C-reactive protein generation in patients with inflammatory bowel disease. Gut 1994, 35, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104. [Google Scholar] [CrossRef]
- Imam, T.; Park, S.; Kaplan, M.H.; Olson, M.R. Effector T helper cell subsets in inflammatory bowel diseases. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Fan, M.; Peng, C.; Peng, Y.; Zhang, M.; Li, X. Analysis of Metabolites of Anthraquinones by Human Fecal Bacteria Using UPLC-Q-TOF-HRMS/MS. Chromatographia 2016, 79, 1593–1604. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, H.; Li, D.; Xiao, N.; Tan, Z. Role of tryptophan-metabolizing microbiota in mice diarrhea caused by Folium sennae extracts. BMC Microbiol. 2020, 20, 185. [Google Scholar] [CrossRef]
- Cui, H.X.; Zhang, L.S.; Luo, Y.; Yuan, K.; Huang, Z.Y.; Guo, Y. A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ayyangar, N.R.; Bapat, D.S.; Joshi, B.S. Anthraquinones and anthrone series: Part XXXVI—a newsynthesis of chrysophanol, rhein, islandicin, emodin and physcion. J. Sci. Ind. Res. 1961, 20B, 493–497. [Google Scholar]
- Horuluoglu, B.H.; Kayraklioglu, N.; Tross, D.; Klinman, D. PAM3 protects against DSS-induced colitis by altering the M2:M1 ratio. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Zong, S.Y.; Pu, Y.Q.; Xu, B.L.; Zhang, T.; Wang, B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2017, 42, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Jeon, B.; Lee, H.; Im, S.; Araki, M.; Araki, K.; Yamamura, K.; Choi, S.; Park, D.; Jun, C. IGSF4 is a novel TCR ζ-chain-interacting protein that enhances TCR-mediated signaling. J. Exp. Med. 2011, 208, 2545–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Choi, E.-J.; Lee, K.-S.; Kim, H.-R.; Na, B.-R.; Kwon, M.-S.; Jeong, G.-S.; Choi, H.G.; Choi, E.Y.; Jun, C.-D. Oral Administration of p-Hydroxycinnamic Acid Attenuates Atopic Dermatitis by Downregulating Th1 and Th2 Cytokine Production and Keratinocyte Activation. PLoS ONE 2016, 11, e0150952. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| human TNFα | CCT ACC AGA CCA AGG TCA AC | AGG GGG TAA TAA AGG GAT TG |
| human IL-1β | GGA TAT GGA GCA ACA AGT GG | ATG TAC CAG TTG GGG AAC TG |
| human IL-8 | GTG CAG TTT TGC CAA GGA GT | TTA TGA ATT CTC AGC CCT CTT CAA AAA |
| human GAPDH | CGG AGT CAA CGG ATT TGG TCG TAT | AGC CTT CTC CAT GGT GGT GAA GAC |
| mouse TNFα | GGC AGG TCT ACT TTG GAG TCA TTG C | ACA TTC GAG GCT CCA GTG AAT TCG G |
| mouse IL-1β | ATA ACC TGC TGG TGT GTG AC | AGG TGC TGA TGT ACC AGT TG |
| mouse IL-8 | ATG GCT GCT CAA GGC TGG TC | AGG CTT TTC ATG CTC AAC ACT AT |
| mouse IL-6 | CCG GAG AGG AGA CTT CAC AG | GGA AAT TGG GGT AGG AAG GA |
| mouse IL-2 | TGA GCA GGA TGG AGA ATT ACA GG | GTC CAA GTT CAT CTT CTA GGC AC |
| mouse IFNγ | TCA AGT GGC ATA GAT GTG GAA GAA | TGG CTC TGC AGG ATT TTC ATG |
| mouse IL-17 | TCC CCT CTG TCA TCT GGG AAG | CTC GAC CCT GAA AGT GAA GG |
| mouse GAPDH | GCA CAG TCA AGG CCG AGA AT | GCC TTC TCC ATG GTG GTG AA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Jeong, G.-S. Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo. Molecules 2021, 26, 1682. https://doi.org/10.3390/molecules26061682
Lee H-S, Jeong G-S. Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo. Molecules. 2021; 26(6):1682. https://doi.org/10.3390/molecules26061682
Chicago/Turabian StyleLee, Hyun-Su, and Gil-Saeng Jeong. 2021. "Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo" Molecules 26, no. 6: 1682. https://doi.org/10.3390/molecules26061682
APA StyleLee, H.-S., & Jeong, G.-S. (2021). Chrysophanol Attenuates Manifestations of Immune Bowel Diseases by Regulation of Colorectal Cells and T Cells Activation In Vivo. Molecules, 26(6), 1682. https://doi.org/10.3390/molecules26061682





