S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells
Abstract
:1. Introduction
2. Results
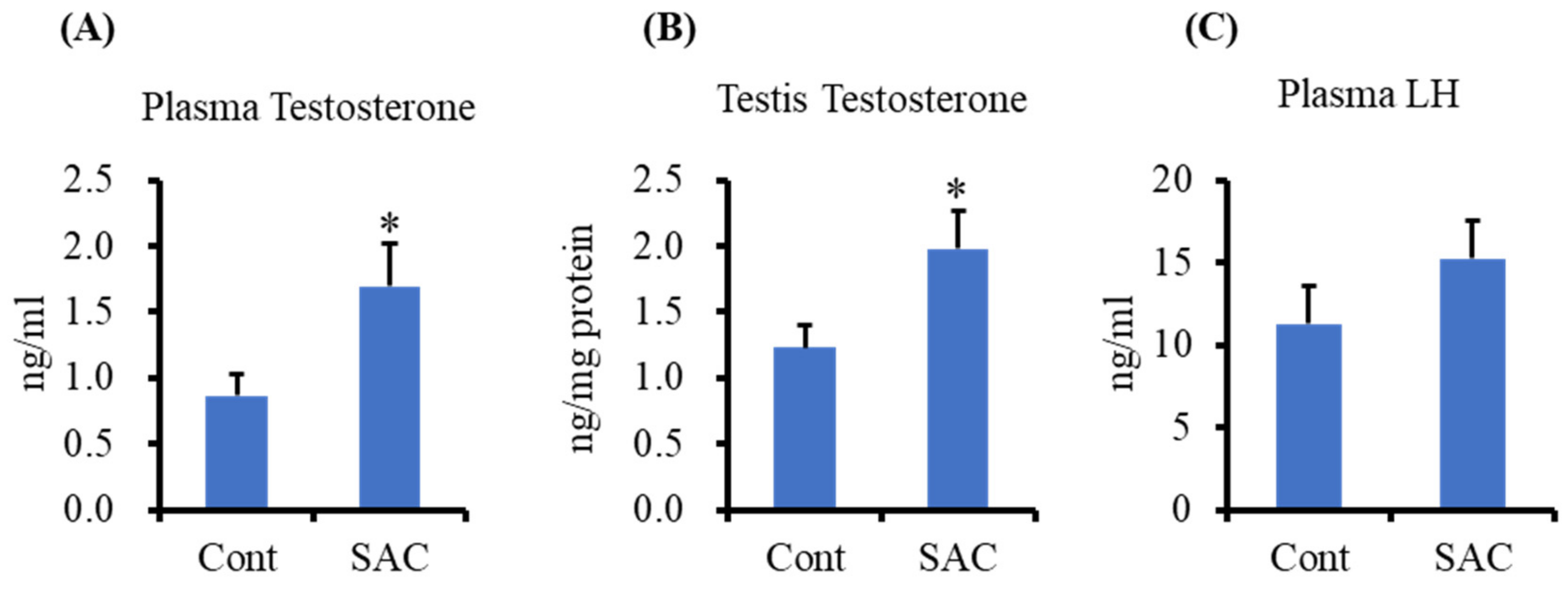
2.1. Effect of SAC on Testosterone Production in Testes and Plasma of Mice
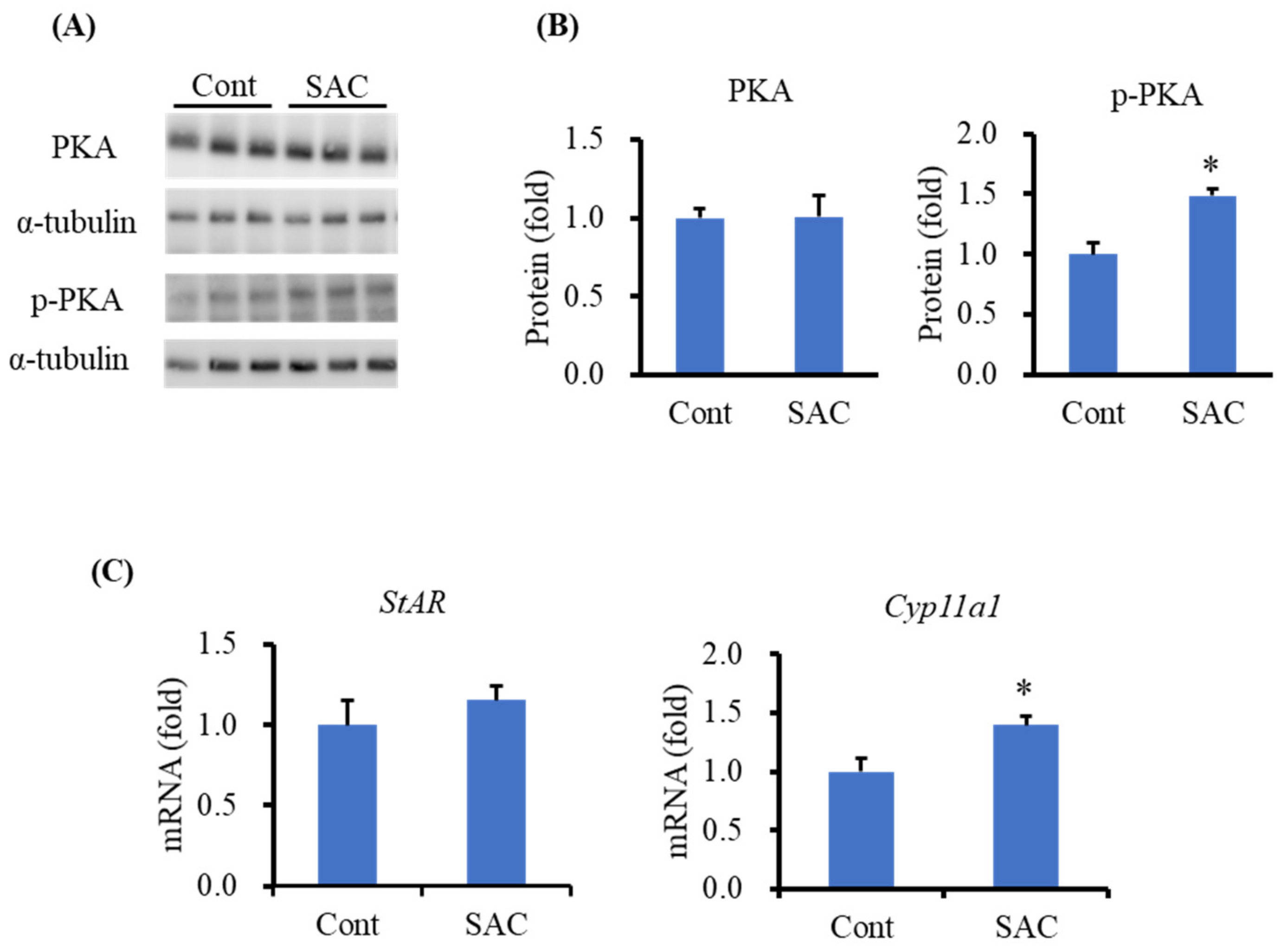
2.2. Effect of SAC on Steroidogenic Protein Levels in the Testes of Mice
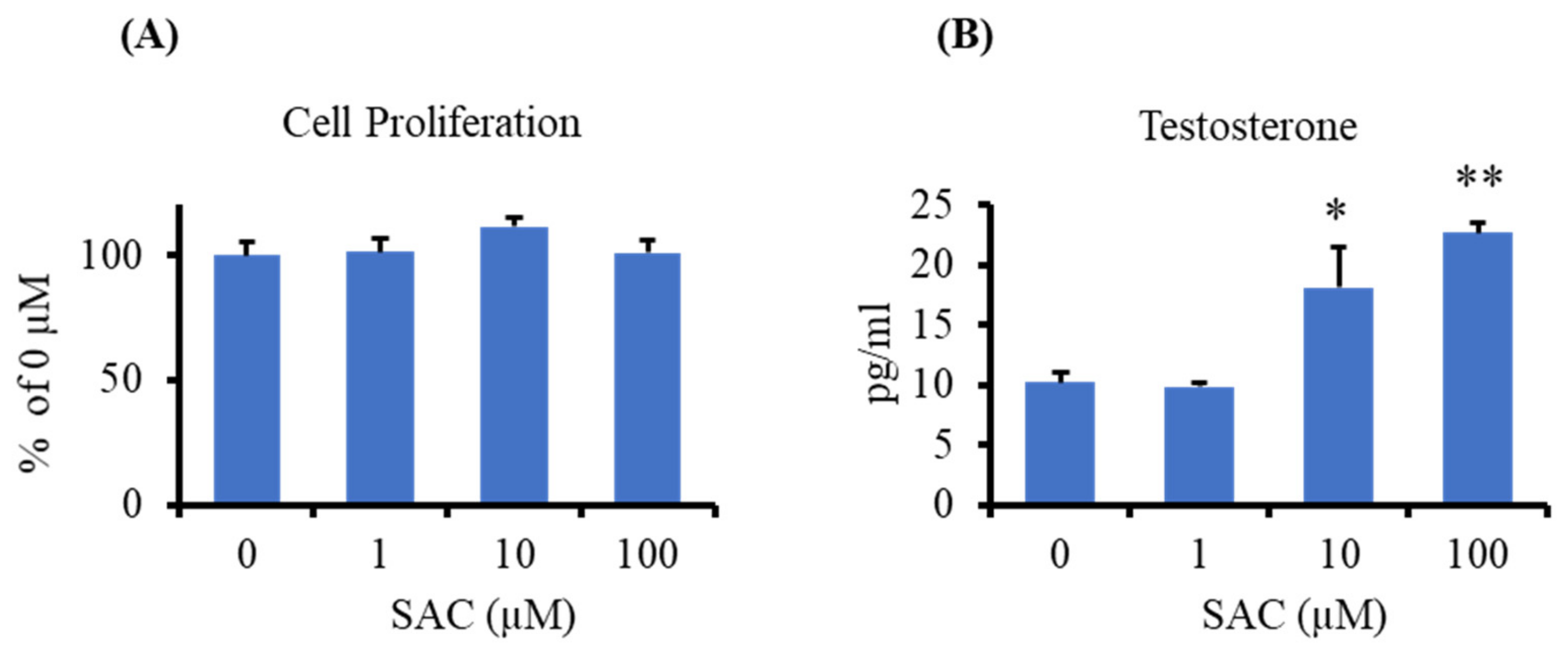
2.3. Effect of SAC on Testosterone Production in I-10 Cells
2.4. Effect of SAC on the Activation of PKA in I-10 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Cell Culture
4.4. Testosterone and Luteinizing Hormone Measurement
4.5. Cell Proliferation Assay
4.6. Western Blot
4.7. RNA Extraction and mRNA Quantification
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [Green Version]
- Nieschlag, E. Late-onset hypogonadism: A concept comes of age. Andrology 2020, 8, 1506–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Yeap, B.B. Testosterone and ill-health in aging men. Nat. Clin. Pr. Endocrinol. Metab. 2009, 5, 113–121. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Ahn, S.T.; Moon, D.G. Evolution of Guidelines for Testosterone Replacement Therapy. J. Clin. Med. 2019, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse Events Associated with Testosterone Administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigen, R.; O’Donnell, C.I.; Barón, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of Testosterone Therapy With Mortality, Myocardial Infarction, and Stroke in Men With Low Testosterone Levels. JAMA 2013, 310, 1829–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Shirakawa, H.; Takumi, N.; Minegishi, Y.; Ohashi, A.; Howlader, Z.H.; Ohsaki, Y.; Sato, T.; Goto, T.; Komai, M. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Heal. Dis. 2011, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.-J.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Ho, H.-J.; Yamagishi, M.; Ikemoto, H.; Komai, M.; Shirakawa, H. Cysteine Sulfoxides Enhance Steroid Hormone Production via Activation of the Protein Kinase A Pathway in Testis-Derived I-10 Tumor Cells. Molecules 2020, 25, 4694. [Google Scholar] [CrossRef]
- Horigome, S.; Maeda, M.; Ho, H.; Shirakawa, H.; Komai, M. Effect of Kaempferia parviflora extract and its polymethoxyflavonoid components on testosterone production in mouse testis-derived tumour cells. J. Funct. Foods 2016, 26, 529–538. [Google Scholar] [CrossRef]
- Banihani, S.A. Ginger and Testosterone. Biomolecules 2018, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Testosterone in Males as Enhanced by Onion (Allium Cepa L.). Biomolecules 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banihani, S.A. Mechanisms of honey on testosterone levels. Heliyon 2019, 5, e02029. [Google Scholar] [CrossRef]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, Chemical, and Biological Properties of S-Allylcysteine, an Amino Acid Derived from Garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The Antioxidant Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection. Oxidative Med. Cell. Longev. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Ali, S.F.; Túnez, I.; Santamaría, A. On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update. Neurochem. Int. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Agbana, Y.L.; Ni, Y.; Zhou, M.; Zhang, Q.; Kassegne, K.; Karou, S.D.; Kuang, Y.; Zhu, Y. Garlic-derived bioactive compound S-allylcysteine inhibits cancer progression through diverse molecular mechanisms. Nutr. Res. 2020, 73, 1–14. [Google Scholar] [CrossRef]
- Asdaq, S.; Inamdar, M. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am. J. Physiol. Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanan, G.; Ponmurugan, P.; Gandhipuram, P.S.; Rajaraja, T. Antidiabetic properties of S-allyl cysteine, a garlic component on streptozotocin-induced diabetes in rats. J. Appl. Biomed. 2009, 7, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, G.; Ponmurugan, P. Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem. Interact. 2011, 189, 100–106. [Google Scholar] [CrossRef]
- Amano, H.; Kazamori, D.; Itoh, K.; Kodera, Y. Metabolism, Excretion, and Pharmacokinetics of S-Allyl-l-Cysteine in Rats and Dogs. Drug Metab. Dispos. 2015, 43, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas 2010, 67, 144–150. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Rao, K.; Zhan, Y.; Chen, R.-B.; Liu, Z.; Li, M.-C.; Zhuan, L.; Zang, G.-H.; Guo, S.-M.; et al. S-allyl cysteine restores erectile function through inhibition of reactive oxygen species generation in diabetic rats. Andrology 2013, 1, 487–494. [Google Scholar] [CrossRef]
- Takemura, S.; Ichikawa, H.; Naito, Y.; Takagi, T.; Yoshikawa, T.; Minamiyama, Y. S-allyl cysteine ameliorates the quality of sperm and provides protection from age-related sperm dysfunction and oxidative stress in rats. J. Clin. Biochem. Nutr. 2014, 55, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.-S.; Lee, S.-H.; Lee, S.; Yang, B.-K. Effects of streptozotocin and S-allyl-l-cysteine on motility, plasma membrane integrity, and mitochondrial activity of boar spermatozoa. Trop. Anim. Heal. Prod. 2019, 52, 437–444. [Google Scholar] [CrossRef]
- Tremblay, J.J. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 2015, 103, 3–10. [Google Scholar] [CrossRef]
- Miller, W.L.; Bose, H.S. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Chandrala, S.P.; Jo, Y.; Stocco, D.M. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J. Mol. Endocrinol. 2006, 37, 81–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef]
- Weng, J.-H.; Chung, B.-C. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids 2016, 111, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Imai, T.; Kosuge, Y.; Endo-Umeda, K.; Miyagishi, H.; Ishige, K.; Makishima, M.; Ito, Y. Protective effect of S-allyl-l-cysteine against endoplasmic reticulum stress-induced neuronal death is mediated by inhibition of calpain. Amino Acids 2013, 46, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Moriguchi, T.; Morihara, N.; Saito, H. Ameliorative effect of S-allylcysteine, a major thioallyl constituent in aged garlic extract, on learning deficits in senescence-accelerated mice. J. Nutr. 2001, 131, 1093S–1095S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Ghasemi, Z.; Roghani, M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur. J. Pharmacol. 2017, 794, 69–76. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, E.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Maldonado, P.D.; Rojas, P. S-allyl Cysteine, a Garlic Compound, Produces an Antidepressant-Like Effect and Exhibits Antioxidant Properties in Mice. Brain Sci. 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.T.; Kowalewski, M.P.; Manna, P.R.; Stocco, D.M. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol. Cell. Endocrinol. 2009, 300, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Park, T.; Oh, J.-H.; Jang, Y.P.; Lee, J.H. Oral Administration of (S)-Allyl-l-Cysteine and Aged Garlic Extract to Rats: Determination of Metabolites and Their Pharmacokinetics. Planta Med. 2017, 83, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.A.; Khamenehi, H.J.; Hamadeh, M. Evaluation of androgenic activity of allium cepa on spermatogenesis in the rat. Folia Morphol. 2009, 68, 45–51. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Cyp11a1 | 5′-CGTGACCTTGCAGAGGTACACT-3′ | 5′-GCTGGAATCTTGTAATTACGAAGCA-3′ |
| StAR | 5′-GGAGCTCTCTGCTTGGTTCTC-3′ | 5′-TTAGCACTTCGTCCCCGTTC-3′ |
| Eef1a1 | 5′-GATGGCCCCAAATTCTTGAAG | 5′-GGACCATGTCAACAATTGCAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.M.; Shiozawa, K.; Mukai, K.; Takayanagi, K.; Eguchi, K.; Sultana, H.; Ohsaki, Y.; Komai, M.; Shirakawa, H. S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells. Molecules 2021, 26, 1697. https://doi.org/10.3390/molecules26061697
Rana MM, Shiozawa K, Mukai K, Takayanagi K, Eguchi K, Sultana H, Ohsaki Y, Komai M, Shirakawa H. S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells. Molecules. 2021; 26(6):1697. https://doi.org/10.3390/molecules26061697
Chicago/Turabian StyleRana, Md Masud, Kota Shiozawa, Katsuyuki Mukai, Katsuhiko Takayanagi, Koichi Eguchi, Halima Sultana, Yusuke Ohsaki, Michio Komai, and Hitoshi Shirakawa. 2021. "S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells" Molecules 26, no. 6: 1697. https://doi.org/10.3390/molecules26061697
APA StyleRana, M. M., Shiozawa, K., Mukai, K., Takayanagi, K., Eguchi, K., Sultana, H., Ohsaki, Y., Komai, M., & Shirakawa, H. (2021). S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10 Cells. Molecules, 26(6), 1697. https://doi.org/10.3390/molecules26061697








