Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection
Abstract
:1. Introduction
2. Rare Sugars in Food Systems and Medicine
3. Role of Rare Sugars in Plants
3.1. Rare Sugars as Herbicides and Plant Growth Regulators
3.2. Rare Sugars as Plant Resistance Inducers
4. Rare Sugars as Sustainable Control Agents against Crop Pests and Diseases
4.1. Rare Sugars as Sustainable Fungicides
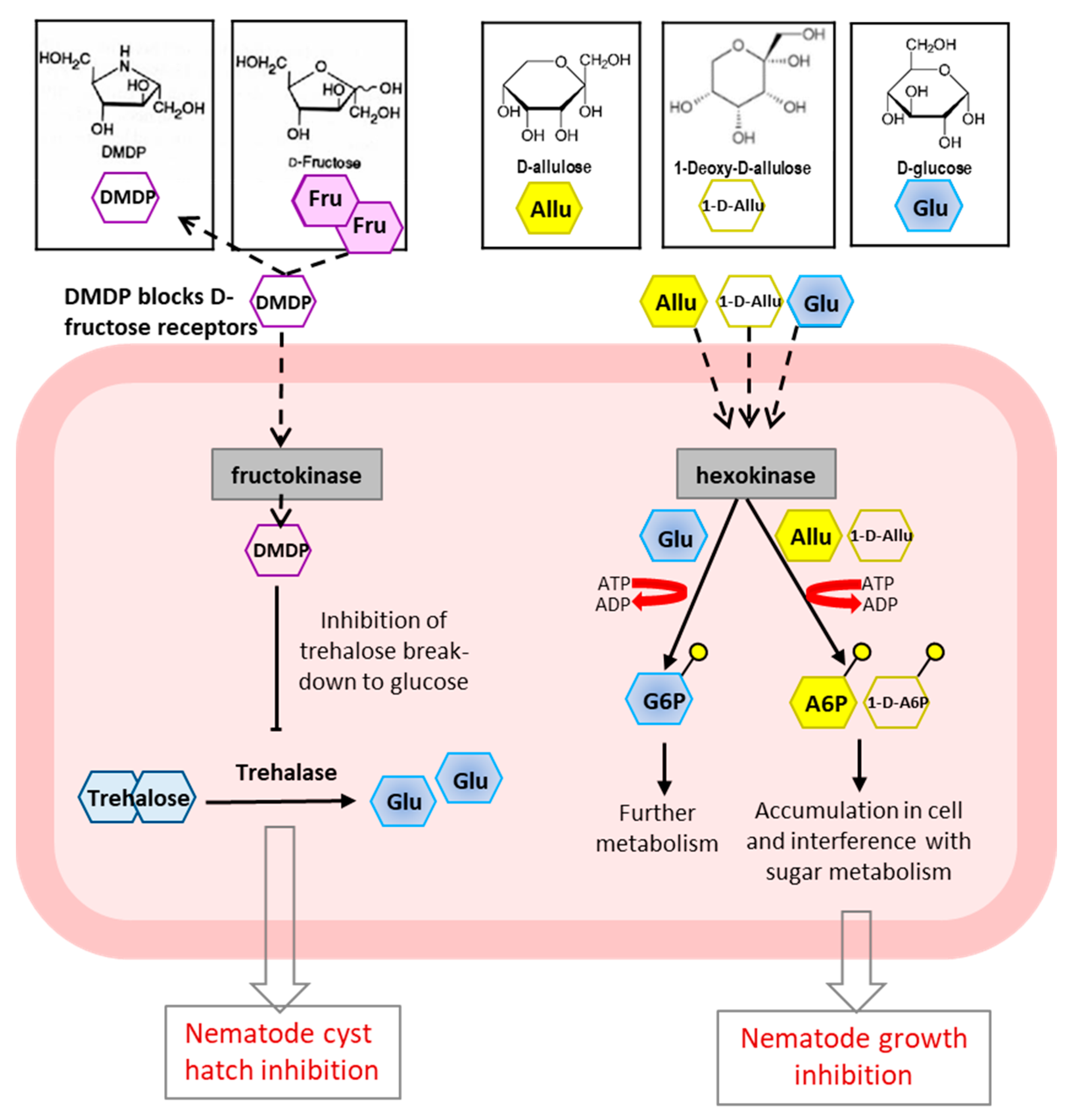
4.2. Rare Sugars as Sustainable Nematicides
4.3. Rare Sugars as Sustainable Insecticides
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakoguchi, H.; Yoshihara, A.; Shintani, T.; Okuma, K.; Izumori, K.; Sato, M. Growth inhibitory effect of d-arabinose against the nematode Caenorhabditis elegans: Discovery of a novel bioactive monosaccharide. Bioorg. Med. Chem. Lett. 2016, 26, 726–729. [Google Scholar] [CrossRef]
- Sakoguchi, H.; Yoshihara, A.; Izumori, K.; Sato, M. Screening of biologically active monosaccharides: Growth inhibitory effects of d-allose, d-taloase, and l-idose against the nematode Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2016, 80, 1058–1061. [Google Scholar] [CrossRef] [Green Version]
- Izumori, K. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 2002, 89, 120–124. [Google Scholar] [CrossRef]
- Izumori, K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol. 2006, 124, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Granström, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef]
- Beerens, K.; Desmet, T.; Soetaert, W. Enzymes for the biocatalytic production of rare sugars. J. Ind. Microbiol. Biotechnol. 2012, 39, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Production of natural and rare pentoses using microorganisms and their enzymes. Electron. J. Biotechnol. 2001, 4, 103–111. [Google Scholar] [CrossRef]
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of D-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef]
- Van Laar, A.D.E.; Grootaert, C.; Van Camp, J. Rare mono- and disaccharides as healthy alternative for traditional sugars and sweeteners? Crit. Rev. Food Sci. Nutr. 2020, 61, 713–741. [Google Scholar] [CrossRef]
- Hirata, Y.; Saito, M.; Tsukamoto, I.; Yamaguchi, F.; Sui, L.; Kamitori, K.; Dong, Y.; Uehara, E.; Konishi, R.; Janjua, N.; et al. Analysis of the inhibitory mechanism of d-allose on MOLT-4F leukemia cell proliferation. J. Biosci. Bioeng. 2009, 107, 562–568. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Nakanishi, H.; Gao, X.; Cai, L. Biosynthesis of rare hexoses using microorganisms and related enzymes. Beilstein. J. Org. Chem. 2013, 9, 2434–2445. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.N. Trehalose—The insect ‘blood’ sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar]
- Wingler, A. The function of trehalose biosynthesis in plants. Phytochemistry 2002, 60, 437–440. [Google Scholar] [CrossRef]
- Grennan, A.K. The role of trehalose biosynthesis in plants. Plant Physiol. 2007, 144, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Best, D. Rare Monosaccharides and Biologically Active Iminosugars from Carbohydrate Chirons. Ph.D. Thesis, Oxford University, Oxford, UK, 2011. [Google Scholar]
- Itoh, H.; Okaya, H.; Khan, A.R.; Tajima, S.; Hayakawa, S.; Izumori, K. Purification and characterization of D -tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci. Biotechnol. Biochem. 1994, 58, 2168–2171. [Google Scholar] [CrossRef]
- Ishida, Y.; Kamiya, T.; Izumori, K. Production of D-tagatose 3-epimerase of Pseudomonas cichorii ST-24 using recombinant Escherichia coli. J. Ferment. Bioeng. 1997, 84, 348–350. [Google Scholar] [CrossRef]
- Ishida, Y.; Kamiya, T.; Itoh, H.; Kimura, Y.; Izumori, K. Cloning and characterization of the D-tagatose 3-epimerase gene from Pseudomonas cichorii ST-24. J. Ferment. Bioeng. 1997, 83, 529–534. [Google Scholar] [CrossRef]
- Sasahara, H.; Mine, M.; Izumori, K. Production of D-talitol from D-psicose by Candida famata R28. J. Ferment. Bioeng. 1998, 85, 84–88. [Google Scholar] [CrossRef]
- Rao, D.; Best, D.; Yoshihara, A.; Gullapalli, P.; Morimoto, K.; Wormald, M.R.; Wilson, F.X.; Izumori, K.; Fleet, G.W.J. A concise approach to the synthesis of all twelve 5-deoxyhexoses: D-tagatose-3-epimerase—A reagent that is both specific and general. Tetrahedron Lett. 2009, 50, 3559–3563. [Google Scholar] [CrossRef]
- Shimonishi, T.; Izumori, K. A new enzyme, L-ribose isomerase from Acinetobacter sp. strain DL-28. J. Ferment. Bioeng. 1996, 81, 493–497. [Google Scholar] [CrossRef]
- Muniruzzaman, S.; Tokunaga, H.; Izumori, K. Conversion of d-psicose to allitol by Enterobacter agglomerans strain 221e. J. Ferment. Bioeng. 1995, 79, 323–327. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, W.; Jiang, B.; Zhang, T. Characterization of d-tagatose-3-epimerase from Rhodobacter sphaeroides that converts d-fructose into d-psicose. Biotechnol. Lett. 2009, 31, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chu, F.; Xing, Q.; Yu, S.; Zhou, L.; Jiang, B. Cloning, expression, and characterization of a D-psicose 3-epimerase from Clostridium cellulolyticum H10. J. Agric. Food Chem. 2011, 59, 7785–7792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, D.; Xing, Q.; Zhou, L.; Jiang, B.; Mu, W. Characterization of a novel metal-dependent D-psicose 3-epimerase from Clostridium scindens 35704. PLoS ONE 2013, 8, e62987. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, E.K.; Kim, Y.S.; Lee, Y.J.; Oh, D.K. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl. Environ. Microbiol. 2006, 72, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Menavuvu, B.T.; Poonperm, W.; Leang, K.; Noguchi, N.; Okada, H.; Morimoto, K.; Granström, T.B.; Takada, G.; Izumori, K. Efficient biosynthesis of D-allose from D-psicose by cross-linked recombinant L-rhamnose isomerase: Separation of product by ethanol crystallization. J. Biosci. Bioeng. 2006, 101, 340–345. [Google Scholar] [CrossRef]
- Park, H.; Park, C.; Kim, H.; Oh, D. Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces d-allose from d-psicose. J. Biotechnol. 2007, 132, 88–95. [Google Scholar] [CrossRef]
- Poonperm, W.; Takata, G.; Okada, H. Cloning, sequencing, overexpression and characterization of L -rhamnose isomerase from Bacillus pallidus Y25 for rare sugar production. Appl. Microbiol. Biotechnol. 2007, 1297–1307. [Google Scholar] [CrossRef]
- Yoon, R.; Yeom, S.; Kim, H.; Oh, D. Novel substrates of a ribose-5-phosphate isomerase from Clostridium thermocellum. J. Biotechnol. 2009, 139, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ryu, S.; Kim, P.; Oh, D. A feasible enzymatic process for D-tagatose production by an immobilized thermostable L-arabinose isomerase in a packed-bed bioreactor. Biotechnol. Prog. 2003, 19, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, Y.; Lee, H.; Lee, D.; Choe, E.; Pyun, Y.R. Cloning, expression and characterization of L-arabinose isomerase from Thermotoga neapolitana: Bioconversion of D-galactose to D-tagatose using the enzyme. FEMS Microbiol. Lett. 2002, 212, 121–126. [Google Scholar] [CrossRef]
- Liang, M.; Chen, M.; Liu, X.; Zhai, Y.; Liu, X.; Zhang, H.; Xiao, M.; Wang, P. Bioconversion of D-galactose to D-tagatose: Continuous packed bed reaction with an immobilized thermostable L-arabinose isomerase and efficient purification by selective microbial degradation. Appl. Microbiol. Biotechnol. 2012, 1469–1474. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, W.; Feng, Y.; Jiang, B.; Zhou, L. Recent advances on applications and biotechnological production of D-psicose. Appl. Microbiol. Biotechnol. 2012, 94, 1461–1467. [Google Scholar] [CrossRef]
- Miller, B.S.; Swain, T. Chromatographic analyses of the free amino-acids, organic acids and sugars in wheat plant extracts. J. Sci. Food Agric. 1960, 11, 344–348. [Google Scholar] [CrossRef]
- Binkley, W.W. The fate of cane juice simple sugars during molasses formation IV. Probable conversion of D-fructose to D-psicose. Int. Sugar J. 1963, 65, 105–106. [Google Scholar]
- Luger, A.; Steinhart, H. Carbohydrates in steam treated coffee. In Proceedings of the 16th International Scientific Colloquium on Coffee, Kyoto, Japan, 9–14 April 1995. [Google Scholar]
- Oshima, H.; Kimura, I.; Izumori, K. Psicose contents in various food products and its origin. Food Sci. Technol. Res. 2006, 12, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Recent advances in D-allulose: Physiological functionalities, applications, and biological production. Trends Food Sci. Technol. 2016, 54, 127–137. [Google Scholar] [CrossRef]
- Matsuo, T.; Suzuki, H.; Hashiguchi, M.; Izumori, K. D-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. 2002, 48, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Hayakawa, S.; Izumori, K. Modification of ovalbumin with a rare ketohexose through the Maillard reaction: Effect on protein structure and gel properties. J. Agric. Food Chem. 2004, 52, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Evaluation of the site specific protein glycation and antioxidant capacity of rare sugar-protein/peptide conjugates. J. Agric. Food Chem. 2005, 53, 10205–10212. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hayakawa, S.; Puangmanee, S.; Izumori, K. Chemical properties and antioxidative activity of glycated α-lactalbumin with a rare sugar, D-allose, by Maillard reaction. Food Chem. 2006, 95, 509–517. [Google Scholar] [CrossRef]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Antioxidant properties of custard pudding dessert containing rare hexose, d-psicose. Food Control 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Fukada, K.; Ishii, T.; Tanaka, K.; Yamaji, M.; Yamaoka, Y.; Kobashi, K.I.; Izumori, K. Crystal structure, solubility, and mutarotation of the rare monosaccharide D-psicose. Bull. Chem Soc. Jpn. 2010, 83, 1193–1197. [Google Scholar] [CrossRef]
- Matsuo, T.; Izumori, K. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 2006, 70, 2081–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Izumori, K. D-psicose inhibits intestinal α-glucosidase and suppresses the glycemic response after ingestion of carbohydrates in rats. J. Clin. Biochem. Nutr. 2009, 45, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, Y.; Mori, S.; Umemura, N.; Futamura, Y.; Inoue, H.; Hata, T.; Miwa, I.; Murao, K.; Nishiyama, A.; Tokuda, M. Suppression of blood glucose levels by D-psicose in glucose tolerance test in diabetic rats. Jpn. Pharmacol. Ther. 2010, 38, 261–269. [Google Scholar]
- Hossain, M.A.; Kitagaki, S.; Nakano, D.; Nishiyama, A.; Funamoto, Y.; Matsunaga, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Dong, Y.; et al. Rare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 2011, 405, 7–12. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Hirose, K.; Matsunaga, T.; Li, S.; Hirata, Y.; Noguchi, C.; Katagi, A.; Kamitori, K.; Dong, Y.; et al. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model otsuka long-evans tokushima fatty rats. Drug Des. Dev. Ther. 2015, 9, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.M.; Hyun Lee, J.; Youl Kim, D.; Hwang, S.H.; Hong, Y.H.; Kim, S.B.; Jin Lee, S.; Hye Park, C. Dietary d-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J. Food Sci. 2012, 77, 53–58. [Google Scholar] [CrossRef]
- Nagata, Y.; Kanasaki, A.; Tamaru, S.; Tanaka, K. D-psicose, an epimer of D-fructose, favorably alters lipid metabolism in sprague-dawley rats. J. Agric. Food Chem. 2015, 63, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yoon, J.; Choi, M.S. Tracing the anti-inflammatory mechanism/triggers of d-allulose: A profile study of microbiome composition and mRNA expression in diet-induced obese mice. Mol. Nutr. Food Res. 2020, 64, 1900982. [Google Scholar] [CrossRef]
- Han, Y.; Park, H.; Choi, B.R.; Ji, Y.; Kwon, E.Y.; Choi, M.S. Alteration of microbiome profile by d-allulose in amelioration of high-fat-diet-induced obesity in mice. Nutrients 2020, 12, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagi, K.; Matsuo, T. The study on long-term toxicity of D-psicose in rats. J. Clin. Biochem. Nutr. 2009, 45, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, T.; Yamada, T.; Hayashi, N.; Okuma, K.; Izumori, K.; Ishii, R.; Matsuo, T. Reduction of abdominal fat accumulation in rats by 8-week ingestion of a newly developed sweetener made from high fructose corn syrup. Food Chem. 2013, 138, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Onishi, K.; Yamada, T.; Iida, T.; Matsuo, T. D-psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int. J. Food Sci. Nutr. 2014, 65, 245–250. [Google Scholar] [CrossRef]
- Moller, D.E.; Berger, J.P. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int. J. Obes. 2003, 27, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Murao, K.; Yu, X.; Cao, W.M.; Imachi, H.; Chen, K.; Muraoka, T.; Kitanaka, N.; Li, J.; Ahmed, R.A.M.; Matsumoto, K.; et al. d-Psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECs. Life Sci. 2007, 81, 592–599. [Google Scholar] [CrossRef]
- Takata, M.K.; Yamaguchi, F.; Nakanose, K.; Watanabe, Y.; Hatano, N.; Tsukamoto, I.; Nagata, M.; Izumori, K.; Tokuda, M. Neuroprotective effect of D-psicose on 6-hydroxydopamine-induced apoptosis in rat pheochromocytoma (PC12) cells. J. Biosci. Bioeng. 2005, 100, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Hasegawa, Y.; Zhang, S.; Yoshihashi, Y.; Yonemochi, E.; Terada, K. Low-density microparticles with petaloid surface structure for pulmonary drug delivery. J. Pharm. Sci. 2014, 103, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Kondo, E.; Hayashi, H.; Suezawa, C.; Suguri, S.; Arai, M. D-Allose and D-psicose reinforce the action of metronidazole on trichomonad. Parasitol. Res. 2012, 110, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kurose, H.; Yamasaki, T.; Izumori, K. Potential anthelmintic: D-psicose inhibits motility, growth and reproductive maturity of L1 larvae of Caenorhabditis elegans. J. Nat. Med. 2008, 62, 244–246. [Google Scholar] [CrossRef]
- Iida, T.; Kishimoto, Y.; Yoshikawa, Y.; Hayashi, N.; Okuma, K.; Tohi, M.; Yagi, K.; Matsuo, T.; Izumori, K. Acute D-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J. Nutr. Sci. Vitaminol. 2008, 54, 511–514. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, N.; Iida, T.; Yamada, T.; Okuma, K.; Takehara, I.; Yamamoto, T.; Yamada, K.; Tokuda, M. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 2010, 74, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perold, G.W.; Beylis, P.; Howard, A.S. Metabolites of proteaceae. 8. The occurrence of (+)-D-allose in nature: Rubropilosin and pilorubrosin from Protea rubropilosa Beard. J. Chem. Soc. Perkin Trans. 1 1973, 6, 643–649. [Google Scholar] [CrossRef]
- O’Neil, M.J.; Heckelman, P.; Koch, C.; Roman, K. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck & Co. Inc.: Whitehouse Station, NJ, USA, 2001; Volume 767, p. 4342. [Google Scholar]
- Chari, V.M.; Grayer-Barkmeijer, R.J.; Harborne, J.B.; Österdahl, B.G. An acylated allose-containing 8-hydroxyflavone glycoside from Veronica filiformis. Phytochemistry 1981, 20, 1977–1979. [Google Scholar] [CrossRef]
- Jensen, S.R.; Mikkelsen, C.B.; Nielsen, B.J. Iridoid mono- and di-glycosides in Mentzelia. Phytochemistry 1981, 20, 71–83. [Google Scholar] [CrossRef]
- Weckwerth, W.; Loureiro, M.E.; Wenzel, K.; Fiehn, O. Differential metabolic networks unravel the effects of silent plant phenotypes. Proc. Natl. Acad. Sci. USA 2004, 101, 7809–7814. [Google Scholar] [CrossRef] [Green Version]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Anantharaman, P. Chemical composition and antibacterial activity of Indian seagrasses against urinary tract pathogens. Food Chem. 2012, 135, 2470–2473. [Google Scholar] [CrossRef]
- Sithara, R.; Selvakumar, P.; Arun, C.; Anandan, S.; Sivashanmugam, P. Economical synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in the detection of Mn(II) ions. J. Adv. Res. 2017, 8, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H.; Shintani, T.; Sato, M. D-Allose, a trace component in human serum, and its pharmaceutical applicability. Int. J. Appl. Biol. Pharm. 2020, 11, 200–213. [Google Scholar]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. eSPen 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent research on the physiological functions, applications, and biotechnological production of d-allose. Appl. Microbiol. Biotechnol. 2018, 102, 4269–4278. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Dong, Y.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Izumori, K.; Tokuda, M. Growth inhibitory effect of D-allose on human ovarian carcinoma cells in vitro. Anticancer Res. 2005, 25, 2639–2644. [Google Scholar]
- Sui, L.; Dong, Y.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Tsukamoto, I.; Izumori, K.; Tokuda, M. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int. J. Oncol. 2005, 27, 907–912. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Kamitori, K.; Sanada, K.; Horii, M.; Dong, Y.; Sui, L.; Tokuda, M. Rare sugar d-allose enhances anti-tumor effect of 5-fluorouracil on the human hepatocellular carcinoma cell line HuH-7. J. Biosci. Bioeng. 2008, 106, 248–252. [Google Scholar] [CrossRef]
- Yokohira, M.; Hosokawa, K.; Yamakawa, K.; Saoo, K.; Matsuda, Y.; Zeng, Y.; Kuno, T.; Imaida, K. Potential inhibitory effects of d-allose, a rare sugar, on liver preneoplastic lesion development in F344 rat medium-term bioassay. J. Biosci. Bioeng. 2008, 105, 545–553. [Google Scholar] [CrossRef]
- Naha, N.; Lee, H.Y.; Jo, M.J.; Chung, B.C.; Kim, S.H.; Kim, M.O. Rare sugar D-allose induces programmed cell death in hormone refractory prostate cancer cells. Apoptosis 2008, 13, 1121–1134. [Google Scholar] [CrossRef]
- Jeong, R.U.; Lim, S.; Kim, M.O.; Moon, M.H. Effect of d-allose on prostate cancer cell lines: Phospholipid profiling by nanoflow liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Indo, K.; Hoshikawa, H.; Kamitori, K.; Yamaguchi, F.; Mori, T.; Tokuda, M.; Mori, N. Effects of d-allose in combination with docetaxel in human head and neck cancer cells. Int. J. Oncol. 2014, 45, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malm, S.W.; Hanke, N.T.; Gill, A.; Carbajal, L.; Baker, A.F. The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. J. Exp. Clin. Cancer Res. 2015, 34, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaji, N.; Kamitori, K.; Hossain, A.; Noguchi, C.; Katagi, A.; Kadowaki, N.; Tokuda, M. Additive antitumour effect of D-allose in combination with cisplatin in non-small cell lung cancer cells. Oncol. Rep. 2018, 39, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Hoshikawa, H.; Indo, K.; Mori, T.; Mori, N. Enhancement of the radiation effects by d-allose in head and neck cancer cells. Cancer Lett. 2011, 306, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Ueki, M.; Ueno, M.; Asaga, T.; Tokuda, M.; Shirakami, G. D-allose ameliorates cisplatin-induced nephrotoxicity in mice. Tohoku J. Exp. Med. 2012, 228, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A.; Wakabayashi, H.; Goda, F.; Kobayashi, S.; Maeba, T.; Maeta, H. Effect of the immunosuppressants FK506 and D-allose on allogenic orthotopic liver transplantation in rats. Transplant. Proc. 2000, 32, 2021–2023. [Google Scholar] [CrossRef]
- Murata, A.; Sekiya, K.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Izumori, K.; Tokuda, M. A novel inhibitory effect of D-allose on production of reactive oxygen species from neutrophils. J. Biosci. Bioeng. 2003, 96, 89–91. [Google Scholar] [CrossRef]
- Hirooka, K.; Miyamoto, O.; Jinming, P.; Du, Y.; Itano, T.; Baba, T.; Tokuda, M.; Shiraga, F. Neuroprotective effects of D-allose against retinal ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1653–1657. [Google Scholar] [CrossRef] [Green Version]
- Akram Hossain, M.; Izuishi, K.; Maeta, H. Protective effects of D-allose against ischemia reperfusion injury of the rat liver. J. Hepatobiliary Pancreat. Surg. 2003, 10, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Wakabayashi, H.; Izuishi, K.; Okano, K.; Yachida, S.; Maeta, H. The role of prostaglandins in liver ischemia-reperfusion injury. Curr. Pharm. Des. 2006, 12, 2935–2951. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kawate, H.; Nomura, M.; Takayanagi, R. Eldecalcitol for the treatment of osteoporosis. Clin. Interv. Aging 2013, 8, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Kawai, N.; Nakamura, T.; Lu, F.; Fei, Z.; Tamiya, T. Anti-inflammatory effect of D-allose in cerebral ischemia/reperfusion injury in rats. Neurol. Med. Chir. 2013, 53, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iga, Y.; Nakamichi, K.; Shirai, Y.; Matsuo, T. Acute and sub-chronic toxicity of D-allose in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1476–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iga, Y.; Matsuo, T. D-allose metabolism in rats. J. Jpn. Soc. Nutr. Food Sci. 2010, 63, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Livesey, G.; Brown, J.C. D-tagatose is a bulk sweetener with zero energy determined in rats. J. Nutr. 1996, 126, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Bär, A.; Lina, B.A.R.; De Groot, D.M.G.; De Bie, B.; Appel, M.J. Effect of D-tagatose on liver weight and glycogen content of rats. Regul. Toxicol. Pharmacol. 1999, 29, S11–S28. [Google Scholar] [CrossRef] [PubMed]
- Vastenavond, C.; Bertelsen, H.; Hansen, S.; Laursen, R.; Saunders, J.; Eriknauer, K. Tagatose (D-Tagatose). In Alternative Sweeteners, 4th ed.; O’brien-Nabors, L., Ed.; CRC Press: New York, NY, USA, 2011; pp. 197–222. [Google Scholar] [CrossRef]
- Pigman, W.; Horton, D. The Carbohydrates: Chemistry and Biochemistry; Academic Press: New York, NY, USA; London, UK, 1972. [Google Scholar]
- Khuwijitjaru, P.; Milasing, N.; Adachi, S. Production of D-tagatose: A review with emphasis on subcritical fluid treatment. Sci. Eng. Health Stud. 2018, 12, 159–167. [Google Scholar] [CrossRef]
- Zehner, L.R. D-tagatose as a Low-Calorie Carbohydrate Sweetener and Bulking Agent. U.S. Patent 4,786,722, 22 November 1988. [Google Scholar]
- Torrico, D.D.; Tam, J.; Fuentes, S.; Viejo, C.G.; Dunshea, F.R. D-tagatose as a sucrose substitute and its effect on the physico-chemical properties and acceptability of strawberry-flavored yogurt. Foods 2019, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, G.W. Reduced-calorie sweeteners and caloric alternatives. In Optimising Sweet Taste in Foods; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. 252–280. [Google Scholar]
- Mitchell, H. Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef]
- Mogha, K.V.; Chaudhari, A.R.; Aparnathi, K.D. Tagatose: A low calorie multifunctional sweetener. Res. Rev. J. Dairy Sci. Technol. 2016, 5, 29–35. [Google Scholar]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering pathways for rare sugars biosynthesis, physiological functionalities, and applications—A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.L.; Whittaker, M.H.; Frankos, V.H.; Trimmer, G.W. 90-Day oral toxicity study of D-tagatose in rats. Regul. Toxicol. Pharmacol. 1999, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.W.; Magder, L.S.; Zarbalian, K. Dietary supplementation with d-tagatose in subjects with type 2 diabetes leads to weight loss and raises high-density lipoprotein cholesterol. Nutr. Res. 2010, 30, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V.; Lu, Y. D-Tagatose as an Anti-Biofilm Agent. U.S. Patent 7,189,351, 13 March 2007. [Google Scholar]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. D-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef]
- Zehner, L.R.; Levin, G.V.; Saunders, J.P.; Beadle, J.R. D-Tagatose as Anti-Hyperglycemic Agent. U.S. Patent 5,447,917, 5 September 1995. [Google Scholar]
- Priya, K.; Gupta, V.R.M.; Srikanth, K. Natural sweeteners: A complete review. J. Pharm. Res. 2011, 4, 2034–2039. [Google Scholar]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Sheet, B.S.; Artık, N.; Ayed, M.A.; Abdulaziz, O.F. Some alternative sweeteners (xylitol, sorbitol, sucralose and stevia): Review. Karaelmas Sci. Eng. J. 2014, 4, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Nabors, L.O.B.; Gelardi, R.C. Alternative Sweeteners; Food Science and Technology; Marcel Dekker: New York, NY, USA, 1991; 461p. [Google Scholar]
- Kano, A.; Gomi, K.; Yamasaki-Kokudo, Y.; Satoh, M.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; Ishida, Y.; et al. A rare sugar, D-allose, confers resistance to rice bacterial blight with upregulation of defense-related genes in Oryza sativa. Phytopathology 2010, 100, 85–90. [Google Scholar] [CrossRef]
- Kano, A.; Hosotani, K.; Gomi, K.; Yamasaki-Kokudo, Y.; Shirakawa, C.; Fukumoto, T.; Ohtani, K.; Tajima, S.; Izumori, K.; Tanaka, K.; et al. D-Psicose induces upregulation of defense-related genes and resistance in rice against bacterial blight. J. Plant Physiol. 2011, 168, 1852–1857. [Google Scholar] [CrossRef]
- Kano, A.; Fukumoto, T.; Ohtani, K.; Yoshihara, A.; Ohara, T.; Tajima, S.; Izumori, K.; Tanaka, K.; Ohkouchi, T.; Ishida, Y.; et al. The rare sugar d-allose acts as a triggering molecule of rice defence via ROS generation. J. Exp. Bot. 2013, 64, 4939–4951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, T.; Kano, A.; Ohtani, K.; Inoue, M.; Yoshihara, A.; Izumori, K.; Tajima, S.; Shigematsu, Y.; Tanaka, K.; Ohkouchi, T.; et al. Phosphorylation of d-allose by hexokinase involved in regulation of OsABF1 expression for growth inhibition in Oryza sativa L. Planta 2013, 237, 1379–1391. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Song, F. D-allose is a critical regulator of inducible plant immunity in tomato. Physiol. Mol. Plant Pathol. 2020, 111, 101507. [Google Scholar] [CrossRef]
- Perazzolli, M.; Nesler, A.; Giovannini, O.; Antonielli, L.; Puopolo, G.; Pertot, I. Ecological impact of a rare sugar on grapevine phyllosphere microbial communities. Microbiol. Res. 2020, 232, 126387. [Google Scholar] [CrossRef]
- Chahed, A.; Nesler, A.; Navazio, L.; Baldan, B.; Busato, I.; Ait Barka, E.; Pertot, I.; Puopolo, G.; Perazzolli, M. The rare sugar tagatose differentially inhibits the growth of Phytophthora infestans and Phytophthora cinnamomi by interfering with mitochondrial processes. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Tanaka, K.; Ebihara, K.; Tajima, S.; Gomi, K.; et al. The rare sugar d-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brilisauer, K.; Rapp, J.; Rath, P.; Schöllhorn, A.; Bleul, L.; Weiß, E.; Stahl, M.; Grond, S.; Forchhammer, K. Cyanobacterial antimetabolite 7-deoxy-sedoheptulose blocks the shikimate pathway to inhibit the growth of prototrophic organisms. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Matheson, N.K.; Myers, D.K. Inhibition of germination by glucose analogues that are hexokinase substrates. Phytochemistry 1998, 48, 241–248. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Takaoka, T.; Izumori, K. Psicose inhibits lettuce root growth via a hexokinase-independent pathway. Physiol. Plant. 2005, 125, 293–298. [Google Scholar] [CrossRef]
- Chowdhury, M.T.I.; Naito, M.; Yanagita, R.C.; Kawanami, Y. Synthesis of 6-O-decanoyl-d-altrose and 6-O-decanoyl-d-gulose and evaluation of their biological activity on plant growth. Plant Growth Regul. 2015, 75, 707–713. [Google Scholar] [CrossRef]
- Suzuki, T. Effects of D-allose and D-psicose on shoot and root growth of thirteen plant species. In Proceedings of the Second Symposium of International Society of Rare Sugars, Takamatsu, Kagawa, Japan, 3 April 2008; pp. 209–213. [Google Scholar]
- Akimitsu, K.; Tsukuda, S.; Suzuku, N.; Ichii, M.; Tajima, S. An elicitor effect of D-psicose for induction of plant defense gene transcriptions. In Proceedings of the International Society of Rare Sugars: Creating a Novel Bio-World with Rare Sugars, Kagawa, Japan, 3 April 2008; pp. 163–168. [Google Scholar]
- Narusaka, Y.; Narusaka, M.; Abe, H.; Hosaka, N.; Kobayashi, M.; Shiraishi, T.; Iwabuchi, M. High-throughput screening for plant defense activators using a β-glucuronidase-reporter gene assay in Arabidopsis thaliana. Plant Biotechnol. 2009, 26, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Akimitsu, K.; Matsudaira, K.; Aki, A.; Mochizuki, S.; Kano, A.; Yoshihara, A.; Gomi, K.; Ichimura, K.; Fukumoto, T.; Ohara, T. A possibility of rare sugar applications for agro-usages. Jpn. J. Pestic. Sci. 2017, 42, 99–103. [Google Scholar] [CrossRef]
- Xue, J.; Wang, S.; You, X.; Dong, J.; Han, L.; Liu, F. Multi-residue determination of plant growth regulators in apples and tomatoes by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jiang, Z.; Shen, W.; Zou, S. Toxicological characteristics of plant growth regulators and their impact on male reproductive health. Natl. J. Androl. 2018, 24, 370–375. [Google Scholar]
- Al-Samarai, G.F.; Mahdi, W.M.; Al-Hilali, B.M. Reducing environmental pollution by chemical herbicides using natural plant derivatives–allelopathy effect. Ann. Agric. Environ. Med. 2018, 25, 449–452. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Bencko, V.; Foong, F.Y.L. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann. Agric. Environ. Med. 2017, 24, 312–316. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, A.N. Natural Bioactive Products in Sustainable Agriculture; Springer Nature Singapore Pte Tld: Singapore, 2020; 307p. [Google Scholar]
- Pego, J.V.; Weisbeek, P.J.; Smeekens, S.C.M. Mannose inhibits Arabidopsis germination via a hexokinase-mediated step. Plant Physiol. 1999, 119, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Baskin, T.I.; Remillong, E.L.; Wilson, J.E. The impact of mannose and other carbon sources on the elongation and diameter of the primary root of Arabidopsis thaliana. Aust. J. Plant Physiol. 2001, 28, 481–488. [Google Scholar] [CrossRef]
- Fukumoto, T.; Kano, A.; Ohtani, K.; Yamasaki-Kokudo, Y.; Kim, B.G.; Hosotani, K.; Saito, M.; Shirakawa, C.; Tajima, S.; Izumori, K.; et al. Rare sugar d-allose suppresses gibberellin signaling through hexokinase-dependent pathway in Oryza sativa L. Planta 2011, 234, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Fitzgerald, H.A.; Canlas, P.E.; Navarre, D.A.; Ronald, P.C. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant-Microbe Interact. 2005, 18, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Q.; Bai, X.Q.; Qian, Q.; Wang, X.J.; Chen, M.S.; Chu, C.C. OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res. 2005, 15, 593–603. [Google Scholar] [CrossRef]
- Takahashi, A.; Agrawal, G.K.; Yamazaki, M.; Onosato, K.; Miyao, A.; Kawasaki, T.; Shimamoto, K.; Hirochika, H. Rice Pti1a negatively regulates RAR1-dependent defense responses. Plant Cell 2007, 19, 2940–2951. [Google Scholar] [CrossRef] [Green Version]
- Shimono, M.; Sugano, S.; Nakayama, A.; Jiang, C.J.; Ono, K.; Toki, S.; Takatsuji, H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 2007, 19, 2064–2076. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, A.K.; Hofmann, M.G.; Römer, U.; Köckenberger, W.; Elling, L.; Roitsch, T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002, 128, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Afach, G.; Kawanami, Y.; Kato-Noguchi, H.; Izumori, K. Practical production of 6-O-octanoyl-D-allose and its biological activity on plant growth. Biosci. Biotechnol. Biochem. 2006, 70, 2010–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T. Biosurfactants. J. Jpn. Oil Chem. Soc. 1996, 45, 1013–1022. [Google Scholar] [CrossRef]
- Ogino, K. Research and development of novel surfactants. J. Jpn. Oil Chem. Soc. 1996, 45, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Ueda, M.; Furumoto, T.; Kawanami, Y. Retarding activity of 6-O-Acyl-d-alloses against plant growth. Biosci. Biotechnol. Biochem. 2010, 74, 216–217. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.T.I.; Ando, H.; Yanagita, R.C.; Kawanami, Y. Syntheses and biological activities of deoxy-D-allose fatty acid ester analogs. Biosci. Biotechnol. Biochem. 2016, 80, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, T.I.; Ando, H.; Yanagita, R.C.; Kawanami, Y. Synthesis and inhibitory activity of deoxy-d-allose amide derivative against plant growth. Biosci. Biotechnol. Biochem. 2018, 82, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Yamaashi, H.; Tazul Islam Chowdhury, M.; Yanagita, R.C.; Kawanami, Y. Inhibitory activity of 6-O-decyl-D-allose and 6-decanoylamino-6-deoxy-D-allose against plant growth. Tech. Bull. Fac. Agric. Kagawa Univ. 2017, 69, 17–21. [Google Scholar]
- Fry, S.C.; Aldington, S.; Hetherington, P.R.; Aitken, J. Oligosaccharides as signals and substrates in the plant cell wall. Plant Physiol. 1993, 103, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell 1996, 8, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Guarnizo, N.; Oliveros, D.; Murillo-Arango, W.; Bermúdez-Cardona, M.B. Oligosaccharides: Defense inducers, their recognition in plants, commercial uses and perspectives. Molecules 2020, 25, 5972. [Google Scholar] [CrossRef]
- Héloir, M.C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of elicitors in grapevine: From MAMP and DAMP perception to induced resistance. Front. Plant Sci. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Plant science review: Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Aziz, A.; Heyraud, A.; Lambert, B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 2004, 218, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Moscatiello, R.; Mariani, P.; Sanders, D.; Maathuis, F.J.M. Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J. Exp. Bot. 2006, 57, 2847–2865. [Google Scholar] [CrossRef]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 2008, 1, 423–445. [Google Scholar] [CrossRef] [Green Version]
- Ait Barka, E.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [Green Version]
- Brulé, D.; Villano, C.; Davies, L.J.; Trdá, L.; Claverie, J.; Héloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1-1 and VvLYK1-2 mediate chitooligosaccharide-triggered immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Trotel-Aziz, P.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan activates defense reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413. [Google Scholar] [CrossRef]
- Trouvelot, S.; Varnier, A.L.; Allègre, M.; Mercier, L.; Baillieul, F.; Arnould, C.; Gianinazzi-Pearson, V.; Klarzynski, O.; Joubert, J.M.; Pugin, A.; et al. A β-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant-Microbe Interact. 2008, 21, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Gauthier, A.; Bézier, A.; Poinssot, B.; Joubert, J.M.; Pugin, A.; Heyraud, A.; Baillieul, F. Elicitor and resistance-inducing activities of β-1,4 cellodextrins in grapevine, comparison with β-1,3 glucans and α-1,4 oligogalacturonides. J. Exp. Bot. 2007, 58, 1463–1472. [Google Scholar] [CrossRef]
- Claverie, J.; Balacey, S.; Lemaître-Guillier, C.; Brulé, D.; Chiltz, A.; Granet, L.; Noirot, E.; Daire, X.; Darblade, B.; Héloir, M.C.; et al. The cell wall-derived xyloglucan is a new DAMP triggering plant immunity in Vitis vinifera and Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1725. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.G.; Meyer, A.; Holst, O.; Pühler, A.; Niehaus, K. Characterization of the Xanthomonas campestris pv. campestris lipopolysaccharide substructures essential for elicitation of an oxidative burst in tobacco cells. Mol. Plant-Microbe Interact. 2005, 18, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Alexandersson, E.; Mulugeta, T.; Lankinen, Å.; Liljeroth, E.; Andreasson, E. Plant resistance inducers against pathogens in Solanaceae species-from molecular mechanisms to field application. Int. J. Mol. Sci. 2016, 17, 1673. [Google Scholar] [CrossRef]
- Desaki, Y.; Kouzai, Y.; Ninomiya, Y.; Iwase, R.; Shimizu, Y.; Seko, K.; Molinaro, A.; Minami, E.; Shibuya, N.; Kaku, H.; et al. OsCERK1 plays a crucial role in the lipopolysaccharide-induced immune response of rice. New Phytol. 2018, 217, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.H.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 2012, 160, 1630–1641. [Google Scholar] [CrossRef] [Green Version]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef] [Green Version]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.; Campo, S.; Rufat, M.; Estopà, M.; Messeguer, J.; San Segundo, B.; Coca, M. Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Mol. Plant-Microbe Interact. 2007, 20, 832–842. [Google Scholar] [CrossRef] [Green Version]
- Mi, S.K.; Song, M.C.; Eun, Y.K.; Yang, J.I.; Hwangbo, H.; Young, C.K.; Ryu, C.M.; Kwang, Y.Y.; Gap, C.C.; Baik, H.C. Galactinol is a signaling component of the induced systemic resistance caused by Pseudomonas chlororaphis O6 root colonization. Mol. Plant-Microbe Interact. 2008, 21, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Dugé De Bernonville, T.; Marolleau, B.; Staub, J.; Gaucher, M.; Brisset, M.N. Using molecular tools to decipher the complex world of plant resistance inducers: An apple case study. J. Agric. Food Chem. 2014, 62, 11403–11411. [Google Scholar] [CrossRef] [PubMed]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [Green Version]
- Doke, N. NADPH-dependent O2- generation in membrane fractions isolated from wounded potato tubers inoculated with Phytophthora infestans. Physiol. Plant Pathol. 1985, 27, 311–322. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Sandroni, M.; Liljeroth, E.; Mulugeta, T.; Alexandersson, E. Plant resistance inducers (PRIs): Perspectives for future disease management in the field. CAB Rev. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Siemens, J.; González, M.C.; Wolf, S.; Hofmann, C.; Greiner, S.; Du, Y.; Rausch, T.; Roitsch, T.; Ludwig-Müller, J. Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Pathol. 2011, 12, 247–262. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Sun, A.; Li, Z. Regulatory role of nitric oxide in lipopolysaccharides-triggered plant innate immunity. Plant Signal. Behav. 2013, 8, 134–136. [Google Scholar] [CrossRef] [Green Version]
- Herbers, K.; Meuwly, P.; Métraux, J.P.; Sonnewald, U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996, 397, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Schaarschmidt, S.; Kopka, J.; Ludwig-Müller, J.; Hause, B. Regulation of arbuscular mycorrhization by apoplastic invertases: Enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J. 2007, 51, 390–405. [Google Scholar] [CrossRef]
- Essmann, J.; Schmitz-Thom, I.; Schön, H.; Sonnewald, S.; Weis, E.; Scharte, J. RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 2008, 147, 1288–1299. [Google Scholar] [CrossRef] [Green Version]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 2008, 148, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Verhaest, M.; Lammens, W.; Le Roy, K.; De Ranter, C.J.; Van Laere, A.; Rabijns, A.; Van Den Ende, W. Insights into the fine architecture of the active site of chicory fructan 1-exohydrolase: 1-Kestose as substrate vs sucrose as inhibitor. New Phytol. 2007, 174, 90–100. [Google Scholar] [CrossRef]
- Müller, J.; Aeschbacher, R.A.; Sprenger, N.; Boller, T.; Wiemken, A. Disaccharide-mediated regulation of sucrose:fructan-6-fructosyltransferase, a key enzyme of fructan synthesis in barley leaves. Plant Physiol. 2000, 123, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.H.; Liu, Y.X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wind, J.J.; Shi, X.; Zhang, H.; Hanson, J.; Smeekens, S.C.; Teng, S. Fructose sensitivity is suppressed in Arabidopsis by the transcription factor ANAC089 lacking the membrane-bound domain. Proc. Natl. Acad. Sci. USA 2011, 108, 3436–3441. [Google Scholar] [CrossRef] [Green Version]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef]
- Myers, D.K.; Matheson, N.K. Hexose-6-kinases in germinating honey locust cotyledons: Substrate specificity of d-fructo-6-kinase. Phytochemistry 1994, 37, 957–969. [Google Scholar] [CrossRef]
- Ohara, T.; Ishida, Y.; Kudou, R.; Kakibuchi, K.; Akimitsu, K.; Izumori, K.; Tajima, S. Plant Disease Control Agent Comprising D-Tagatose as Active Ingredient, and Plant Disease Control Method. U.S. Patent 9,125,409-B2, 18 August 2008. [Google Scholar]
- Ohara, T. Effect of D-tagatose on plant disease; downy mildew and powdery mildew. In Proceedings of the 5th Symposium of International Society of Rare Sugars, Takamatsu and Miki, Kagawa, Japan, 9–12 November 2011. [Google Scholar]
- Evans, S.V.; Gatehouse, A.M.; Fellows, L.E. Detrimental effects of 2,5-Dihydroxymethyl-3,4-dihydroxypyrrolidine in some tropical legume seeds on larvae of the bruchid Callosobruchus maculatus. Entomol. Exp. Appl. 1985, 37, 257–261. [Google Scholar] [CrossRef]
- Blaney, W.M.; Simmonds, M.S.J.; Evans, S.V.; Fellows, L.E. The role of the secondary plant compound 2, 5-dihydroxymethyl 3, 4-dihydroxypyrrolidine as a feeding inhibitor for insects. Entomol. Exp. Appl. 1984, 36, 209–216. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Blaney, W.M.; Fellows, L.E. Behavioral and electrophysiological study of antifeedant mechanisms associated with polyhydroxy alkaloids. J. Chem. Ecol. 1990, 16, 3167–3196. [Google Scholar] [CrossRef]
- Izumori, K.; Akimitsu, K.; Tajima, S.; Agarie, M.; Yanagi, T.; Mochioka, R. Utilization of Rare Sugars in Plant or Microorganism. U.S. Patent 8,017,828, 13 September 2011. [Google Scholar]
- Hayer, K.; Stratford, M.; Archer, D.B. Structural features of sugars that trigger or support conidial germination in the filamentous fungus Aspergillus Niger. Appl. Environ. Microbiol. 2013, 79, 6924–6931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komoń-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elgawad, M.M.M.; Askary, T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 2018, 28. [Google Scholar] [CrossRef]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Yoshihara, A.; Sakoguchi, H.; Shintani, T.; Fleet, G.W.J.; Izumori, K.; Sato, M. Growth inhibition by 1-deoxy-D-allulose, a novel bioactive deoxy sugar, screened using Caenorhabditis elegans assay. Bioorg. Med. Chem. Lett. 2019, 29, 2483–2486. [Google Scholar] [CrossRef] [PubMed]
- Diasio, R.B. Pyrimidine and purine antimetabolites. Holland-Frei Cancer MedHolland-Frei Cancer Med. 2017, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Huang, D.; McArthur, D.L.; Boros, L.G.; Nissen, N.; Heaney, A.P. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010, 70, 6368–6376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, C.; Kamitori, K.; Hossain, A.; Hoshikawa, H.; Katagi, A.; Dong, Y.; Sui, L.; Tokuda, M.; Yamaguchi, F. D-allose inhibits cancer cell growth by reducing GLUT1 expression. Tohoku J. Exp. Med. 2016, 238, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Fellows, L.E.; Phillips, M.S.; Alphey, T.J.W.; McGavin, W.J.; Geoghegan, I.E.; Simmonds, M.S.J.; Robertson, W.M.; Watson, A.A.; Birch, A.N.E.; Porter, E.A. DMDP-a plant-derived sugar analogue with systemic activity against plant parasitic nematodes. Nematologica 1993, 39, 521–535. [Google Scholar] [CrossRef]
- Fellows, L.E. Distribution and biological activity of alkaloidal glycosidase inhibitors from plants. Proc. Phytochem. Soc. Eur. 1993, 33, 271–282. [Google Scholar]
- Sharma, S.; Kooner, R.; Arora, R. Insect Pests and Crop Losses. Breeding Insect Resistant Crops for Sustainable Agriculture; Springer: Singapore, 2017; pp. 45–66. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [Green Version]
- Chowański, S.; Kudlewska, M.; Marciniak, P.; Rosiński, G. Synthetic insecticides—Is there an alternative? Pol. J. Environ. Stud. 2014, 23, 291–302. [Google Scholar]
- Derridj, S.; Elad, Y.; Birch, A.N.E. Sugar signaling as a new way for vegetable and fruit induced resistance against insects, pathogens and nematodes. IOBC-WPRS Bull. 2012, 83, 2012. [Google Scholar]
- Arnault, I.; Lombarkia, N.; Joy-Ondet, S.; Romet, L.; Brahim, I.; Meradi, R.; Nasri, A.; Auger, J.; Derridj, S. Foliar application of microdoses of sucrose to reduce codling moth Cydia pomonella L. (Lepidoptera: Tortricidae) damage to apple trees. Pest Manag. Sci. 2016, 72, 1901–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, G.V.; Zehner, L.R. Method for Killing Pests. U.S. Patent 5,166,193, 24 November 1992. [Google Scholar]
- Ohara, T.; Tanaka, K.; Ishizaki, S.; Kato, S.; Akimitsu, K.; Izumori, K. Composition for Enhancing Plant Disease Control Effect of Monosaccharides. U.S. Patent 10,638,752, 5 May 2020. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijailovic, N.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection. Molecules 2021, 26, 1720. https://doi.org/10.3390/molecules26061720
Mijailovic N, Nesler A, Perazzolli M, Aït Barka E, Aziz A. Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection. Molecules. 2021; 26(6):1720. https://doi.org/10.3390/molecules26061720
Chicago/Turabian StyleMijailovic, Nikola, Andrea Nesler, Michele Perazzolli, Essaid Aït Barka, and Aziz Aziz. 2021. "Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection" Molecules 26, no. 6: 1720. https://doi.org/10.3390/molecules26061720
APA StyleMijailovic, N., Nesler, A., Perazzolli, M., Aït Barka, E., & Aziz, A. (2021). Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection. Molecules, 26(6), 1720. https://doi.org/10.3390/molecules26061720








