Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease
Abstract
1. Introduction
1.1. Inflammatory Bowel Disease
1.2. The Inflammasomes
1.3. The Inflammasomes Are Implicated in the Pathogenesis of IBD
2. NLRP3 Inflammasome Activation Contributes to Gut Homeostasis
3. Inhibition of the NLRP3 Inflammasome Ameliorates Colitis
3.1. Genetic Factors
3.2. Natural Products
3.3. Stem Cells
3.4. Probiotics
3.5. Hypoxia
4. Regulation of Inflammasomes Other Than NLRP3 in Colitis
4.1. The NLRP6 Inflammasome
4.2. The NLRP1 Inflammasome
4.3. The Pyrin Inflammasome
4.4. The AIM2 Inflammasome
4.5. The NLRC4 Inflammasome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An update on inflammatory bowel disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Click, B.H. The burden of cost in inflammatory bowel disease: A medical economic perspective. Curr. Opin. Gastroenterol. 2020, 36, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L.; EpiCom, E. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Knutson, C.G.; Mangerich, A.; Zeng, Y.; Raczynski, A.R.; Liberman, R.G.; Kang, P.; Ye, W.; Prestwich, E.G.; Lu, K.; Wishnok, J.S.; et al. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2013, 110, E2332–E2341. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Ogata, H.; Hibi, T. Innate immunity in inflammatory bowel disease: State of the art. Curr. Opin. Gastroenterol. 2008, 24, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.D. Inflammatory bowel disease and the NLRP3 inflammasome. N. Engl. J. Med. 2017, 377, 694–696. [Google Scholar] [CrossRef]
- Liu, L.; Li, X. NLRP3 inflammasome in inflammatory bowel disease: Friend or foe? Dig. Dis. Sci. 2017, 62, 2211–2214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 2010, 10, 688–698. [Google Scholar] [CrossRef]
- Khare, S.; Luc, N.; Dorfleutner, A.; Stehlik, C. Inflammasomes and their activation. Crit. Rev. Immunol. 2010, 30, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Latz, E. The inflammasomes: Mechanisms of activation and function. Curr. Opin. Immunol. 2010, 22, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Munoz-Planillo, R.; Reimer, T.; Eigenbrod, T.; Nunez, G. Inflammasomes as microbial sensors. Eur. J. Immunol. 2010, 40, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.; Wu, H.; Kagan, J.C. Gasdermin D activity in inflammation and host defense. Sci. Immunol. 2019, 4, eaav1447. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Hollingsworth, L.R.T.; Wu, H. Mechanism and regulation of gasdermin-mediated cell death. Cold Spring Harb Perspect. Biol. 2020, 12, a036400. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Aspects Med. 2020, 76, 100924. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Smith, J.; Vance, R.E. The NLRP1 inflammasomes. Immunol. Rev. 2015, 265, 22–34. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef]
- Duncan, J.A.; Canna, S.W. The NLRC4 Inflammasome. Immunol. Rev. 2018, 281, 115–123. [Google Scholar] [CrossRef]
- Gong, Y.N.; Shao, F. Sensing bacterial infections by NAIP receptors in NLRC4 inflammasome activation. Protein Cell 2012, 3, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Lamkanfi, M.; Kanneganti, T.D. The Nlrp3 inflammasome: Contributions to intestinal homeostasis. Trends Immunol. 2011, 32, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tartey, S.; Kanneganti, T.D. Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology 2019, 156, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the role of NLRP3 inflammasome in diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Booshehri, L.M.; Hoffman, H.M. CAPS and NLRP3. J. Clin. Immunol. 2019, 39, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Strober, W. New insights into the nature of autoinflammatory diseases from mice with Nlrp3 mutations. Eur. J. Immunol. 2010, 40, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhang, F.; Fuss, I.; Kitani, A.; Strober, W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 2009, 30, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, M.; Simon, P.L.; Karmeli, F.; Rachmilewitz, D. Role of interleukin 1 in inflammatory bowel disease—Enhanced production during active disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Lehr, H.A.; Endres, S.; Schnurr, M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: Influence of genetic and environmental factors. Dig. Dis. 2012, 30 (Suppl. 1), 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Dupaul-Chicoine, J.; Yeretssian, G.; Doiron, K.; Bergstrom, K.S.; McIntire, C.R.; LeBlanc, P.M.; Meunier, C.; Turbide, C.; Gros, P.; Beauchemin, N.; et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010, 32, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, K.; Nakase, H. Contradictory effects of NLRP3 inflammasome regulatory mechanisms in colitis. Int. J. Mol. Sci. 2020, 21, 8145. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The role of NLRP3 and IL-1beta in the pathogenesis of inflammatory bowel disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Zenewicz, L.A.; Huber, F.J.; Bosurgi, L.; Hu, B.; Hedl, M.; Zhang, W.; O’Connor, W., Jr.; Murphy, A.J.; et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012, 491, 259–263. [Google Scholar] [CrossRef]
- Song, H.; Liu, B.; Huai, W.; Yu, Z.; Wang, W.; Zhao, J.; Han, L.; Jiang, G.; Zhang, L.; Gao, C.; et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016, 7, 13727. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, C.; Bai, W.; Li, J.; Pan, Y.; Huang, X.; Yang, H.; Feng, Z.; Xiang, Q.; Fei, L.; et al. CD1d1 intrinsic signaling in macrophages controls NLRP3 inflammasome expression during inflammation. Sci. Adv. 2020, 6, eaaz7290. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Raselli, T.; Frey-Wagner, I.; Gutte, P.M.; et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Invest. 2016, 126, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, C.; Xing, Y.; Xue, G.; Zhang, Q.; Pan, F.; Wu, G.; Hu, Y.; Guo, Q.; Lu, A.; et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 2017, 8, 1896. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.T.; Yao, Y.; Yang, L.; Xing, Y.; Wang, Y.; You, J.H. Correlation between IRGM genetic polymorphisms and Crohn’s disease risk: A meta-analysis of case-control studies. Genet. Mol. Res. 2014, 13, 10741–10753. [Google Scholar] [CrossRef]
- Mehto, S.; Jena, K.K.; Nath, P.; Chauhan, S.; Kolapalli, S.P.; Das, S.K.; Sahoo, P.K.; Jain, A.; Taylor, G.A.; Chauhan, S. The Crohn’s disease risk factor IRGM limits NLRP3 inflammasome activation by impeding its assembly and by mediating its selective autophagy. Mol. Cell 2019, 73, 429–445.e7. [Google Scholar] [CrossRef] [PubMed]
- Newton, K. Multitasking kinase RIPK1 regulates cell death and inflammation. Cold Spring Harb Perspect Biol. 2020, 12, a036368. [Google Scholar] [CrossRef]
- Li, Y.; Fuhrer, M.; Bahrami, E.; Socha, P.; Klaudel-Dreszler, M.; Bouzidi, A.; Liu, Y.; Lehle, A.S.; Magg, T.; Hollizeck, S.; et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Barmettler, S.; Otani, I.M.; Minhas, J.; Abraham, R.S.; Chang, Y.; Dorsey, M.J.; Ballas, Z.K.; Bonilla, F.A.; Ochs, H.D.; Walter, J.E. Gastrointestinal manifestations in X-linked agammaglobulinemia. J. Clin. Immunol. 2017, 37, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Hiejima, E.; Montgomery-Recht, K.; Zhou, W.; Fuss, I.; Wiestner, A.; Strober, W. Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1beta-mediated colitis. J. Clin. Invest. 2020, 130, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Manzini, R.; Hering, L.; Riggs, J.B.; Gottier, C.; Lang, S.; Atrott, K.; Fettelschoss, A.; Olomski, F.; Kundig, T.M.; et al. PTPN2 regulates inflammasome activation and controls onset of intestinal inflammation and colon cancer. Cell Rep. 2018, 22, 1835–1848. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Q.; Long, F.; Di, Y.; Wang, J.; Zhun Zhu, Y.; Liu, X. Jmjd3 regulates inflammasome activation and aggravates DSS-induced colitis in mice. FASEB J. 2020, 34, 4107–4119. [Google Scholar] [CrossRef] [PubMed]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, Z.T.; Lu, X.X.; Wang, Y.G.; Zhong, J.; Liu, J. NLRP3 gene is associated with ulcerative colitis (UC), but not Crohn’s disease (CD), in Chinese Han population. Inflamm. Res. 2014, 63, 979–985. [Google Scholar] [CrossRef]
- Villani, A.C.; Lemire, M.; Fortin, G.; Louis, E.; Silverberg, M.S.; Collette, C.; Baba, N.; Libioulle, C.; Belaiche, J.; Bitton, A.; et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 2009, 41, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Sadr, M.; Rezaei, A.; Shahkarami, S.; Ebrahimi Daryani, N.; Bidoki, A.Z.; Rezaei, N. Association of NLRP3 single nucleotide polymorphisms with ulcerative colitis: A case-control study. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 269–275. [Google Scholar] [CrossRef]
- Lewis, G.J.; Massey, D.C.; Zhang, H.; Bredin, F.; Tremelling, M.; Lee, J.C.; Berzuini, C.; Parkes, M. Genetic association between NLRP3 variants and Crohn’s disease does not replicate in a large UK panel. Inflamm. Bowel Dis. 2011, 17, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, T.; Huang, B.; Luo, M.; Chen, Z.; Zhao, Z.; Wang, J.; Leung, D.; Yang, X.; Chan, K.W.; et al. Excessive deubiquitination of NLRP3-R779C variant contributes to very-early-onset inflammatory bowel disease development. J. Allergy Clin. Immunol. 2021, 147, 267–279. [Google Scholar] [CrossRef]
- Mouhadeb, O.; Ben Shlomo, S.; Cohen, K.; Farkash, I.; Gruber, S.; Maharshak, N.; Halpern, Z.; Burstein, E.; Gluck, N.; Varol, C. Impaired COMMD10-mediated regulation of Ly6C(hi) monocyte-driven inflammation disrupts gut barrier function. Front. Immunol 2018, 9, 2623. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Similuk, M.; Oler, A.J.; Albenberg, L.; Kelsen, J.; Aktay, A.; Quezado, M.; Yao, M.; Montgomery-Recht, K.; et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J. Clin. Invest. 2018, 128, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.S.; Biswas, A.; Kang, Y.H.; Griffith, A.E.; Konnikova, L.; Mascanfroni, I.D.; Redhu, N.S.; Frei, S.M.; Field, M.; Doty, A.L.; et al. Interleukin 1beta mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology 2016, 151, 1100–1104. [Google Scholar] [CrossRef]
- de Luca, A.; Smeekens, S.P.; Casagrande, A.; Iannitti, R.; Conway, K.L.; Gresnigt, M.S.; Begun, J.; Plantinga, T.S.; Joosten, L.A.; van der Meer, J.W.; et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 3526–3531. [Google Scholar] [CrossRef]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia 2015, 101, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Oliveira, G.A.; Alarcon de la Lastra, C.; Rosillo, M.A.; Castejon Martinez, M.L.; Sanchez-Hidalgo, M.; Rolim Medeiros, J.V.; Villegas, I. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem. Biol. Interact. 2019, 297, 25–33. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.T.; Wu, M.M.; Wang, C.L.; Su, Z.R.; Cheng, Y.Y.; Zhang, X.J. Palmatine attenuated dextran sulfate sodium (DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol. Immunol. 2019, 105, 76–85. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Le, T.H.; Du, Q.; Zhao, Z.; Liu, Y.; Zou, J.; Hua, W.; Liu, C.; Zhu, Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int. Immunopharmacol. 2019, 71, 144–154. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of alpha-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Yin, P.; Zou, W.; Li, J.; Jin, N.; Gao, Q.; Liu, F. Using high-throughput sequencing to explore the anti-inflammatory effects of alpha-mangostin. Sci. Rep. 2019, 9, 15626. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, S.; Luo, W.; Shen, Z.; Meng, X.; Xiao, M.; Tan, B.; Nie, K.; Tong, T.; Wang, X. Roseburia intestinalisderived flagellin ameliorates colitis by targeting miR2233pmediated activation of NLRP3 inflammasome and pyroptosis. Mol. Med. Rep. 2020, 22, 2695–2704. [Google Scholar]
- Banerjee, S.; Ghosh, S.; Sinha, K.; Chowdhury, S.; Sil, P.C. Sulphur dioxide ameliorates colitis related pathophysiology and inflammation. Toxicology 2019, 412, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- Zong, S.; Ye, Z.; Zhang, X.; Chen, H.; Ye, M. Protective effect of Lachnum polysaccharide on dextran sulfate sodium-induced colitis in mice. Food Funct. 2020, 11, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Dimethyl fumarate: A review in relapsing-remitting MS. Drugs 2019, 79, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Cordaro, M.; Impellizzeri, D.; Bruschetta, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl fumarate reduces inflammatory responses in experimental colitis. J. Crohn’s Colitis 2016, 10, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, W.; Zhang, X.; Lu, P.; Du, Q.; Tao, L.; Ding, Y.; Wang, Y.; Hu, R. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem. Pharmacol. 2016, 112, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Takasu, C.; Lau, H.; Robles, L.; Vo, K.; Farzaneh, T.; Vaziri, N.D.; Stamos, M.J.; Ichii, H. Dimethyl fumarate alleviates dextran sulfate sodium-induced colitis, through the activation of Nrf2-mediated antioxidant and anti-inflammatory pathways. Antioxidants 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shang, C.; Wang, H.; Yun, J. Isatin-azole hybrids and their anticancer activities. Arch. Pharm. 2020, 353, e1900272. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, W.; Wang, Y.; Chen, C.; Guo, L.; Ju, R.; Li, J.; Zhang, D.; Zhu, L.; Ye, C. Therapeutic efficacy of carboxyamidotriazole on 2,4,6-trinitrobenzene sulfonic acid-induced colitis model is associated with the inhibition of NLRP3 inflammasome and NF-kappaB activation. Int. Immunopharmacol. 2017, 45, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gong, Z.; Zhou, J.; Tian, C.; Gao, Y.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Deoxycholic acid triggers NLRP3 inflammasome activation and aggravates DSS-induced colitis in mice. Front. Immunol. 2016, 7, 536. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, Z.; Du, X.; Tian, C.; Wang, L.; Zhou, J.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Deoxycholic acid-mediated sphingosine-1-phosphate receptor 2 signaling exacerbates DSS-induced colitis through promoting cathepsin B release. J. Immunol. Res. 2018, 2018, 2481418. [Google Scholar] [CrossRef]
- Colica, C.; Di Renzo, L.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. Rosmarinic acid as potential anti-inflammatory agent. Rev. Recent Clin. Trials 2018, 13, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-kappaB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar]
- Formiga, R.O.; Alves Junior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araujo, A.; Carvalho, T.G.; Garcia, V.B.; de Araujo Junior, R.F.; Gadelha, F.; Vieira, G.C.; et al. p-cymene and rosmarinic acid ameliorate TNBS-induced intestinal inflammation upkeeping ZO-1 and MUC-2: Role of antioxidant system and immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Avila-Roman, J.; Motilva, V.; Talero, E. Anti-inflammatory effects of rosmarinic acid-loaded Nanovesicles in acute colitis through modulation of NLRP3 inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]
- Ding, W.; Ding, Z.; Wang, Y.; Zhu, Y.; Gao, Q.; Cao, W.; Du, R. Evodiamine attenuates experimental colitis injury via activating autophagy and inhibiting NLRP3 inflammasome assembly. Front. Pharmacol. 2020, 11, 573870. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; Zhu, K.; Cao, H.; Liu, J.; Lu, X.; Li, Y.; Jing, Y.; Yuan, X.; Fu, Y.; et al. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-kappaB and NLRP3 inflammasome. Biomed. Pharmacother. 2019, 110, 786–795. [Google Scholar] [CrossRef]
- Li, C.G.; Zeng, Q.Z.; Chen, M.Y.; Xu, L.H.; Zhang, C.C.; Mai, F.Y.; Zeng, C.Y.; He, X.H.; Ouyang, D.Y. Evodiamine augments NLRP3 inflammasome activation and anti-bacterial responses through inducing alpha-tubulin acetylation. Front. Pharmacol 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.C.; OuYang, C.N.; Yuan, S.N.; Lin, H.C.; Huang, K.Y.; Wu, P.S.; Liu, C.Y.; Tsai, K.J.; Loi, L.K.; Chen, Y.J.; et al. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Cosin-Roger, J.; Simmen, S.; Melhem, H.; Atrott, K.; Frey-Wagner, I.; Hausmann, M.; de Valliere, C.; Spalinger, M.R.; Spielmann, P.; Wenger, R.H.; et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat. Commun. 2017, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalova, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Chen, G.Y.; Liu, M.; Wang, F.; Bertin, J.; Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 2011, 186, 7187–7194. [Google Scholar] [CrossRef]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 protects Il10(-/-) mice from colitis by limiting colonization of akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kumar, R.; Tsakem Lenou, E.; Basrur, V.; Kontoyiannis, D.L.; Ioakeimidis, F.; Mosialos, G.; Theiss, A.L.; Flavell, R.A.; Venuprasad, K. Deubiquitination of NLRP6 inflammasome by Cyld critically regulates intestinal inflammation. Nat. Immunol. 2020, 21, 626–635. [Google Scholar] [CrossRef]
- Williams, T.M.; Leeth, R.A.; Rothschild, D.E.; Coutermarsh-Ott, S.L.; McDaniel, D.K.; Simmons, A.E.; Heid, B.; Cecere, T.E.; Allen, I.C. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J. Immunol. 2015, 194, 3369–3380. [Google Scholar] [CrossRef]
- Tye, H.; Yu, C.H.; Simms, L.A.; de Zoete, M.R.; Kim, M.L.; Zakrzewski, M.; Penington, J.S.; Harapas, C.R.; Souza-Fonseca-Guimaraes, F.; Wockner, L.F.; et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 2018, 9, 3728. [Google Scholar] [CrossRef]
- Sharma, D.; Malik, A.; Guy, C.S.; Karki, R.; Vogel, P.; Kanneganti, T.D. Pyrin inflammasome regulates tight junction integrity to restrict colitis and tumorigenesis. Gastroenterology 2018, 154, 948–964.e8. [Google Scholar] [CrossRef]
- Ratsimandresy, R.A.; Indramohan, M.; Dorfleutner, A.; Stehlik, C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol. Immunol. 2017, 14, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Nalbantoglu, I.; Aitken, J.D.; Uchiyama, R.; Su, Y.; Doho, G.H.; Vijay-Kumar, M.; Gewirtz, A.T. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal. Immunol. 2012, 5, 288–298. [Google Scholar] [CrossRef] [PubMed]

| Gene | Function to Inflammasome | Mechanism | Deficiency or Variant | Variant Impact to Disease | Disease or Model | Reference |
|---|---|---|---|---|---|---|
| IRGM | Inhibit NLRP3 inflammasome assambly | Interact with NLRP3 and Promote NLRP3 autophagic degradation | Deficiency | Exacerbate colitis | DSS-colitis in mouse | [49] |
| RIPK1 | Inhibit NLRP3 inflammasome activation upon LPS stimulation | Not clear | Deficiency | Primary immunodeficiency and/or colitis | Human colitis | [51] |
| BTK | Inhibit NLRP3 inflammasome activation | Inhibit PP2A mediated NLRP3 dephosphorylation | Deficiency | Exacerbate colitis | DSS-colitis, TNBS-colitis in mouse | [53] |
| PTPN2 | Inhibit NLRP3 inflammasome activation | Inhibit JNK and ASC phosphorylation | Deficiency in myeloid cell | Exacerbate colitis | DSS-colitis in mouse | [54] |
| Jmjd3 | Enhance NLRP3 inflammasome activation | Prevent H3K27me3 mediated inhibition of Nrf2 | Inhibition or Knock-down | Ameliorate colitis | DSS-colitis | [55] |
| miR-223 | Inhibit NLRP3 inflammasome activation | Bind to NLRP3 3’ untranslated region to inhibit inflammasome assembly | Deficiency | Exacerbate colitis | DSS-colitis in mouse | [56] |
| NLRP3 mutation | Enhance NLRP3 inflammasome activation | Enhance deubiquitination of NLRP3 via binding with BRCC3 and JOSD2 | R779C | Exacerbate colitis | DSS-colitis in mouse | [61] |
| COMMD10 | Inhibit NLRP3 inflammasome activation | Inhibit transcription of inflammasome components | Deficiency in monocytes | Exacerbate colitis | DSS-colitis in mouse | [62] |
| CARD8 | Inhibit NLRP3 inflammasome assembly | Interact with NLRP3 and inhibit NLRP3 oligomerization | V44I | Exacerbate colitis | Crohn’s disease | [63] |
| IL-10R | Inhibit NLRP3 inflammasome activation | Inhibit expression of NLRP3 and IL-1β | Deficiency | Spontaneous colitis in mouse and infant-onset IBD in human | Mouse spontaneous colitis and human IBD | [64] |
| NADPH oxidase | Inhibit NLRP3 inflammasome activation | Induce autophagy | Deficiency | Exacerbate colitis | TNBS-colitis in mouse | [65] |
| CD1d1 | Reduce transcription of NLRP3 inflammasome components | Reduce peroxiredoxin 1/ATK/STAT1 mediated NF-kB signaling | Deficiency in macrophage | Ameliorate colitis | DSS-colitis in mouse | [44] |
| PTPN22 | Enhance NLRP3 inflammasome activation | Induce NLRP3 dephosphorylation at Tyr861 | Deficiency | Exacerbate colitis | DSS-colitis in mouse | [45] |
| V619W | Ameliorate colitis | DSS-colitis in mouse | [45] |
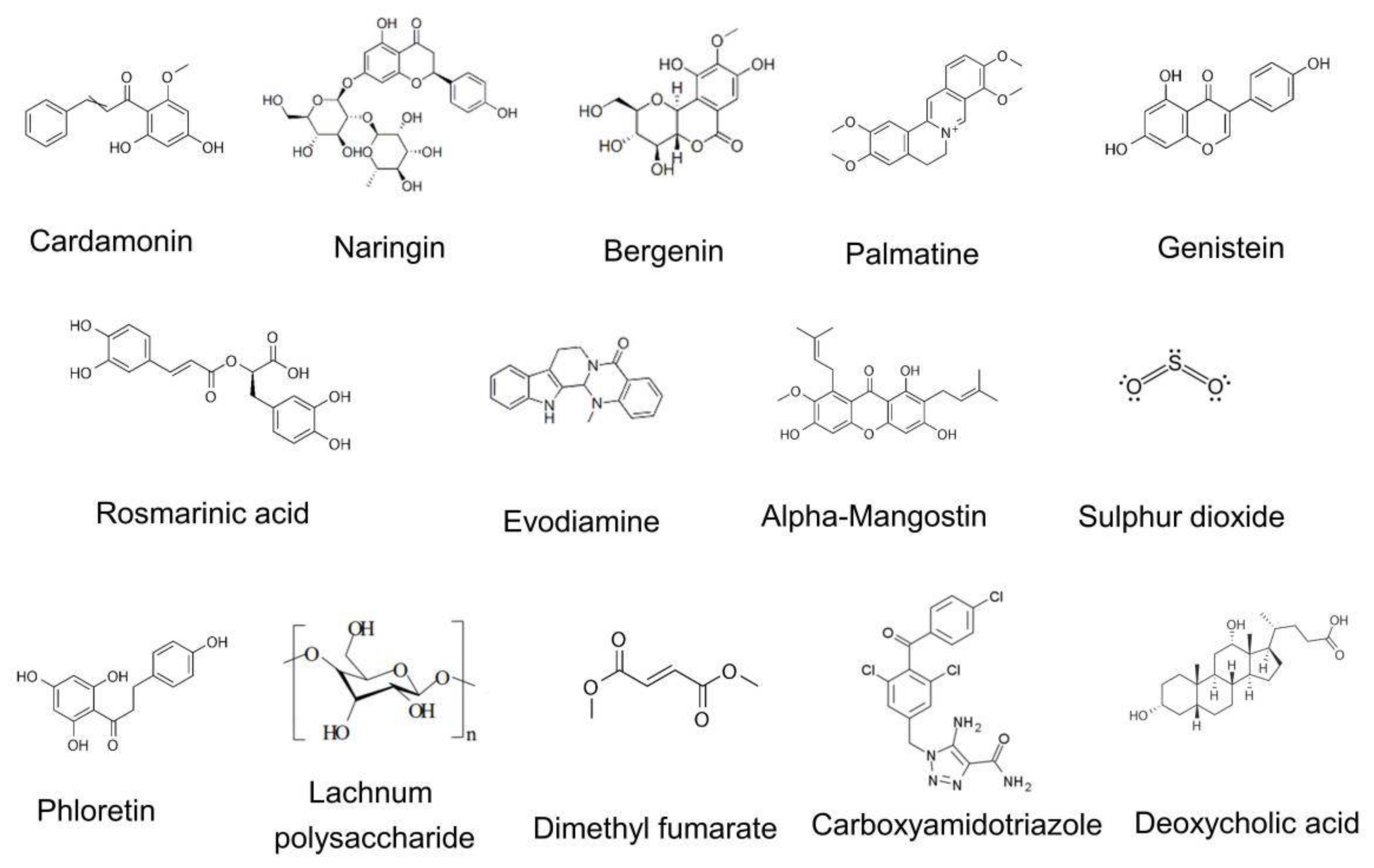
| Natural Product | Source | Mechanism | Disease or Model | Reference |
|---|---|---|---|---|
| Cardamonin | Cardamom | Activate AhR/Nrf2/NQO1 pathway | DSS-colitis and TNBS-colitis in mouse | [67] |
| Naringin | Citrus fruit | Activate PPARγ and suppresse NF-kB activation | DSS-colitis in mouse | [69] |
| Bergenin | Plant metabolite | Inhibit COX-2, iNOS, IkB-α, and pSTAT3 expression | TNBS-colitis in rat | [71] |
| Palmatine | Herbal plant | Elevated mitophagy proteins LC3, PINK1 and Parkin | DSS-colitis in mouse | [73] |
| Genistein | Plant | Elevate intracellular cAMP | DSS-colitis in mouse | [75] |
| Rosmarinic acid | Rosmarinus officinalis L. | Reduce the expression of inflammasome components | DSS-colitis in mouse | [94] |
| Evodiamine | Evodiae fructus | Regulate NF-kB and autophagy | DSS-colitis in mouse | [95,96] |
| α-mangostin | Mangosteen fruit | Promote expression of NLRP3, caspase 1, IL-18, and IL-1β | LPS-induced colitis in rat | [77] |
| Flagellin | Roseburia intestinalis | Elevate expression of miR-223-3p | DSS-colitis in mouse | [78] |
| Sulphur dioxide | Metabolism of sulfur-containing amino acids | Reduce oxidative stress, ER stress and autophagy | TNBS-colitis in rat | [79] |
| Phloretin | Fruits and vegetables | Suppressed NF-kB, PPARγ and oxidative stress | DSS-colitis in mouse | [80] |
| Lachnum polysaccharide | Lachnum sp. | ER stress and oxidative/nitrosative stress | DSS-colitis in mouse | [81] |
| Dimethyl fumarate | Derivative of fumarate | Induced GSH, activate Nrf2 and suppress mitochondrial ROS | DNBS-colitis in mouse | [84] |
| Carboxyamidotriazole | Noncytotoxic chemotherapy agent | Reduce activation of NF-kB | TNBS-colitis in rat | [87] |
| Deoxycholic Acid | High fat diet | Promote cathepsin B release by engagement of S1PR2 | DSS-colitis in mouse | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Zhou, X.; Strober, W.; Mao, L. Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease. Molecules 2021, 26, 1725. https://doi.org/10.3390/molecules26061725
Xu Q, Zhou X, Strober W, Mao L. Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease. Molecules. 2021; 26(6):1725. https://doi.org/10.3390/molecules26061725
Chicago/Turabian StyleXu, Qiuyun, Xiaorong Zhou, Warren Strober, and Liming Mao. 2021. "Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease" Molecules 26, no. 6: 1725. https://doi.org/10.3390/molecules26061725
APA StyleXu, Q., Zhou, X., Strober, W., & Mao, L. (2021). Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease. Molecules, 26(6), 1725. https://doi.org/10.3390/molecules26061725






