Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
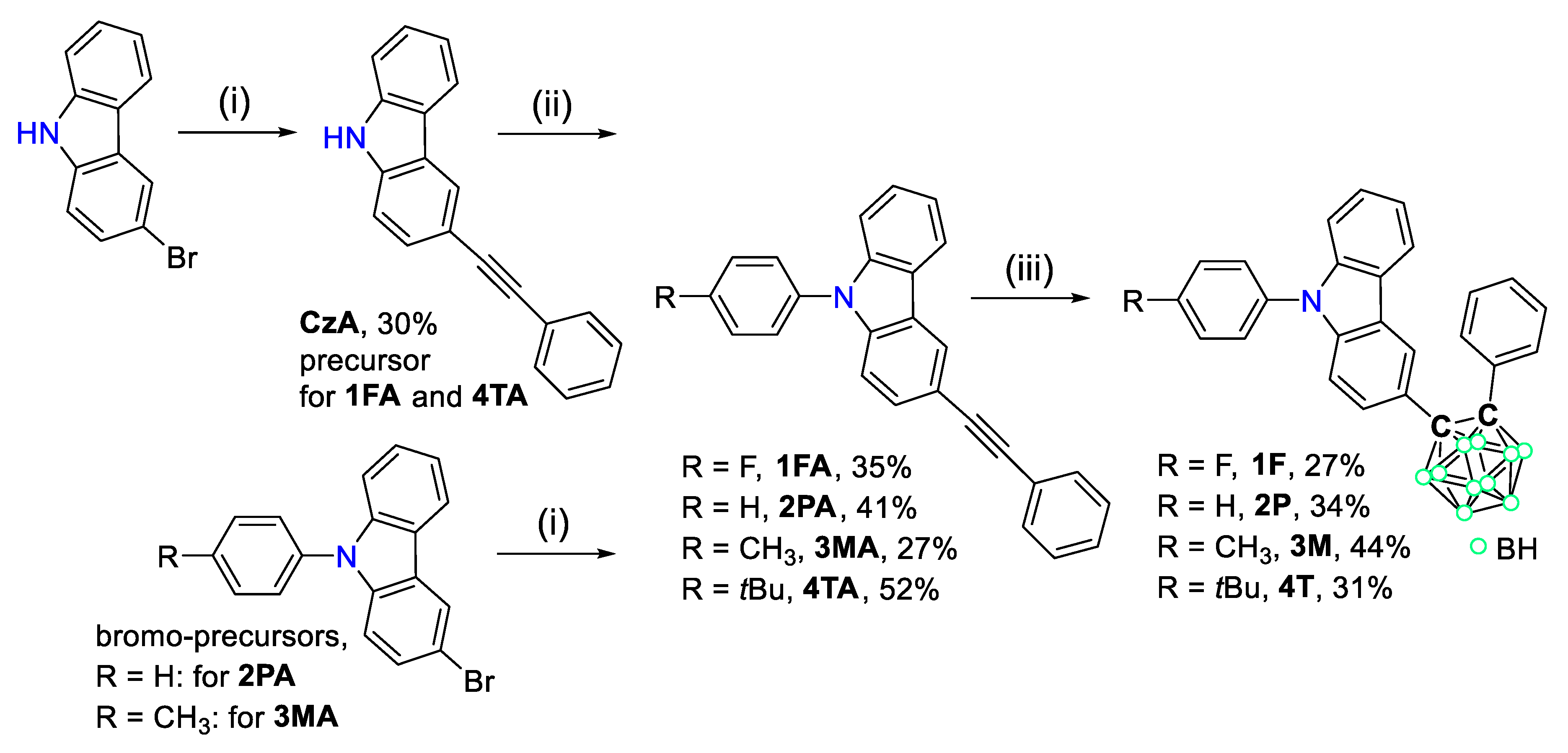
2.2. General Synthetic Procedure for Acetylene Precursors (CzA, 2PA, and 3MA)
2.2.1. Data for CzA
2.2.2. Data for 2PA
2.2.3. Data for 3MA
2.3. Synthesis of 1FA
2.4. Synthesis of 4TA
2.5. General Synthetic Procedure for Carbazole-Based o-Carborane Compounds (1F, 2P, 3M, and 4T)
2.5.1. Data for 1F
2.5.2. Data for 2P
2.5.3. Data for 3M
2.5.4. Data for 4T
2.6. UV/Vis Absorption and Photoluminescence (PL) Measurements
2.7. X-ray Crystallography
2.8. Computational Calculations
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Experimental and Theoretical Analysis of Photophysical Properties
3.3. Electronic Effect on ICT-Based Radiative Decay Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and Their Derivatives. Chem. Rev. 1992, 92, 209–223. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; Fabrizi de Biani, F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef] [PubMed]
- Ochi, J.; Tanaka, K.; Chujo, Y. Recent Progress in the Development of Solid-state Luminescent o-Carboranes with Stimuli Responsivity. Angew. Chem. Int. Ed. 2020, 59, 9841–9855. [Google Scholar] [CrossRef]
- Poater, J.; Viñas, C.; Bennour, I.; Escayola, S.; Solà, M.; Teixidor, F. Too Persistent to Give Up: Aromaticity in Boron Clusters Survives Radical Structural Changes. J. Am. Chem. Soc. 2020, 142, 9396–9407. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Machan, C.W.; Clingerman, D.J.; Rosen, M.S.; Wiester, M.J.; Kennedy, R.D.; Stern, C.L.; Sarjeant, A.A.; Mirkin, C.A. A coordination chemistry dichotomy for icosahedral carborane-based ligands. Nat. Chem. 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Wee, K.-R.; Cho, Y.-J.; Jeong, S.; Kwon, S.; Lee, J.-D.; Suh, I.-H.; Kang, S.O. Carborane-Based Optoelectronically Active Organic Molecules: Wide Band Gap Host Materials for Blue Phosphorescence. J. Am. Chem. Soc. 2012, 134, 17982–17990. [Google Scholar] [CrossRef] [PubMed]
- Furue, R.; Nishimoto, T.; Park, I.S.; Lee, J.; Yasuda, T. Aggregation-Induced Delayed Fluorescence Based on Donor/Acceptor-Tethered Janus Carborane Triads: Unique Photophysical Properties of Nondoped OLEDs. Angew. Chem. Int. Ed. 2016, 55, 7171–7175. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Zhang, J.; Zhang, J.; Miao, Q.; Xie, Z. o-carborane functionalized pentacenes: Synthesis, molecular packing and ambipolar organic thin-film transistors. Chem. Commun. 2015, 51, 12004–12007. [Google Scholar] [CrossRef] [PubMed]
- Nar, I.; Atsay, A.; Altındal, A.; Hamuryudan, E. o-Carborane, Ferrocene, and Phthalocyanine Triad for High-Mobility Organic Field-Effect Transistors. Inorg. Chem. 2018, 57, 2199–2208. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Gaillard, E.R.; Maguire, J.A.; Hosmane, N.S. Synthesis and Properties of Carborane-Appended C3-Symmetrical Extended π Systems. J. Am. Chem. Soc. 2010, 132, 6578–6587. [Google Scholar] [CrossRef] [PubMed]
- Kokado, K.; Chujo, Y. Multicolor Tuning of Aggregation-Induced Emission through Substituent Variation of Diphenyl-o-carborane. J. Org. Chem. 2011, 76, 316–319. [Google Scholar] [CrossRef]
- Wee, K.-R.; Han, W.-S.; Cho, D.W.; Kwon, S.; Pac, C.; Kang, S.O. Carborane Photochemistry Triggered by Aryl Substitution: Carborane-Based Dyads with Phenyl Carbazoles. Angew. Chem. Int. Ed. 2012, 51, 2677–2680. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Fox, M.A. Luminescence Properties of C-Diazaborolyl-ortho-Carboranes as Donor-Acceptor Systems. Chem. Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Núñez, R. Synthesis, Characterization, and Thermal Behavior of Carboranyl-Styrene Decorated Octasilsesquioxanes: Influence of the Carborane Clusters on Photoluminescence. Chem. Eur. J. 2013, 19, 17021–17030. [Google Scholar] [CrossRef]
- Wee, K.-R.; Cho, Y.-J.; Song, J.K.; Kang, S.O. Multiple Photoluminescence from 1,2-Dinaphthyl-ortho-Carborane. Angew. Chem. Int. Ed. 2013, 52, 9682–9685. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; González-Campo, A.; Viñas, C.; Rodríguez-Romero, J.; Santillan, R.; Farfán, N.; Sillanpää, R.; Sousa-Pedrares, A.; Núñez, R.; Teixidor, F. Fluorescence of New o-Carborane Compounds with Different Fluorophores: Can it be Tuned? Chem. Eur. J. 2014, 20, 9940–9951. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π Aromaticity and Three-Dimensional Aromaticity: Two sides of the Same Coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef]
- Naito, H.; Morisaki, Y.; Chujo, Y. o-Carborane-Based Anthracene: A Variety of Emission Behaviors. Angew. Chem. Int. Ed. 2015, 54, 5084–5087. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef]
- Kirlikovali, K.O.; Axtell, J.C.; Gonzalez, A.; Phung, A.C.; Khan, S.I.; Spokoyny, A.M. Luminescent metal complexes featuring photophysically innocent boron cluster ligands. Chem. Sci. 2016, 7, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Yamamoto, H.; Tanaka, K.; Chujo, Y. Development of Solid-State Emissive Materials Based on Multifunctional o-Carborane–Pyrene Dyads. Org. Lett. 2016, 18, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-State Emission of the Anthracene-o-Carborane Dyad from the Twisted-Intramolecular Charge Transfer in the Crystalline State. Angew. Chem. Int. Ed. 2017, 56, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Highly-efficient solid-state emissions of anthracene–o-carborane dyads with various substituents and their thermochromic luminescence properties. J. Mater. Chem. C 2017, 5, 10047–10054. [Google Scholar] [CrossRef]
- Tu, D.; Leong, P.; Guo, S.; Yan, H.; Lu, C.; Zhao, Q. Highly Emissive Organic Single-Molecule White Emitters by Engineering o-Carborane-Based Luminophores. Angew. Chem. Int. Ed. 2017, 56, 11370–11374. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically triggered reversible stepwise tricolor switching and thermochromism of anthracene-o-carborane dyad. Chem. Sci. 2018, 9, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Peng, X.; Chen, Y.; Qi, Q.; Luo, X.; Lai, W.-Y.; Huang, W. Stimuli-responsive solid-state emission from o-carborane–tetraphenylethene dyads induced by twisted intramolecular charge transfer in the crystalline state. J. Mater. Chem. C 2018, 6, 19–28. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Zhao, J.; Che, Y.; Jia, D.; Chen, Y.M. Multifunctional luminescent molecules of o-carborane-pyrene dyad/triad: Flexible synthesis and study of the photophysical properties. Dyes Pigm. 2018, 154, 44–51. [Google Scholar] [CrossRef]
- Marsh, A.V.; Cheetham, N.J.; Little, M.; Dyson, M.; White, A.J.P.; Beavis, P.; Warriner, C.N.; Swain, A.C.; Stavrinou, P.N.; Heeney, M. Carborane-Induced Excimer Emission of Severely Twisted Bis-o-Carboranyl Chrysene. Angew. Chem. Int. Ed. 2018, 57, 10640–10645. [Google Scholar] [CrossRef]
- So, H.; Kim, J.H.; Lee, J.H.; Hwang, H.; An, D.K.; Lee, K.M. Planarity of terphenyl rings possessing o-carborane cages: Turning on intramolecular-charge-transfer-based emission. Chem. Commun. 2019, 55, 14518–14521. [Google Scholar] [CrossRef]
- Martin, K.L.; Smith, J.N.; Young, E.R.; Carter, K.R. Synthetic Emission Tuning of Carborane-Containing Poly(dihexylfluorene)s. Macromolecules 2019, 52, 7951–7960. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.H.; So, H.; Ryu, J.; Lee, J.; Hwang, H.; Kim, Y.; Park, M.H.; Lee, K.M. Spirobifluorene-Based o-Carboranyl Compounds: Insights into the Rotational Effect of Carborane Cages on Photoluminescence. Chem. Eur. J. 2020, 26, 548–557. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.H.; So, H.; Kim, M.; Mun, M.S.; Hwang, H.; Park, M.H.; Lee, K.M. Insights into the effects of substitution position on the photophysics of mono-o-carborane-substituted pyrenes. Inorg. Chem. Front. 2020, 7, 2949–2959. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, C.H.; Hong, J.H.; Lee, J.H.; Hwang, H.; Lee, K.M. Planarity of N-aryl in appended 1,2,4-triazole-based o-carboranyl luminophores: A key factor to control intramolecular charge transfer. Inorg. Chem. Front. 2020, 7, 4180–4189. [Google Scholar] [CrossRef]
- Mun, M.S.; Ryu, C.H.; So, H.; Kim, M.; Lee, J.H.; Hwang, H.; Lee, K.M. Multiple Photoluminescence of Spiro[acridine-fluorene]-based o-Carboranyl Compounds and Potential as a Visual Sensory Material. J. Mater. Chem. C 2020, 8, 16896–16906. [Google Scholar] [CrossRef]
- Núñez, R.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Kivekäs, R. Contribution of the o-carboranyl fragment to the chemical stability and the 31P-NMR chemical shift in closo-carboranylphosphines. Crystal structure of bis(1-yl-2-methyl-1,2-dicarba-closo-dodecaborane)phenylphosphine. J. Organomet. Chem. 1999, 592, 22–28. [Google Scholar] [CrossRef]
- Teixidor, F.; Núñez, R.; Viñas, C.; Sillanpää, R.; Kivekäs, R. The Distinct Effect of the o-Carboranyl Fragment: Its Influence on the I−I Distance in R3PI2 Complexes. Angew. Chem. Int. Ed. 2000, 39, 4290–4292. [Google Scholar] [CrossRef]
- Núñez, R.; Farràs, P.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Kivekäs, R. A Discrete P⋅⋅⋅I—I⋅⋅⋅P Assembly: The Large Influence of Weak Interactions on the 31P NMR Spectra of Phosphane–Diiodine Complexes. Angew. Chem. Int. Ed. 2006, 45, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.O.; Kim, H.; Lee, K.M.; Lee, Y.S.; Do, Y.; Lee, M.H. o-Carborane-assisted Lewis acidity enhancement of triarylboranes. Chem. Commun. 2010, 46, 1138–1140. [Google Scholar] [CrossRef]
- Lee, K.N.; Huh, J.O.; Kim, T.; Do, Y.; Lee, M.H. A highly Lewis acidic triarylborane bearing peripheral o-carborane cages. Dalton Trans. 2011, 40, 11758–11764. [Google Scholar] [CrossRef]
- Fox, M.A.; Gill, P.L.; Herbertson, P.L.; MacBride, J.A.H.; Wade, K.; Colquhoun, H.M. Deboronation of C-substituted ortho- and meta- closo-carboranes using “wet” fluoride ion solutions. Polyhedron 1996, 15, 565–571. [Google Scholar] [CrossRef]
- Yoo, J.; Hwang, J.-W.; Do, Y. Facile and Mild Deboronation of o-Carboranes Using Cesium Fluoride. Inorg. Chem. 2001, 40, 568–570. [Google Scholar] [CrossRef]
- Song, K.C.; Kim, H.; Lee, K.M.; Lee, Y.S.; Do, Y.; Lee, M.H. Dual sensing of fluoride ions by the o-carborane–triarylborane dyad. Dalton Trans. 2013, 42, 2351–2354. [Google Scholar] [CrossRef]
- You, D.K.; Lee, J.H.; Hwang, H.; Kwon, H.; Park, M.H.; Lee, K.M. Deboronation-induced ratiometric emission sensing of fluoride by 1,3,5-tris-(o-carboranyl-methyl)benzene. Tetrahedron Lett. 2017, 58, 3246–3250. [Google Scholar] [CrossRef]
- Nghia, N.V.; Oh, J.; Sujith, S.; Jung, J.; Lee, M.H. Tuning the photophysical properties of carboranyl luminophores by closo- to nido-carborane conversion and application to OFF–ON fluoride sensing. Dalton Trans. 2018, 47, 17441–17449. [Google Scholar] [CrossRef]
- Nghia, N.V.; Jana, S.; Sujith, S.; Ryu, J.Y.; Lee, J.; Lee, S.U.; Lee, M.H. Nido-Carboranes: Donors for Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2018, 57, 12483–12488. [Google Scholar] [CrossRef]
- So, H.; Mun, M.S.; Kim, M.; Kim, J.H.; Lee, J.H.; Hwang, H.; An, D.K.; Lee, K.M. Deboronation-Induced Ratiometric Emission Variations of Terphenyl-Based Closo-o-Carboranyl Compounds: Applications to Fluoride-Sensing. Molecules 2020, 25, 2413. [Google Scholar] [CrossRef] [PubMed]
- Sujith, S.; Nam, E.B.; Lee, J.; Lee, S.U.; Lee, M.H. Enhancing the thermally activated delayed fluorescence of nido-carborane-appended triarylboranes by steric modification of the phenylene linker. Inorg. Chem. Front. 2020, 7, 3456–3464. [Google Scholar] [CrossRef]
- Teixidor, F.; Casabó, J.; Viñas, C.; Sanchez, E.; Escriche, L.; Kivekäs, R. Macrocycles incorporating sulfur and nido-carborane cages: Reactivity toward nickel(II) and palladium(II). Molecular structures of Pd{7,8-μ-(S(CH2CH2OCH2CH2OCH2CH2OCH2CH2)S)C2B9H10}2 and Pd{P(C6H5)3}Cl{7,8-μ-(SCH2CH2S)C2B9H10}. Inorg. Chem. 1991, 30, 3053–3058. [Google Scholar] [CrossRef]
- Teixidor, F.; Viñas, C.; Sillanpää, R.; Kivekäs, R.; Casabó, J. Nido-Carborane-Containing Compounds Resulting from the Reaction of closo-Carboranes with Transition Metal Complexes. Inorg. Chem. 1994, 33, 2645–2650. [Google Scholar] [CrossRef]
- Teixidor, F.; Núñez, R.; Viñas, C.; Sillanpää, R.; Kivekäs, R. Contribution of the nido-[7,8-C2B9H10]− Anion to the Chemical Stability, Basicity, and 31P NMR Chemical Shift in nido-o- Carboranylmonophosphines. Inorg. Chem. 2001, 40, 2587–2594. [Google Scholar] [CrossRef]
- Lerouge, F.; Ferrer-Ugalde, A.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Abreu, A.; Xochitiotzi, E.; Farfán, N.; Santillan, R.; Núñez, R. Synthesis and fluorescence emission of neutral and anionic di- and tetra-carboranyl compounds. Dalton Trans. 2011, 40, 7541–7550. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, K.M.; Kim, T.; Do, Y.; Lee, M.H. Ortho-Carborane-Functionalized Luminescent Polyethylene: Potential Chemodosimeter for the Sensing of Nucleophilic Anions. Chem. Asian J. 2011, 6, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Binkley, J.S.; People, J.A.; Hehre, W.J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 1980, 102, 939–947. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16 Revision B.01; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Berry, T.E.; Wegner, P.A. The Electronic Properties of the 1,2- and 1,7-Dicarbaclovododecaborane(12) Groups Bonded at Carbon. J. Am. Chem. Soc. 1965, 87, 4746–4750. [Google Scholar] [CrossRef]
- Paxson, T.E.; Callahan, K.P.; Hawthorne, M.F. Improved synthesis of biscarborane and its precursor ethynylcarborane. Inorg. Chem. 1973, 12, 708–709. [Google Scholar] [CrossRef]
- Jiang, W.; Knobler, C.B.; Hawthorne, M.F. Synthesis and Structural Characterization of Bis- and Tris(closo-1,2-C2B10H11-1-yl)-Substituted Biphenyl and Benzene. Inorg. Chem. 1996, 35, 3056–3058. [Google Scholar] [CrossRef]
- Llop, J.; Viñas, C.; Teixidor, F.; Victori, L.; Kivekäs, R.; Sillanpää, R. C−C Plasticity in Boron Chemistry: Modulation of the Cc···Cc Distance in Mixed Pyrrolyl/Dicarbollide Complexes. Organometallics 2001, 19, 4024–4030. [Google Scholar] [CrossRef]
- Oliva, J.M.; Allan, N.L.; Schleyer, P.v.R.; Viñas, C.; Teixidor, F. Strikingly Long C···C Distances in 1,2-Disubstituted ortho-Carboranes and Their Dianions. J. Am. Chem. Soc. 2005, 127, 13538–13547. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Sillanpää, R.; Pérez-Inestrosa, E.; Núñez, R. Synthesis and Characterization of New Fluorescent Styrene-Containing Carborane Derivatives: The Singular Quenching Role of a Phenyl Substituent. Chem. Eur. J. 2012, 18, 544–553. [Google Scholar] [CrossRef] [PubMed]




| Compd. | λabs1/nm (ε × 10−3 M−1 cm−1) | λex/nm | λem/nm | |||
|---|---|---|---|---|---|---|
| THF2 | 77 K1 | film3 | fw = 90%4 | |||
| 1F | 329 (3.5), 279 (26.1) | 329 | -8 | 541 | 543 | 552 |
| 2P | 328 (4.9), 279 (30.3) | 328 | -8 | 528 | 545 | 557 |
| 3M | 330 (3.2), 279 (22.2) | 330 | -8 | 544 | 542 | 559 |
| 4T | 333 (3.8), 279 (30.9) | 333 | -8 | 543 | 549 | 556 |
| Compd. | Φem5 | τobs3/ns | kr3,6/× 108 s−1 | knr3,7/× 107 s−1 | ||
| film3 | fw = 90%4 | |||||
| 1F | 0.34 | 0.10 | 6.8 | 0.50 | 9.7 | |
| 2P | 0.44 | 0.20 | 5.9 | 0.75 | 9.5 | |
| 3M | 0.51 | 0.35 | 5.2 | 0.98 | 9.4 | |
| 4T | 0.61 | 0.47 | 4.2 | 1.5 | 9.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lee, J.H.; Mun, M.S.; Yi, S.; Yoo, E.; Hwang, H.; Lee, K.M. Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores. Molecules 2021, 26, 1763. https://doi.org/10.3390/molecules26061763
Lee SH, Lee JH, Mun MS, Yi S, Yoo E, Hwang H, Lee KM. Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores. Molecules. 2021; 26(6):1763. https://doi.org/10.3390/molecules26061763
Chicago/Turabian StyleLee, Seok Ho, Ji Hye Lee, Min Sik Mun, Sanghee Yi, Eunji Yoo, Hyonseok Hwang, and Kang Mun Lee. 2021. "Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores" Molecules 26, no. 6: 1763. https://doi.org/10.3390/molecules26061763
APA StyleLee, S. H., Lee, J. H., Mun, M. S., Yi, S., Yoo, E., Hwang, H., & Lee, K. M. (2021). Influence of Electronic Environment on the Radiative Efficiency of 9-Phenyl-9H-carbazole-Based ortho-Carboranyl Luminophores. Molecules, 26(6), 1763. https://doi.org/10.3390/molecules26061763






