LC and NMR Studies for Identification and Characterization of Degradation Byproducts of Olmesartan Acid, Elucidation of Their Degradation Pathway and Ecotoxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug and Reagents
2.2. Chlorination Reaction
2.2.1. Apparatus and Equipment
2.2.2. Chlorination Experiments
2.2.3. Chlorination Procedure and Product Isolation
2.3. Ecotoxicity Data
3. Results and Discussion
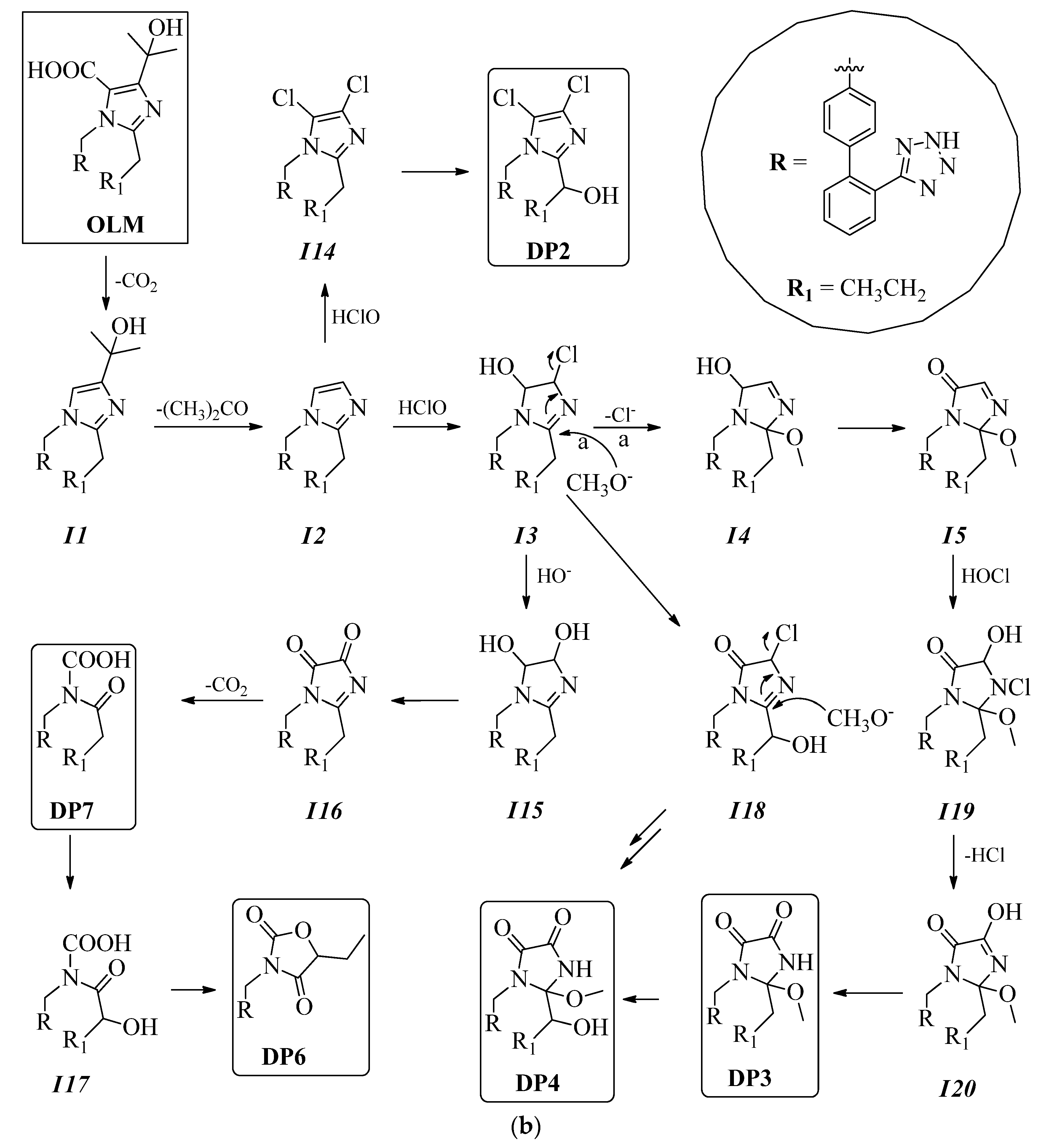
3.1. Chlorination Experiments
3.2. Structure Elucidation of Degradation Byproducts DP1–DP9
3.3. Spectral Data
3.4. Ecotoxicity Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- Kahn, G.; Vercellotti, J.R. CARB 100-Commercial applications of powdered activated carbons for decolorizing food products such as fruit juice concentrates and sugar. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2008; Volume 235, p. 1155. [Google Scholar]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Endocrine-disrupting compounds: Occurrence, detection methods, effects and promising treatment pathways—A critical review. J. Environ. Chem. Eng. 2020, 9, 104558. [Google Scholar] [CrossRef]
- Valdez-Carrillo, M.; Abrell, L.; Ramírez-Hernández, J.; Reyes-López, J.A.; Carreón-Diazconti, C. Pharmaceuticals as emerging contaminants in the aquatic environment of Latin America: A review. Environ. Sci. Pollut. Res. 2020, 27, 44863–44891. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.D.; Cassada, D.A.; Biswas, S.; Malakar, A.; D’Alessio, M.; Marshall, A.H.; Sallach, J.B. Detection, occurrence, and fate of emerging contaminants in agricultural environments. Water Environ. Res. 2020, 92, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Niu, L.; Zhu, S.; Lu, H.; Liu, W. Occurrence, abundance, and distribution of sulfonamide and tetracycline resistance genes in agricultural soils across China. Sci. Total Environ. 2017, 599, 1977–1983. [Google Scholar] [CrossRef]
- Li, P.; Wu, Y.; He, Y.; Zhang, B.; Huang, Y.; Yuan, Q.; Chen, Y. Occurrence and fate of antibiotic residues and antibiotic resistance genes in a reservoir with ecological purification facilities for drinking water sources. Sci. Total Environ. 2020, 707, 135276. [Google Scholar] [CrossRef]
- Mathew, R.A.; Kanmani, S. A review on emerging contaminants in indian waters and their treatment technologies. Nat. Environ. Pollut. Technol. 2020, 19, 549–562. [Google Scholar] [CrossRef]
- Jean, J.; Perrodin, Y.; Pivot, C.; Trepo, D.; Perraud, M.; Droguet, J.; Tissot-Guerraz, F.; Locher, F. Identification and prioritization of bioaccumulable pharmaceutical substances discharged in hospital effluents. J. Environ. Manag. 2012, 103, 113–121. [Google Scholar] [CrossRef]
- Fowler, P.A.; Bellingham, M.; Sinclair, K.D.; Evans, N.P.; Pocar, P.; Fischer, B.; Schaedlich, K.; Schmidt, J.-S.; Amezaga, M.R.; Bhattacharya, S.; et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 2012, 355, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hess-Wilson, J.; Knudsen, K. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006, 241, 1–12. [Google Scholar] [CrossRef]
- Villa, S.; Di Nica, V.; Castiglioni, S.; Finizio, A. Environmental risk classification of emerging contaminants in an alpine stream influenced by seasonal tourism. Ecol. Indic. 2020, 115, 106428. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghmaeian, K.; Moussavi, G.; Alahabadi, A. Removal of amoxicillin from contaminated water using NH 4 Cl-activated carbon: Continuous flow fixed-bed adsorption and catalytic ozonation regeneration. Chem. Eng. J. 2014, 236, 538–544. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M. Ángeles; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Lor, E.G.; Bijlsma, L.; Morales, E.; Pastor, L.; Hernández, F. Removal of emerging contaminants in sewage water subjected to advanced oxidation with ozone. J. Hazard. Mater. 2013, 260, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.; Park, C.M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Nogales, R.; Romero, E. Innovative application of biobed bioremediation systems to remove emerging contaminants: Adsorption, degradation and bioaccesibility. Sci. Total Environ. 2019, 651, 990–997. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Letzel, T.; Bayer, A.; Schulz, W.; Heermann, A.; Lucke, T.; Greco, G.; Grosse, S.; Schüssler, W.; Sengl, M.; Letzel, M. LC—MS screening techniques for wastewater analysis and analytical data handling strategies: Sartans and their transformation products as an example. Chemosphere 2015, 137, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef]
- Schwocho, L.R.; Masonson, H.N. Pharmacokinetics of CS-866, a New Angiotensin II Receptor Blocker, in Healthy Subjects. J. Clin. Pharmacol. 2001, 41, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, U.; Paffrath, D. Report on Pharmaceutical Prescriptions; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Al-Rajab, A.J.; Al Bratty, M.; Hakami, O.; A Alhazmi, H.; Sharma, M.; Reddy, D.N. Investigation of the presence of pharmaceuticals and personal care products (PPCPs) in groundwater of Jazan area, Saudi Arabia. Trop. J. Pharm. Res. 2018, 17, 2061. [Google Scholar] [CrossRef] [Green Version]
- Zarrelli, A.; DellaGreca, M.; Iesce, M.R.; Lavorgna, M.; Temussi, F.; Schiavone, L.; Criscuolo, E.; Parrella, A.; Previtera, L.; Isidori, M. Ecotoxicological evaluation of caffeine and its derivatives from a simulated chlorination step. Sci. Total Environ. 2014, 470, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Chusaksri, S.; Sutthivaiyakit, S.; Sedlak, D.L.; Sutthivaiyakit, P. Reactions of phenylurea compounds with aqueous chlorine: Implications for herbicide transformation during drinking water disinfection. J. Hazard. Mater. 2012, 209, 484–491. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Sandín-España, P.; Magrans, J.O.; García-Baudín, J.M. Study of clethodim degradation and by-product formation in chlorinated water by HPLC. Chromatographia 2005, 62, 133–137. [Google Scholar] [CrossRef]
- Luongo, G.; Previtera, L.; Ladhari, A.; Di Fabio, G.; Zarrelli, A. Peracetic Acid vs. Sodium Hypochlorite: Degradation and Transformation of Drugs in Wastewater. Molecules 2020, 25, 2294. [Google Scholar] [CrossRef]
- Luongo, G.; Guida, M.; Siciliano, A.; Libralato, G.; Saviano, L.; Amoresano, A.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Oxidation of diclofenac in water by sodium hypochlorite: Identification of new degradation by-products and their ecotoxicological evaluation. J. Pharm. Biomed. Anal. 2021, 194, 113762. [Google Scholar] [CrossRef]
- Bedner, M.; MacCrehan, W.A. Transformation of Acetaminophen by Chlorination Produces the Toxicants 1,4-Benzoquinone and N-Acetyl-p-benzoquinone Imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, I.; Castro, G.; Rodríguez, I.; Cela, R. Free chlorine reactions of angiotensin II receptor antagonists: Kinetics study, transformation products elucidation and in-silico ecotoxicity assessment. Sci. Total Environ. 2019, 647, 1000–1010. [Google Scholar] [CrossRef]
- ISO. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Aliivibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria; 30ISO 11348-3; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- ISO. Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae; ISO 8692; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Romanucci, V.; Siciliano, A.; Galdiero, E.; Guida, M.; Luongo, G.; Liguori, R.; Di Fabio, G.; Previtera, L.; Zarrelli, A. Disinfection by-Products and Ecotoxic Risk Associated with Hypochlorite Treatment of Tramadol. Molecules 2019, 24, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrelli, A.; DellaGreca, M.; Parolisi, A.; Iesce, M.R.; Cermola, F.; Temussi, F.; Isidori, M.; Lavorgna, M.; Passananti, M.; Previtera, L. Chemical fate and genotoxic risk associated with hypochlorite treatment of nicotine. Sci. Total Environ. 2012, 426, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trampuž, M.; Stavber, G.; Likozar, B. Catalyst-free aza-Michael addition for C–N coupling in active pharmaceutical ingredient synthesis: Modelling of thermodynamic, reaction kinetics and mass transfer considerations. Chem. Eng. J. 2019, 374, 924–936. [Google Scholar] [CrossRef]
- Grom, M.; Stavber, G.; Drnovšek, P.; Likozar, B. Modelling chemical kinetics of a complex reaction network of active pharmaceutical ingredient (API) synthesis with process optimization for benzazepine heterocyclic compound. Chem. Eng. J. 2016, 283, 703–716. [Google Scholar] [CrossRef]
- Robnik, B.; Likozar, B.; Wang, B.; Ljubin, T.S.; Časar, Z. Understanding and Kinetic Modeling of Complex Degradation Pathways in the Solid Dosage Form: The Case of Saxagliptin. Pharmaceutics 2019, 11, 452. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Konno, H.; Fukutsu, N.; Onodera, M.; Kawasaki, T.; Kusu, F. Identification of a degradation product in stressed tablets of olmesartan medoxomil by the complementary use of HPLC hyphenated techniques. J. Pharm. Biomed. Anal. 2008, 47, 553–559. [Google Scholar] [CrossRef]
- Lofrano, G.; Libralato, G.; Carotenuto, M.; Guida, M.; Inglese, M.; Siciliano, A.; Meriç, S. Emerging Concern from Short-Term Textile Leaching: A Preliminary Ecotoxicological Survey. Bull. Environ. Contam. Toxicol. 2016, 97, 646–652. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luongo, G.; Siciliano, A.; Libralato, G.; Serafini, S.; Saviano, L.; Previtera, L.; Di Fabio, G.; Zarrelli, A. LC and NMR Studies for Identification and Characterization of Degradation Byproducts of Olmesartan Acid, Elucidation of Their Degradation Pathway and Ecotoxicity Assessment. Molecules 2021, 26, 1769. https://doi.org/10.3390/molecules26061769
Luongo G, Siciliano A, Libralato G, Serafini S, Saviano L, Previtera L, Di Fabio G, Zarrelli A. LC and NMR Studies for Identification and Characterization of Degradation Byproducts of Olmesartan Acid, Elucidation of Their Degradation Pathway and Ecotoxicity Assessment. Molecules. 2021; 26(6):1769. https://doi.org/10.3390/molecules26061769
Chicago/Turabian StyleLuongo, Giovanni, Antonietta Siciliano, Giovanni Libralato, Sara Serafini, Lorenzo Saviano, Lucio Previtera, Giovanni Di Fabio, and Armando Zarrelli. 2021. "LC and NMR Studies for Identification and Characterization of Degradation Byproducts of Olmesartan Acid, Elucidation of Their Degradation Pathway and Ecotoxicity Assessment" Molecules 26, no. 6: 1769. https://doi.org/10.3390/molecules26061769







