Solvent-Controlled Self-Assembled Oligopyrrolic Receptor †
Abstract
:1. Introduction
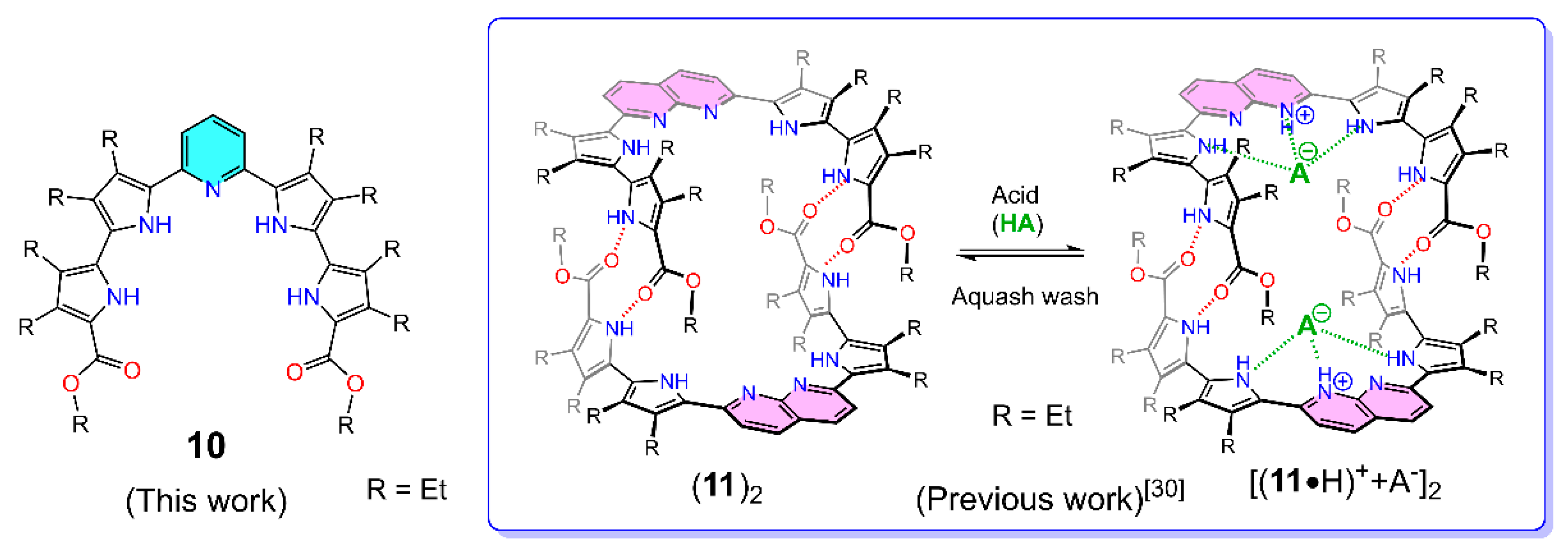
2. Results and Discussion
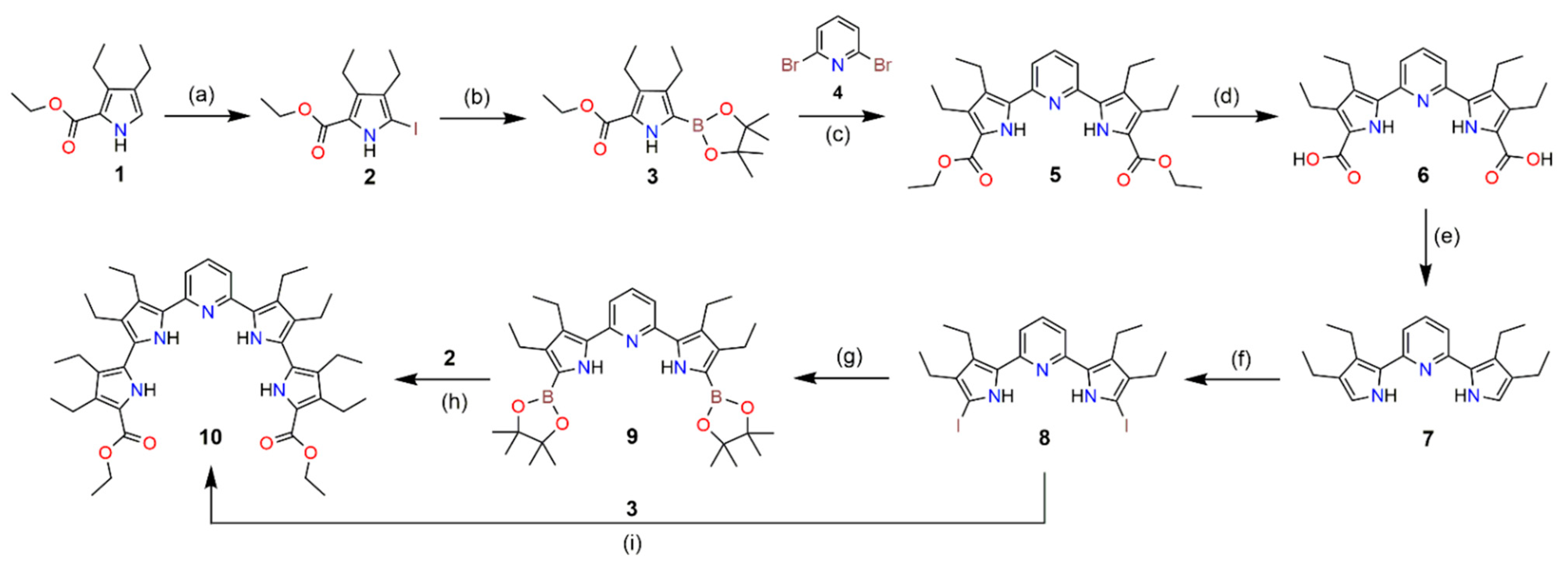
2.1. Synthesis
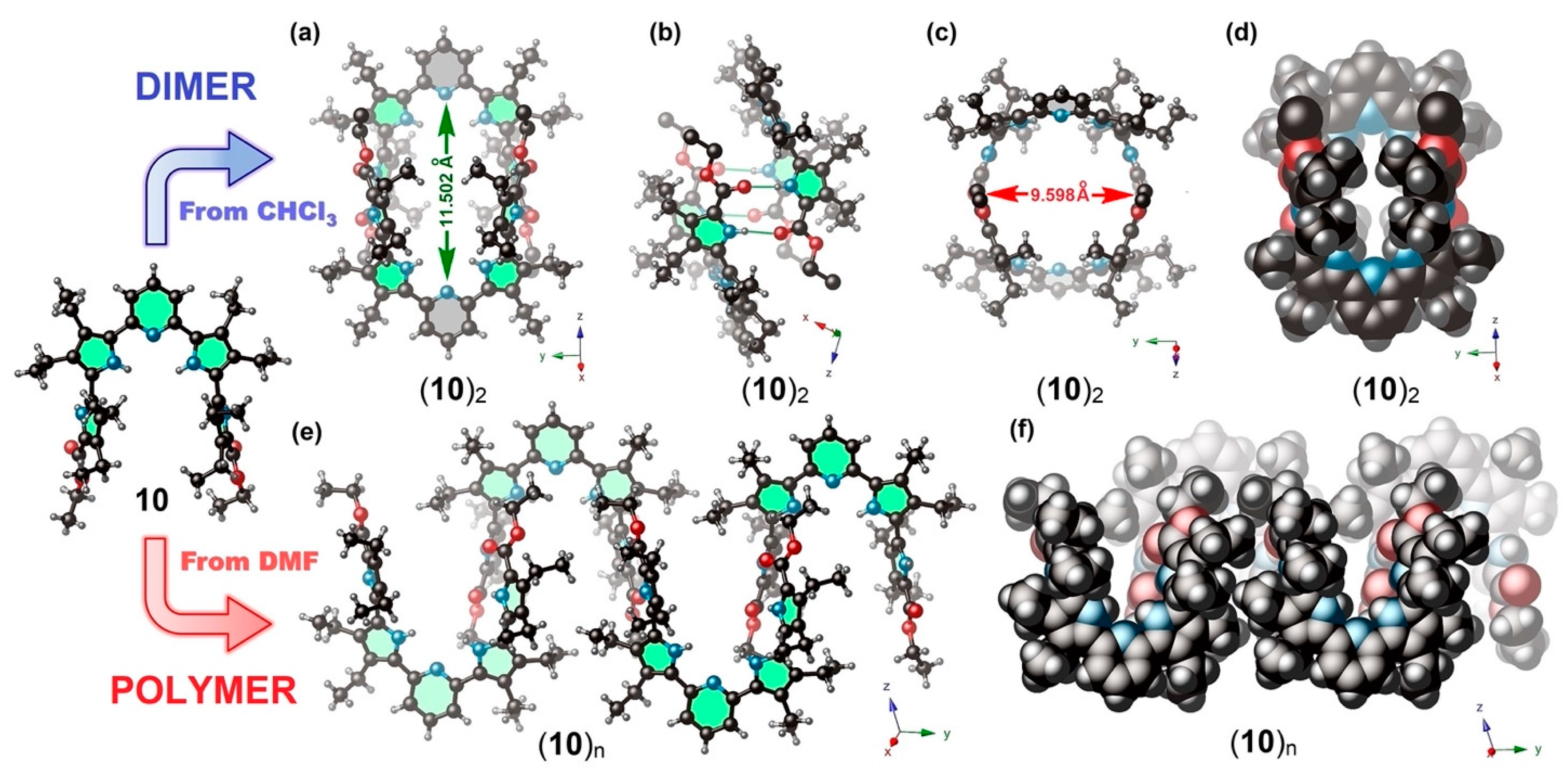
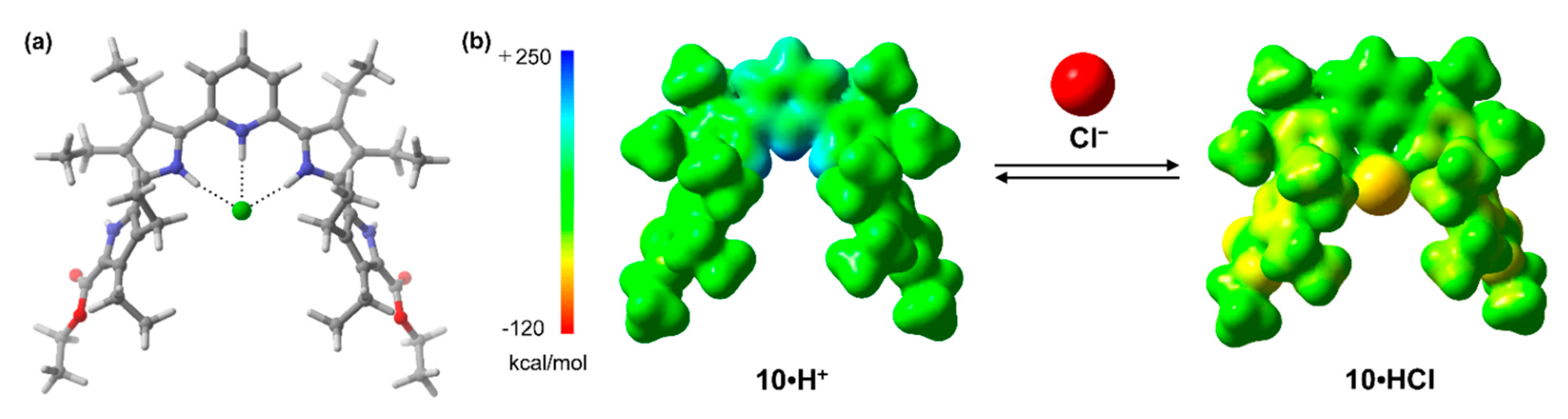
2.2. X-ray Crystallography
2.3. Structural Behavior in Solution
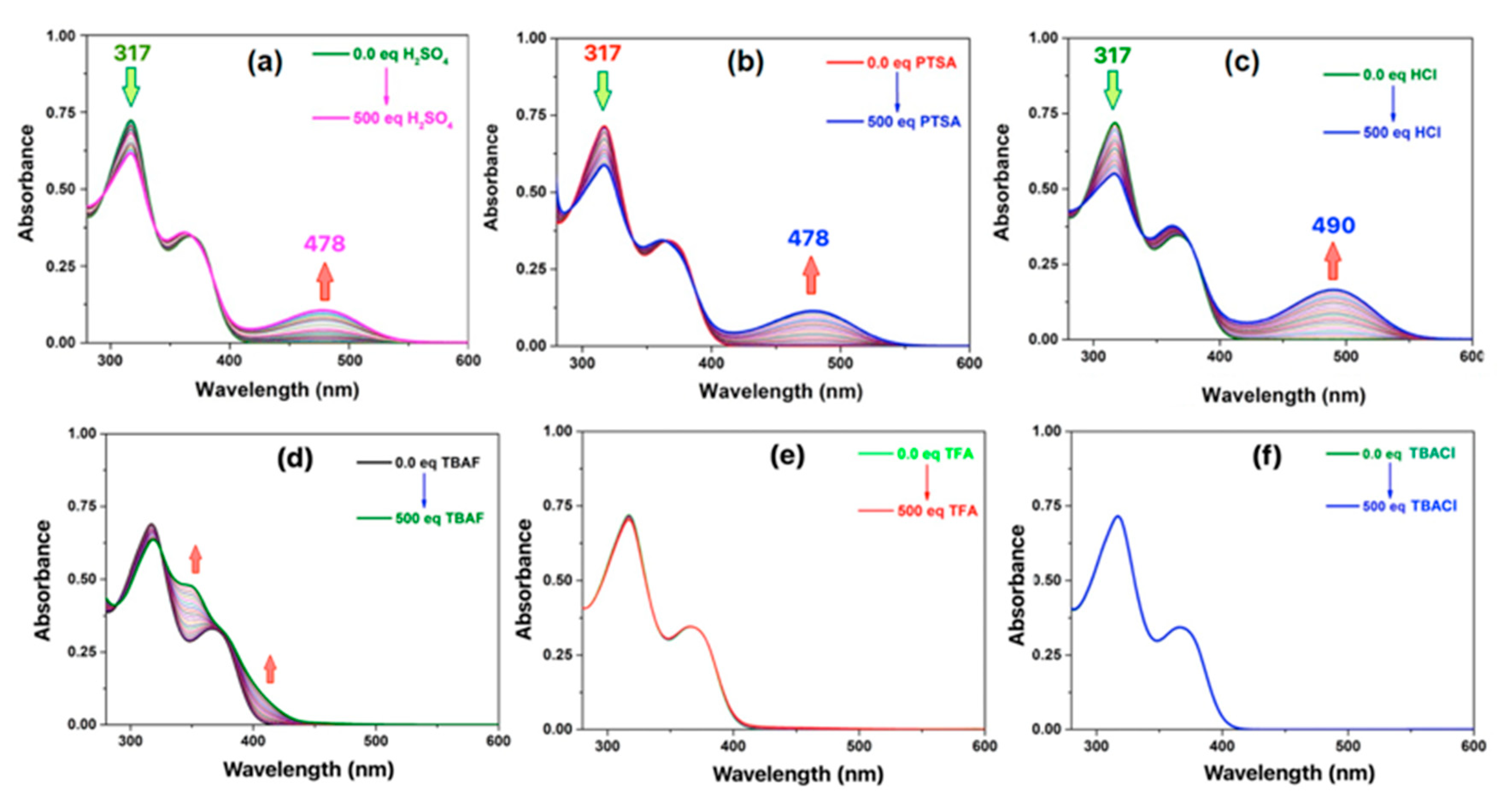
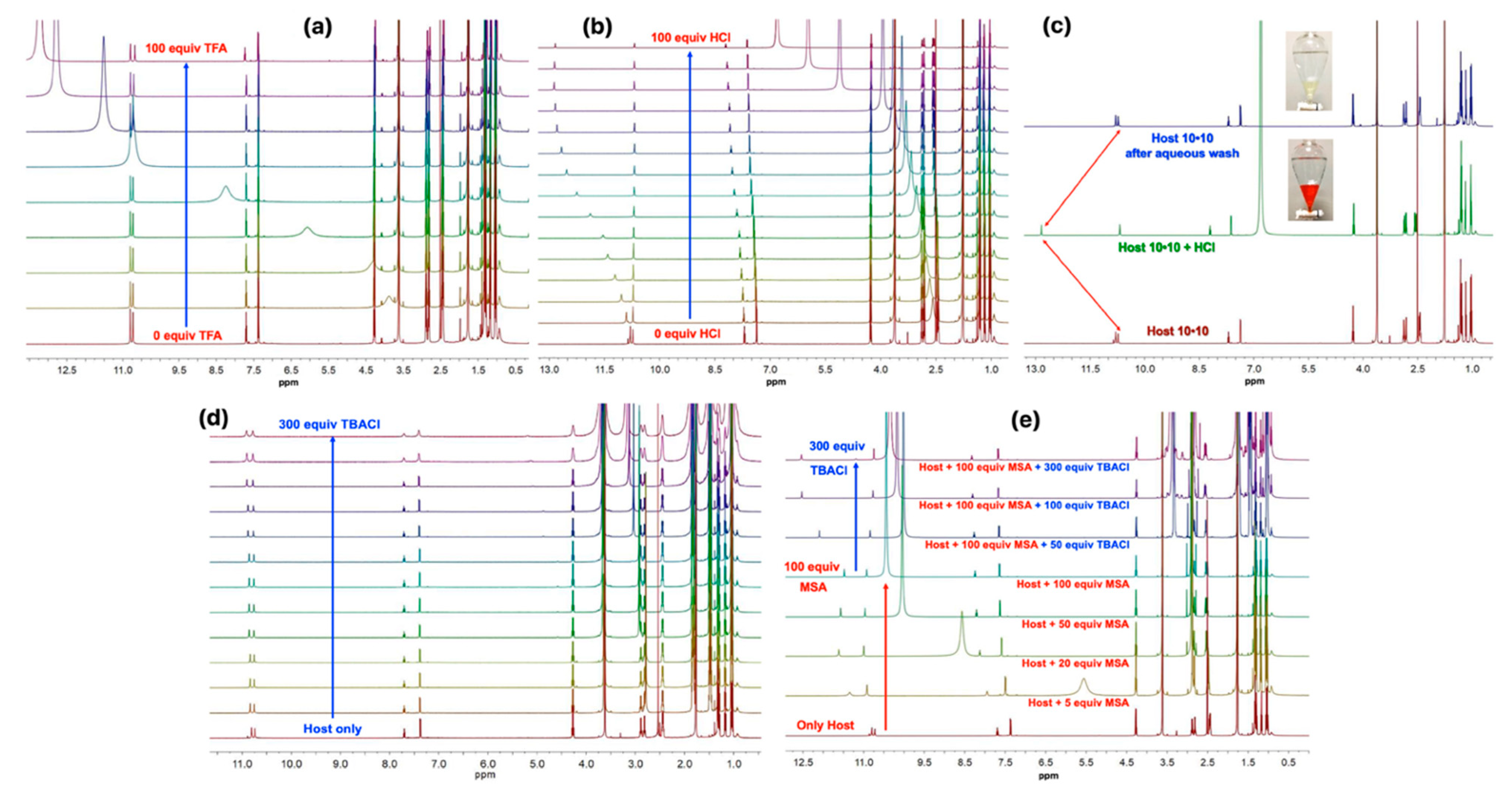
2.4. Acid Recognition Studies Using Spectroscopy
2.5. Theoretical Studies
3. Materials and Methods
3.1. General Experiments
3.1.1. Chemicals and Consumables
- Iodine (I2) (CAS No: 7553-56-2), Triethylamine (Et3N) (CAS No: 121-44-8), Potassium carbonate (K2CO3) (CAS No: 584-08-7), Sodium bicarbonate (NaHCO3) (CAS No: 144-55-8), Sodium thiosulfate (Na2S2O3) (CAS No: 7772-98-7), Dichloromethane (CH2Cl2) (CAS No: 75-09-2) were purchased from Adamas-beta®, Shanghai, China.
- Sodium hydroxide (NaOH) (CAS No: 1310-73-2), Ethanol (EtOH) (CAS No: 64-17-5) were purchased from Energy Chemical, Shanghai, China.
- Palladium(II) acetate, Pd(OAc)2 (CAS No: 3375-31-3), Tetrakis(triphenylphosphine)palladium, Pd(PPh3)4 (CAS No: 14221-01-3), Triphenylphosphine, PPh3 (CAS No: 603-35-0), Dichlorobis(triphenylphosphine)palladium(II) (PdCl2(PPh3)2) (CAS No: 13965-03-2) were purchased from Merck-Sigma, Shanghai, China.
- Potassium iodide (KI) (CAS No: 7681-11-0), N,N-Dimethylformamide (DMF) (CAS No: 68-12-2), Ethylene glycol (EG) (CAS No: 107-21-1), 1,4-Dioxane (CAS No: 123-91-1) were purchased from Acros Organics, NJ, USA.
- Pinacolborane, HB(pin) (CAS No: 25015-63-8), 2,6-Dibromopyridine (CAS No: 626-05-1) were purchased from TCI, Tokyo, Japan.
- All anhydrous solvents were either purchased from Sigma Aldrich, Shanghai, China or Acros Organics Shanghai China or collected from the solvent purification plant at the Center for Supramolecular Chemistry and Catalyses, Shanghai University. All reactions were carried out under an argon atmosphere unless noted otherwise.
- Chromatographic separations were performed by using 100–200 mesh silica gel obtained from Merck-Sigma, Darmstadt, Germany.
- The thin-layer chromatographic (TLC) analyses were carried out using Silica Gel 60 F245 glass sheets, which were used to monitor the progress of the reactions and were purchased from Merck-Sigma, Darmstadt, Germany.
- Final separations of the compounds were performed using a recycling preparative gel permeation chromatography (GPC) from Japan Analytical Industries (JAI), Hiroshima Japan using THF as the mobile phase. The oligopyrrole derivatives 1–9 were synthesized with moderate to good yields by following the procedures in the literature [30,31,32].
3.1.2. Investigation and Characterization
3.2. Synthesis
Synthesis of Acyclic Receptor 10
3.3. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hof, F.; Rebek, J.J. Molecules within molecules: Recognition through self-assembly. Proc. Natl. Acad. Sci. USA 2002, 99, 4775–4777. [Google Scholar] [CrossRef] [Green Version]
- Ebbing, M.H.; Villa, M.J.; Valpuesta, J.M.; Prados, P.; de Mendoza, J. Resorcinarenes with 2-benzimidazolone bridg-ES: Self-aggregation, self-assembled dimeric capsules, and guest encapsulation. Proc. Natl. Acad. Sci. USA 2002, 99, 4962–4966. [Google Scholar] [CrossRef] [Green Version]
- Ajami, D.; Rebek, J., Jr. More chemistry in small space. Acc. Chem. Res. 2013, 46, 990–999. [Google Scholar] [CrossRef]
- Wu, N.-W.; Rebek, J.J. Cavitands as chaperones for monofunctional and ring-forming reactions in water. J. Am. Chem. Soc. 2016, 138, 7512–7515. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Du, L.; He, Y.; Jin, X.-H.; Pearce, S.; Eloi, J.-C.; Harniman, R.L.; Alibhai, D.; Ye, R.; et al. Tailored self-assembled photocatalytic nanofibres for visible-light-driven hydrogen production. Nat. Chem. 2020, 12, 1150–1156. [Google Scholar] [CrossRef]
- Fujita, D.; Suzuki, K.; Sato, S.; Yagi-Utsumi, M.; Yamaguchi, Y.; Mizuno, N.; Kumasaka, T.; Takata, M.; Noda, M.; Uchiyama, S.; et al. Protein encapsulation within synthetic molecular hosts. Nat. Commun. 2012, 3, 1093. [Google Scholar] [CrossRef]
- Schmidt, A.; Molano, V.; Hollering, M.; Pöthig, A.; Casini, A.; Kühn, F.E. Evaluation of new palladium cages as potential delivery systems for the anticancer drug cisplatin. Chem. A Eur. J. 2016, 22, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Woods, B.; Wenzel, M. The promise of self-assembled 3D supramolecular coordination complexes for biomedical applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J.L.; Meng, Q.; Li, C. Supramolecular combination chemotherapy: A PH-responsive co-encapsulation drug delivery system. Chem. Sci. 2020, 11, 6275–6282. [Google Scholar] [CrossRef]
- He, Q.; Tu, P.; Sessler, J.L. Supramolecular chemistry of anionic dimers, trimers, tetramers, and clusters. Chem 2018, 4, 46–93. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; He, Q.; Vargas-Zúñiga, G.I.; Qin, L.; Hwang, I.; Kim, S.K.; Heo, N.J.; Lee, C.-H.; Dutta, R.; Sessler, J.L. Strapped Calix[4]pyrroles: From synthesis to applications. Chem. Soc. Rev. 2020, 49, 865–907. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host–guest interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Wyler, R.; De Mendoza, J.; Rebek, J. A synthetic cavity assembles through self-complementary hydrogen bonds. Angew. Chem. Int. Ed. 1993, 32, 1699–1701. [Google Scholar] [CrossRef]
- Ajami, D.; Rebek, J. Longer guests drive the reversible assembly of hyperextended capsules. Angew. Chem. Int. Ed. 2007, 46, 9283–9286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-D.; Ajami, D.; Rebek, J. Hydrogen-bonded capsules in water. J. Am. Chem. Soc. 2013, 135, 18064–18066. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M. Metal-directed self-assembly of two- and three-dimensional synthetic receptors. Chem. Soc. Rev. 1998, 27, 417–425. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-assembly of discrete cyclic nanostructures mediated by transition met-als. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Dalgarno, S.J.; Power, N.P.; Atwood, J.L. Metallo-supramolecular capsules. Co-ord. Chem. Rev. 2008, 252, 825–841. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.; Brewster, J.T.; Chi, X.; Lynch, V.M.; Sessler, J.L. Cation-based structural tuning of pyridine dipyrrolate cages and morphological control over their self-assembly. J. Am. Chem. Soc. 2019, 141, 4749–4755. [Google Scholar] [CrossRef]
- Kusukawa, T.; Fujita, M. “Ship-in-a-bottle” formation of stable hydrophobic dimers of cis-azobenzene and -stilbene derivatives in a self-assembled coordination nanocage. J. Am. Chem. Soc. 1999, 121, 1397–1398. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Cacity-directed, highly stereoselective [2+2] photodimeri-zation of olefins within self-assembled coordination cages. Angew. Chem. Int. Ed. 2002, 41, 1347–1349. [Google Scholar] [CrossRef]
- Ueda, Y.; Ito, H.; Fujita, D.; Fujita, M. Permeable self-assembled molecular containers for catalyst isolation ena-bling two-step cascade reactions. J. Am. Chem. Soc. 2017, 139, 6090–6093. [Google Scholar] [CrossRef]
- Chi, X.; Peters, G.M.; Hammel, F.; Brockman, C.; Sessler, J.L. Molecular recognition under interfacial conditions: Calix[4]pyrrole-based cross-linkable micelles for ion pair extraction. J. Am. Chem. Soc. 2017, 139, 9124–9127. [Google Scholar] [CrossRef]
- Ji, X.; Chi, X.; Ahmed, M.; Long, L.; Sessler, J.L. Soft materials constructed using calix[4]pyrrole- and “Texas-sized” box-based anion receptors. Acc. Chem. Res. 2019, 52, 1915–1927. [Google Scholar] [CrossRef]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion pair receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirata, O.; Takeuchi, M.; Shinkai, S. Highly enantioselective recognition of dicarboxylic acid substrates by the control of nonlinear responses. J. Am. Chem. Soc. 2006, 128, 16008–16009. [Google Scholar] [CrossRef] [PubMed]
- Engle, J.M.; Lakshminarayanan, P.S.; Carroll, C.N.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W. Molecular self-assembly: Solvent guests tune the conformation of a series of 2,6-bis(2-anilinoethynyl)pyridine-based ureas. Cryst. Growth Des. 2011, 11, 5144–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Kim, D.S.; Lin, C.-Y.; Zhang, H.; Lammer, A.D.; Lynch, V.M.; Popov, I.; Miljanić, O.Š.; Anslyn, E.V.; Sessler, J.L. Expanded porphyrin-anion supramolecular assemblies: Environmentally responsive sensors for organic SOL-vents and anions. J. Am. Chem. Soc. 2015, 137, 7769–7774. [Google Scholar] [CrossRef] [PubMed]
- Vonnegut, C.L.; Shonkwiler, A.M.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W. Harnessing solid-state packing for selective detection of chloride in a macrocyclic anionophore. Chem. Commun. 2016, 52, 9506–9509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Sen, S.; Chen, C.; Bähring, S.; Lei, C.; Duan, Z.; Zhang, Z.; Sessler, J.L.; Jana, A. Self-assembled cagelike receptor that binds biologically relevant dicarboxylic acids via proton-coupled anion recognition. J. Am. Chem. Soc. 2019, 142, 1987–1994. [Google Scholar] [CrossRef]
- Setsune, J.-I.; Toda, M.; Watanabe, K.; Panda, P.K.; Yoshida, T. Synthesis of bis(pyrrol-2-yl)arenes by Pd-catalyzed cross coupling. Tetrahedron Lett. 2006, 47, 7541–7544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lim, J.M.; Ishida, M.; Roznyatovskiy, V.V.; Lynch, V.M.; Gong, H.Y.; Yang, X.; Kim, D.; Sessler, J.L. Cy-clo[m]Pyridine[n]Pyrroles: Hybrid macrocycles that display expanded π-conjugation upon protonation. J. Am. Chem. Soc. 2012, 134, 4076–4079. [Google Scholar] [CrossRef]
- Spek, A.L. Platon squeeze: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindfit-Fit Data to 1:1, 1:2 and 2:1 Host-Guest Equilibria. Available online: http://supramolecular.org (accessed on 3 January 2021).
- Thordarson, P. Determining association constants from titration experiments in supremolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 16, Revision, C.01; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interaction by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Turi, L.; Dannenberg, J.J. Correcting for basis set superposition error in aggregates containing more than two molecules: Ambiguities in the calculation of the counterpoise correction. J. Phys. Chem. 1993, 97, 2488–2490. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, 1.0 b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: http://www.cylview.org (accessed on 7 January 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liang, K.; Larsen, M.C.; Bähring, S.; Ishida, M.; Furuta, H.; Jana, A. Solvent-Controlled Self-Assembled Oligopyrrolic Receptor. Molecules 2021, 26, 1771. https://doi.org/10.3390/molecules26061771
Wang F, Liang K, Larsen MC, Bähring S, Ishida M, Furuta H, Jana A. Solvent-Controlled Self-Assembled Oligopyrrolic Receptor. Molecules. 2021; 26(6):1771. https://doi.org/10.3390/molecules26061771
Chicago/Turabian StyleWang, Fei, Kejiang Liang, Mads Christian Larsen, Steffen Bähring, Masatoshi Ishida, Hiroyuki Furuta, and Atanu Jana. 2021. "Solvent-Controlled Self-Assembled Oligopyrrolic Receptor" Molecules 26, no. 6: 1771. https://doi.org/10.3390/molecules26061771






