PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors
Abstract
1. Introduction
1.1. Multimeric Radioligands
1.2. Multimerization of Cyclic RGD Peptides
2. Preclinical Studies of Multimeric Cyclic RGD Peptides
2.1. 18F Labeled Cyclic RGD Multimers
2.2. PEG Linkers on 18F Labeled Cyclic RGD Multimers
2.3. Sugar Amino Acid Linkers on 18F Labeled c(RGD) Multimers
2.4. The Effect of Linkers on the Stability and Production of 18F Labeled Cyclic RGD Multimers
2.5. 64Cu Labeled c(RGD) Multimers
2.6. 68Ga-Labeled RGD Multimers
2.7. Clinically Applied RGD Multimers
3. Discussion
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Handl, H.L.; Vagner, J.; Han, H.; Mash, E.; Hruby, V.J.; Gillies, R.J. Hitting multiple targets with multimeric ligands. Expert Opin. Ther. Targets. 2004, 8, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Crothers, D.M.; Metzger, H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry 1972, 9, 341–357. [Google Scholar] [CrossRef]
- Yim, C.-B.; Van Der Wildt, B.; Dijkgraaf, I.; Joosten, L.; Eek, A.; Versluis, C.; Rijkers, D.T.S.; Boerman, O.C.; Liskamp, R.M.J. Spacer Effects on in vivo Properties of DOTA-Conjugated Dimeric [Tyr3]Octreotate Peptides Synthesized by a “CuI-Click” and “Sulfo-Click” Ligation Method. ChemBioChem 2011, 12, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.-C.; Schäfer, M.; Bauder-Wüst, U.; Wacker, A.; Schmidt, J.; Liolios, C.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Kopka, K.; et al. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjugate Chem. 2017, 28, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.C.; Fragogeorgi, E.A.; Zikos, C.; Loudos, G.; Xanthopoulos, S.; Bouziotis, P.; Paravatou-Petsotas, M.; Livaniou, E.; Varvarigou, A.D.; Sivolapenko, G.B. Structural modifications of 99mTc-labelled bombesin-like peptides for optimizing pharmacokinetics in prostate tumor targeting. Int. J. Pharm. 2012, 430, 1–17. [Google Scholar] [CrossRef]
- Kubas, H.; Schäfer, M.; Bauder-Wüst, U.; Eder, M.; Oltmanns, D.; Haberkorn, U.; Mier, W.; Eisenhut, M. Multivalent cyclic RGD ligands: Influence of linker lengths on receptor binding. Nucl. Med. Biol. 2010, 37, 885–891. [Google Scholar] [CrossRef]
- Maresca, K.P.; Hillier, S.M.; Femia, F.J.; Keith, D.; Barone, C.; Joyal, J.L.; Zimmerman, C.N.; Kozikowski, A.P.; Barrett, J.A.; Eckelman, W.C.; et al. A Series of Halogenated Heterodimeric Inhibitors of Prostate Specific Membrane Antigen (PSMA) as Radiolabeled Probes for Targeting Prostate Cancer. J. Med. Chem. 2009, 52, 347–357. [Google Scholar] [CrossRef]
- Liolios, C.; Buchmuller, B.; Bauder-Wüst, U.; Schäfer, M.; Leotta, K.; Haberkorn, U.; Eder, M.; Kopka, K.; Buchmuler, B.; Schaefer, M. Monomeric and Dimeric 68Ga-Labeled Bombesin Analogues for Positron Emission Tomography (PET) Imaging of Tumors Expressing Gastrin-Releasing Peptide Receptors (GRPrs). J. Med. Chem. 2018, 61, 2062–2074. [Google Scholar] [CrossRef]
- Liolios, C.; Shegani, A.; Roupa, I.; Kiritsis, C.; Makarem, A.; Paravatou-Petsotas, M.; Pelecanou, M.; Bouziotis, P.; Papadopoulos, M.; Kopka, K.; et al. Synthesis, characterization and evaluation of 68Ga labelled monomeric and dimeric quinazoline derivatives of the HBED-CC chelator targeting the epidermal growth factor receptor. Bioorganic Chem. 2020, 100, 103855. [Google Scholar] [CrossRef]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef]
- Cai, W.; Rao, J.; Gambhir, S.S.; Chen, X. How molecular imaging is speeding up antiangiogenic drug development. Mol. Cancer Ther. 2006, 5, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chen, K.; Conti, P.S. Target-specific delivery of peptide-based probes for PET imaging. Adv. Drug Deliv. Rev. 2010, 62, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Shinderman-Maman, E.; Cohen, K.J.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.J.; et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: Regulation of gene transcription and MAPK-dependent proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef]
- Meyer, A.; Van Golen, C.M.; Kim, B.; Van Golen, K.L.; Feldman, E.L. Integrin Expression Regulates Neuroblastoma Attachment and Migration. Neoplasia 2004, 6, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, R.; Bach-Gansmo, T.; Castell-Conesa, J.; McParland, B.J. An open-label, multicenter, phase 2a study to assess the feasibility of imaging metastases in late-stage cancer patients with the αvβ3-selective angiogenesis imaging agent 99mTc-NC100692. Acta Radiol. 2010, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, Y.; Gao, S.; Ji, T.; Zhang, H.; Ji, B.; Chen, B.; Jia, B.; Wang, F.; Xu, Z.; et al. 99mTc-3PRGD2 scintimammography in palpable and nonpalpable breast lesions. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Chen, X.; Park, R.; Tohme, M.; Shahinian, A.H.; Bading, J.R.; Conti, P.S. MicroPET and Autoradiographic Imaging of Breast Cancer αv-Integrin Expression Using 18F- and 64Cu-Labeled RGD Peptide. Bioconjugate Chem. 2004, 15, 41–49. [Google Scholar] [CrossRef]
- Huang, R.; Rofstad, E.K. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–14. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjugate Chem. 2009, 20, 2199–2213. [Google Scholar] [CrossRef]
- Shi, J.; Wang, F.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Poethko, T.; Schottelius, M.; Thumshirn, G.; Hersel, U.; Herz, M.; Henriksen, G.; Kessler, H.; Schwaiger, M.; Wester, H.J. Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J. Nucl. Med. 2004, 45, 892–902. [Google Scholar]
- Kaeopookum, P.; Petrik, M.; Summer, D.; Klinger, M.; Zhai, C.; Rangger, C.; Haubner, R.; Haas, H.; Hajduch, M.; Decristoforo, C. Comparison of 68Ga-labeled RGD mono- and multimers based on a clickable siderophore-based scaffold. Nucl. Med. Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Höltke, C.; Faust, A. Molecular imaging of integrins in oncology. Rep. Med. Imaging 2017, 10, 17–30. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled Peptides: Valuable Tools for the Detection and Treatment of Cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Rokugawa, T.; Konishi, H.; Ito, M.; Iimori, H.; Nagai, R.; Shimosegawa, E.; Hatazawa, J.; Abe, K. Evaluation of hepatic integrin αvβ3 expression in non-alcoholic steatohepatitis (NASH) model mouse by 18F-FPP-RGD2 PET. EJNMMI Res. 2018, 8, 40. [Google Scholar] [CrossRef]
- Lucie, S.; Elisabeth, G.; Stéphanie, F.; Guy, S.; Amandine, H.; Corinne, A.R.; Didier, B.; Catherine, S.; Alexeï, G.; Pascal, D.; et al. Clustering and Internalization of Integrin αvβ3 With a Tetrameric RGD-synthetic. Peptide. Mol. Ther. 2009, 17, 837–843. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; Kruijtzer, J.A.W.; Liu, S.; Soede, A.C.; Oyen, W.J.G.; Corstens, F.H.M.; Liskamp, R.M.J.; Boerman, O.C. Improved targeting of the αvβ3 integrin by multimerisation of RGD peptides. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 267–273. [Google Scholar] [CrossRef]
- Li, Z.-B.; Cai, W.; Cao, Q.; Chen, K.; Wu, Z.; Elhendy, A.; Chen, X. 64Cu-Labeled Tetrameric and Octameric RGD Peptides for Small-Animal PET of Tumor v 3 Integrin Expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Ermert, J.; Benešová, M.; Hugenberg, V.; Gupta, V.; Spahn, I.; Pietzsch, H.-J.; Liolios, C.; Kopka, K. Radiopharmaceutical Sciences. In Clinical Nuclear Medicine; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–191. [Google Scholar]
- Pascali, G.; Matesic, L.; Collier, T.L.; Wyatt, N.; Fraser, B.H.; Pham, T.Q.; Salvadori, P.A.; Greguric, I. Optimization of nucleophilic 18F radiofluorinations using a microfluidic reaction approach. Nat. Protoc. 2014, 9, 2017–2029. [Google Scholar] [CrossRef]
- Poethko, T.; Schottelius, M.; Thumshirn, G.; Herz, M.; Haubner, R.; Henriksen, G.; Kessler, H.; Schwaiger, M.; Wester, H.-J. Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochim. Acta 2004, 92, 317–327. [Google Scholar] [CrossRef]
- Chen, X.; Tohme, M.; Park, R.; Hou, Y.; Bading, J.R.; Conti, P.S. Micro-PET Imaging of αvβ3-Integrin Expression with 18F-Labeled Dimeric RGD Peptide. Mol. Imaging 2004, 3, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Jiang, H.; Xu, Y.; Zhang, H.; Cheng, Z. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.-B.; Cai, W.; He, L.; Chin, F.T.; Li, F.; Chen, X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): Synthesis and microPET imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Liu, S.; Gowrishankar, G.; Yaghoubi, S.; Wedgeworth, J.P.; Chin, F.; Berndorff, D.; Gekeler, V.; Gambhir, S.S.; Cheng, Z. Reproducibility study of [18F]FPP(RGD)2 uptake in murine models of human tumor xenografts. Eur. J. Nucl. Med. Mol. Imaging 2010, 38, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Li, W.; Guo, N.; Ma, Y.; Zhu, L.; Kiesewetter, D.O.; Shen, B.; Niu, G.; Chen, X. Comparison Study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET Imaging of U87MG Tumors in Mice. Bioconjugate Chem. 2011, 22, 2415–2422. [Google Scholar] [CrossRef]
- Guo, N.; Lang, L.; Li, W.; Kiesewetter, D.O.; Gao, H.; Niu, G.; Xie, Q.; Chen, X. Quantitative Analysis and Comparison Study of [18F]AlF-NOTA-PRGD2, [18F]FPPRGD2 and [68Ga]Ga-NOTA-PRGD2 Using a Reference Tissue Model. PLoS ONE 2012, 7, e037506. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Meng, X.; Sun, X.; Yuan, S.; Yu, J. 18F-alfatide positron emission tomography may predict anti-angiogenic responses. Oncol. Rep. 2018, 40, 2896–2905. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Gao, Y.; Zhang, J.; Fu, Z.; Zheng, J.; Liu, N.; Hu, X.; Hou, W.; Yu, J.; Yuan, S. Stereotactic Comparison Study of 18F-Alfatide and 18F-FDG PET Imaging in an LLC Tumor-Bearing C57BL/6 Mouse Model. Sci. Rep. 2016, 6, 28757. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, N.; Lang, L.; Kiesewetter, D.O.; Xie, Q.; Li, Q.; Eden, H.S.; Niu, G.; Chen, X. 18F-Alfatide II and 18F-FDG Dual Tracer Dynamic PET for Parametric, Early Prediction of Tumor Response to Therapy. J. Nucl. Med. 2013, 55, 154–160. [Google Scholar] [CrossRef]
- Bao, X.; Wang, M.-W.; Luo, J.-M.; Wang, S.-Y.; Zhang, Y.-P.; Zhang, Y.-J. Optimization of Early Response Monitoring and Prediction of Cancer Antiangiogenesis Therapy via Noninvasive PET Molecular Imaging Strategies of Multifactorial Bioparameters. Theranostics 2016, 6, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yue, X.; Lang, L.; Kiesewetter, D.O.; Li, F.; Zhu, Z.; Niu, G.; Chen, X. Longitudinal PET Imaging of Muscular Inflammation Using 18F-DPA-714 and 18F-Alfatide II and Differentiation with Tumors. Theranostics 2014, 4, 546–555. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Wang, F.; Liu, S.; Chen, X. Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1296–1307. [Google Scholar] [CrossRef]
- Guo, J.; Lang, L.; Hu, S.; Guo, N.; Zhu, L.; Sun, Z.; Ma, Y.; Kiesewetter, D.O.; Niu, G.; Xie, Q.; et al. Comparison of Three Dimeric 18F-AlF-NOTA-RGD Tracers. Mol. Imaging Biol. 2013, 16, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tang, X.; Tang, G.; Yao, S.; Yao, B.; Wang, H.; Nie, D.; Liang, X.; Tang, C.; He, S. 18F-FP-PEG2-β-Glu-RGD2: A Symmetric Integrin αvβ3-Targeting Radiotracer for Tumor PET Imaging. PLoS ONE 2015, 10, e0138675. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Chen, K.; Yan, Y.; Watzlowik, P.; Wester, H.-J.; Chin, F.T.; Chen, X. 18F-Labeled Galacto and PEGylated RGD Dimers for PET Imaging of αvβ3 Integrin Expression. Mol. Imaging Biol. 2009, 12, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Ma, Y.; Kiesewetter, D.O.; Chen, X. Stability Analysis of Glutamic Acid Linked Peptides Coupled to NOTA through Different Chemical Linkages. Mol. Pharm. 2014, 11, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Bouhlel, A.; Cantelli, C.; Garrigue, P.; Lisowski, V.; Guillet, B. A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling? Molecules 2019, 24, 2866. [Google Scholar] [CrossRef]
- Kang, F.; Wang, S.; Tian, F.; Zhao, M.; Zhang, M.; Wang, Z.; Li, G.; Liu, C.; Yang, W.; Li, X.; et al. Comparing the Diagnostic Potential of 68Ga-Alfatide II and 18F-FDG in Differentiating Between Non-Small Cell Lung Cancer and Tuberculosis. J. Nucl. Med. 2015, 57, 672–677. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zhang, X.; Teng, Z.; Wang, J.; Yung, B.C.; Niu, G.; Zhu, H.; Lu, G.; Chen, X. 18F-Alfatide II PET/CT for Identification of Breast Cancer: A Preliminary Clinical Study. J. Nucl. Med. 2018, 59, 1809–1816. [Google Scholar] [CrossRef]
- Mi, B.; Yu, C.; Pan, D.; Yang, M.; Wan, W.; Niu, G.; Chen, X. Pilot Prospective Evaluation of 18F-Alfatide II for Detection of Skeletal Metastases. Theranostics 2015, 5, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Pan, D.; Mi, B.; Xu, Y.; Lang, L.; Niu, G.; Yang, M.; Wan, W.; Chen, X. (18)F-Alfatide II PET/CT in healthy human volunteers and patients with brain metastases. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2021–2028. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Hou, Y.; Tohme, M.; Park, R.; Bading, J.R.; Conti, P.S. MicroPET imaging of breast cancer αv-integrin expression with Cu-labeled dimeric RGD peptides. Mol. Imaging Biol. 2004, 6, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Xiong, Z.; Cheng, Z.; Fisher, D.R.; Liu, S.; Gambhir, S.S.; Chen, X. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005, 46, 1707–1718. [Google Scholar] [PubMed]
- Hedhli, J.; Czerwiński, A.; Schuelke, M.; Płoska, A.; Sowinski, P.; La Hood, L.; Mamer, S.B.; Cole, J.A.; Czaplewska, P.; Banach, M.; et al. Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of αVβ3 Integrin Activity. Sci. Rep. 2017, 7, 3185. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kim, Y.-S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving Tumor Uptake and Pharmacokinetics of 64Cu-Labeled Cyclic RGD Peptide Dimers with Gly3 and PEG4 linkers. Bioconjugate Chem. 2009, 20, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Z.; Huang, C.-W.; Park, R.; Conti, P.S. 64Cu Labeled AmBaSar-RGD2 for micro-PET Imaging of Integrin αvβ3 Expression. Curr. Radiopharm. 2011, 4, 68–74. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Yap, L.-P.; Huang, C.-W.; Park, R.; Conti, P.S. Efficient Preparation and Biological Evaluation of a Novel Multivalency Bifunctional Chelator for 64Cu Radiopharmaceuticals. Chem. A Eur. J. 2011, 17, 10222–10225. [Google Scholar] [CrossRef] [PubMed]
- Siitonen, R.; Peuhu, E.; Autio, A.K.; Liljenbäck, H.; Mattila, E.; Metsälä, O.; Käkelä, M.; Saanijoki, T.; Dijkgraaf, I.; Jalkanen, S.; et al. 68Ga-DOTA-E[c(RGDfK)]2 PET Imaging of SHARPIN-Regulated Integrin Activity in Mice. J. Nucl. Med. 2019, 60, 1380–1387. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, G.; Shi, J.; Liu, S.; Wang, F.; Liu, S.; Chen, X. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: Promising agents for tumor integrin αvβ3 PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Niu, G.; Wang, F.; Chen, X. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liang, W.; Kang, F.; Yang, W.; Ma, X.; Li, G.; Zong, S.; Chen, K.; Wang, J. A direct comparison of tumor angiogenesis with 68Ga-labeled NGR and RGD peptides in HT-1080 tumor xenografts using microPET imaging. AMINO Acids 2014, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Liang, W.; Kang, F.; Yang, W.; Ma, X.; Li, G.; Zong, S.; Chen, K.; Wang, J. 68Ga-Labeled Cyclic NGR Peptide for MicroPET Imaging of CD13 Receptor Expression. Molecules 2014, 19, 11600–11612. [Google Scholar] [CrossRef]
- Li, Z.-B.; Chen, K.; Chen, X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Oxboel, J.; Brandt-Larsen, M.; Schjoeth-Eskesen, C.; Myschetzky, R.; El-Ali, H.H.; Madsen, J.; Kjaer, A. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl. Med. Biol. 2014, 41, 259–267. [Google Scholar] [CrossRef]
- Knetsch, P.A.; Zhai, C.; Rangger, C.; Blatzer, M.; Haas, H.; Kaeopookum, P.; Haubner, R.; Decristoforo, C. [68Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging αvβ3 integrin expression based on a novel siderophore derived chelating scaffold—synthesis and evaluation. Nucl. Med. Biol. 2015, 42, 115–122. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Wester, H.-J. Be spoilt for choice with radiolabelled RGD peptides: Preclinical evaluation of 68Ga-TRAP(RGD)3. Nucl. Med. Biol. 2013, 40, 33–41. [Google Scholar] [CrossRef]
- Lobeek, D.; Franssen, G.M.; Ma, M.T.; Wester, H.-J.; Decristoforo, C.; Oyen, W.J.G.; Boerman, O.C.; Terry, S.Y.A.; Rijpkema, M. In Vivo Characterization of 4 68Ga-Labeled Multimeric RGD Peptides to Image αvβ3 Integrin Expression in 2 Human Tumor Xenograft Mouse Models. J. Nucl. Med. 2018, 59, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, S.; Wang, L.; Sun, X.; Hu, X.; Meng, X.; Yu, J. Diagnostic and Predictive Value of Using RGD PET/CT in Patients with Cancer: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Mittra, E.S.; Goris, M.L.; Iagaru, A.H.; Kardan, A.; Burton, L.; Berganos, R.; Chang, E.; Liu, S.; Shen, B.; Chin, F.T.; et al. Pilot Pharmacokinetic and Dosimetric Studies of 18F-FPPRGD2: A PET Radiopharmaceutical Agent for Imaging αvβ3 Integrin Levels. Radiology 2011, 260, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A.; Mosci, C.; Shen, B.; Chin, F.T.; Mittra, E.; Telli, M.L.; Gambhir, S.S. 18F-FPPRGD2 PET/CT: Pilot Phase Evaluation of Breast Cancer Patients. Radiology 2014, 273, 549–559. [Google Scholar] [CrossRef]
- Minamimoto, R.; Jamali, M.; Barkhodari, A.; Mosci, C.; Mittra, E.; Shen, B.; Chin, F.; Gambhir, S.S.; Iagaru, A. Biodistribution of the 18F-FPPRGD2 PET radiopharmaceutical in cancer patients: An atlas of SUV measurements. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Karam, A.; Jamali, M.; Barkhodari, A.; Gambhir, S.S.; Dorigo, O.; Iagaru, A. Pilot prospective evaluation of 18F-FPPRGD2 PET/CT in patients with cervical and ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, N.; Li, W.; Zhao, S.; Teng, X.; Fu, Z.; Sun, X.; Zheng, J.; Ma, L.; Lu, H.; et al. A Pilot Study on Imaging of Integrin αvβ3 With RGD PET/CT in Suspected Lung Cancer Patients. Int. J. Radiat. Oncol. 2014, 90, S648–S649. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, S.; Huang, Y.; Zheng, J.; Dong, Y.; Zhang, B.; Zhao, S.; Lu, H.; Liu, Z.; Yu, J.; et al. A Pilot Study of 18F-Alfatide PET/CT Imaging for Detecting Lymph Node Metastases in Patients with Non-Small Cell Lung Cancer. Sci. Rep. 2017, 7, 2877. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, N.; Zhang, J.; Lang, L.; Zhang, W.; Li, S.; Zhao, J.; Niu, G.; Li, F.; Zhu, Z.; et al. 68Ga-NOTA-PRGD2 PET/CT for Integrin Imaging in Patients with Lung Cancer. J. Nucl. Med. 2015, 56, 1823–1827. [Google Scholar] [CrossRef]
- Kang, F.; Wang, Z.; Li, G.; Wang, S.; Liu, D.; Zhang, M.; Zhao, M.; Yang, W.; Wang, J. Inter-heterogeneity and intra-heterogeneity of αvβ3 in non-small cell lung cancer and small cell lung cancer patients as revealed by 68Ga-RGD2 PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1520–1528. [Google Scholar] [CrossRef]
- Arbizu, J.; Tejada, S.; Martí-Climent, J.M.; Díez-Valle, R.; Prieto, E.; Quincoces, G.; Vigil, C.; Idoate, M.A.; Zubieta, J.L.; Penuelas, I.; et al. Quantitative volumetric analysis of gliomas with sequential MRI and 11C-methionine PET assessment: Patterns of integration in therapy planning. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Langen, K.-J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of Treatment Response in Patients with Glioblastoma Using O-(2-18F-Fluoroethyl)-L-Tyrosine PET in Comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Withofs, N.; Martinive, P.; Vanderick, J.; Bletard, N.; Scagnol, I.; Mievis, F.; Giacomelli, F.; Coucke, P.; Delvenne, P.; Cataldo, D.; et al. [18F]FPRGD2 PET/CT imaging of integrin αvβ3 levels in patients with locally advanced rectal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 654–662. [Google Scholar] [CrossRef] [PubMed]











| Imaging Agent | Year | # Patients | Confirmation | Neoplasm | Ref. |
|---|---|---|---|---|---|
| [18F]FP-PRGD2 PET/C 8 | 2014 | 8 | HP | BCa | [73] |
| [18F]Alfatide I 10 | 2015 | 26/16 | HP | LCa/Lnd | [76] |
| [18F]Alfatide I PET/CT 10 | 2017 | 13 | HP | Lnd | [77] |
| [18F]Alfatide II PET/CT 14 | 2015 | 5 (HV) 9 | MRI/CT | BrCa | [54] |
| [18F]Alfatide II PET/CT 14 | 2015 | 30 | BnCa | [53] | |
| [18F]Alfatide II PET/CT 14 | 2018 | 44 | HP | BCa | [52] |
| [68Ga]Ga-NOTA-PRGD2 PET/CT 12 | 2015 | 91 159 | HP | Lnd | [78] |
| [68Ga]Ga-RGD2 PET/CT 15 | 2017 | 31 (21/10) | HP | NSCLC/SCLC | [79] |
| [68Ga]Ga-RGD2 PET/CT 15 | 2016 | 21/13 | HP | NSCLC/TB | [51] |
| # | Name | Cell & Tumor Model | Ref. | Figure |
|---|---|---|---|---|
| 1 | [18F]FBOA-Dpr-HEG-c(RGDfE) | M21 Human melanoma U87MG human glioblastoma | [22,33] | Figure 2 |
| 2 | [18F]FBOA-Dpr-K(HEG-c(RGDfE))2 | |||
| 3 | [18F]FBOA-Dpr-K{K[HEG-(c(RGDfE)]2}2 | |||
| 4 | [18F]FB-E[c(RGDyK)]2 | HBCECs human brain capillary endothelial cells, U87MG | [34,35] | Figure 3 |
| 5 | [18F]FP-E[c(RGDyK)]2 18F-FP-RGD2 | U87MG | ||
| 6 | [18F]AlF-NOTA-E[c(RGDyK)]2 [18F]AlF-NOTA-RGD2 | U87MG | [35,46] | |
| 7 | [18F]FB-PEG3-E[c(RGDyK)]2 [18F]FB-PRGD2 | U87MG | [36,37] | |
| 8 | [18F]FP-PEG3-E[c(RGDyK)]2 [18F]FP-PRGD2 | HCT116 human colon cancer, U87MG | [27,39,72,73,74] | |
| 9 | [68Ga]Ga-NOTA-PEG3-E[c(RGDyK)]2 | U87MG | [38,39] | |
| 10 | [18F]AlF-NOTA-PEG3-E[c(RGDyK)]2 [18F]Alfatide I | U87MG, A549 adenocarcinomic human alveolar basal epithelial cells, PC-3 prostate cancer, LLC Lewis Lung Carcinoma | [38,39,40,41,76,77] | |
| 11 | [18F]FP-PEG4-E[c(RGDfK)]2 [18F]FP-PRGD2 | U87MG, MDA-MB-435 | [45] | Figure 4 |
| 12 | [68Ga]Ga-NOTA-PEG4-E[c(RGDfK)]2 | U87MG | [78] | |
| 13 | [18F]AlF-NOTA-PEG4-E[c(RGDfK)]2 | U87MG | [46] | |
| 14 | [18F]AlF-NOTA-E[PEG4-c(RGDfK)]2, [18F]Alfatide II | U87MG, MDA-MB-435 human breast cancer | [43,44,46,52,53,54] | |
| 15 | [68Ga]Ga-NOTA-E[PEG4-c(RGDfK)]2 [68Ga]Ga-NOTA-PRGD2, | - | [51,79] | |
| 16 | [18F]FP-PEG4-E[PEG4-c(RGDfK)]2 [18F]FP-PPRGD2 | U87MG, MDA-MB-435 | [45] | |
| 17 | [18F]FP-PEG2-β-E[c(RGDyK)]2 | A549, PC-3 | [47] | Figure 5 |
| 18 | [18F]FP-SAA-E[c(RGDyK)2 | U87MG | [48] | Figure 6 |
| 19 | [18F]FB-SAA-E[c(RGDyK)2 | |||
| 20 | NOTA-E[c(RGDyK)]2 | - | [49] | Figure 7 |
| 21 | [68Ga]Ga-NOTA-E[c(RGDyK)]2 | |||
| 22 | [68Ga]Ga-NOTA-Y-c(RGDyK)] (Y =2-(4-anilinyl-methyl)-4-(3-oxopropyl)thiazol-5(4H)-one) | |||
| 23 | [64Cu]Cu-DOTA-E[c(RGDfK)]2 | U87MG, MDA-MB-435 | [55,56] | Figure 8 |
| 24 | [68Ga]Ga-DOTA-E[c(RGDfK)]2 | B16-F10-luc melanoma tumors, SK-RC-52, FaDu | [61,70] | |
| 25 | [64Cu]Cu-DOTA-E[c(RGDyK)]2 | MDA-MB-435 | [55] | |
| 26 | [64Cu]Cu-DOTA-E{E[c(RGDfK)]2}2 | U87MG | [56,58] | |
| 27 | [64Cu]Cu-DOTA-E{E[c(RGDyK)]2}2 | U87MG, c-neu onco-mice | [30] | |
| 28 | [64Cu]Cu-DOTA-E(E{E[c(RGDfK)]2}2)2 | U87MG, c-neu onco-mice | [30] | |
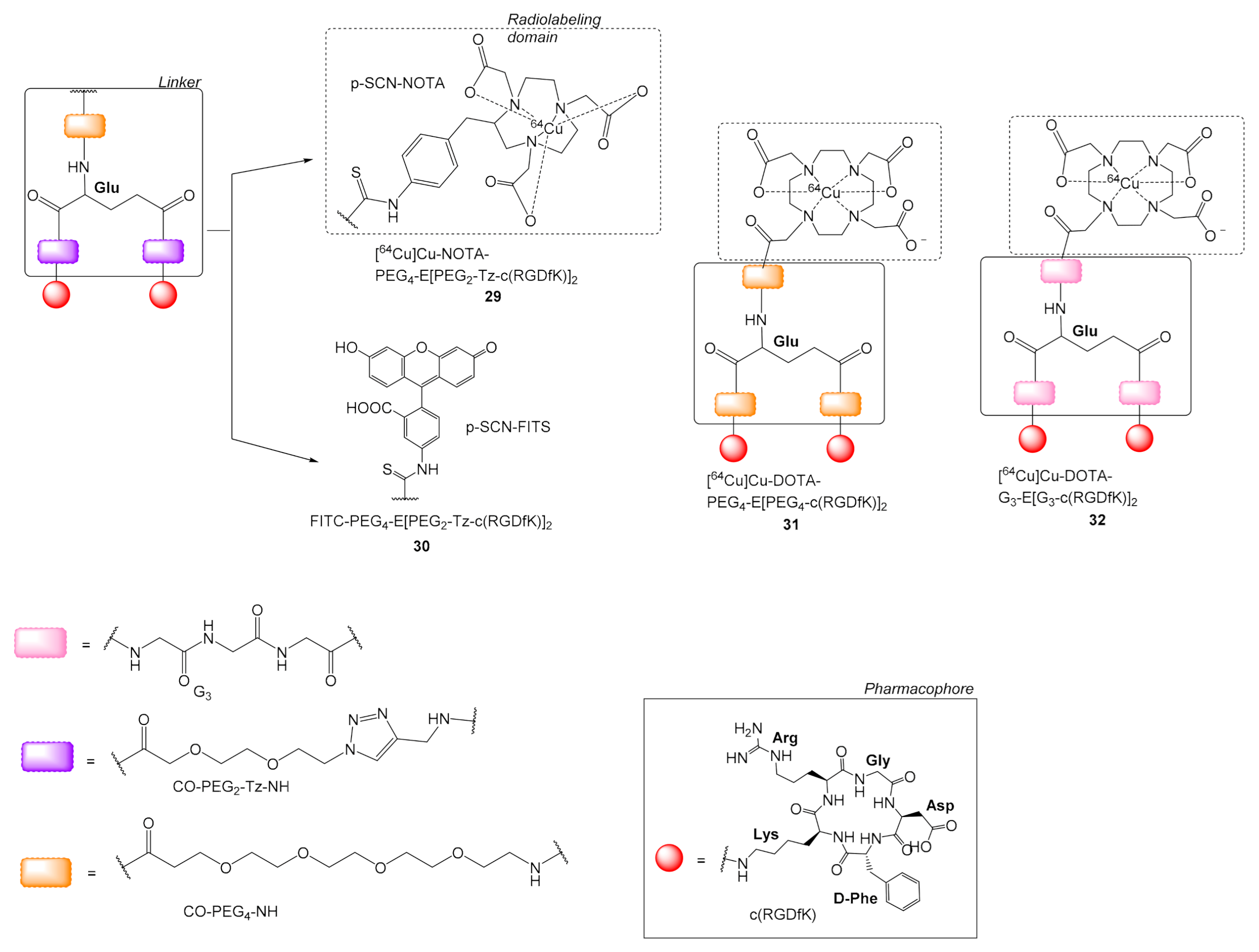
| 29 | [64Cu]Cu-NOTA-PEG4-E[(PEG2-Tz-c(RGDfK)]2 | HUVEC human umbilical vein endothelial cells | [57] | Figure 9 |
| 30 | FITC-PEG4-E[PEG2-Tz-c(RGDfK)]2 | |||
| 31 | [64Cu]Cu-DOTA-PEG4-E[PEG4-c(RGDfK)]2 | U87MG | [58] | |
| 32 | [64Cu]Cu-DOTA-G3-E[G3-c(RGDfK)]2 | |||
| 33 | [64Cu]Cu-AmBaSar-E[c(RGDyK)]2 | U87MG | [59,60] | Figure 10 |
| 34 | [64Cu]Cu-AmBaBaSar-c(RGDyK)2 | |||
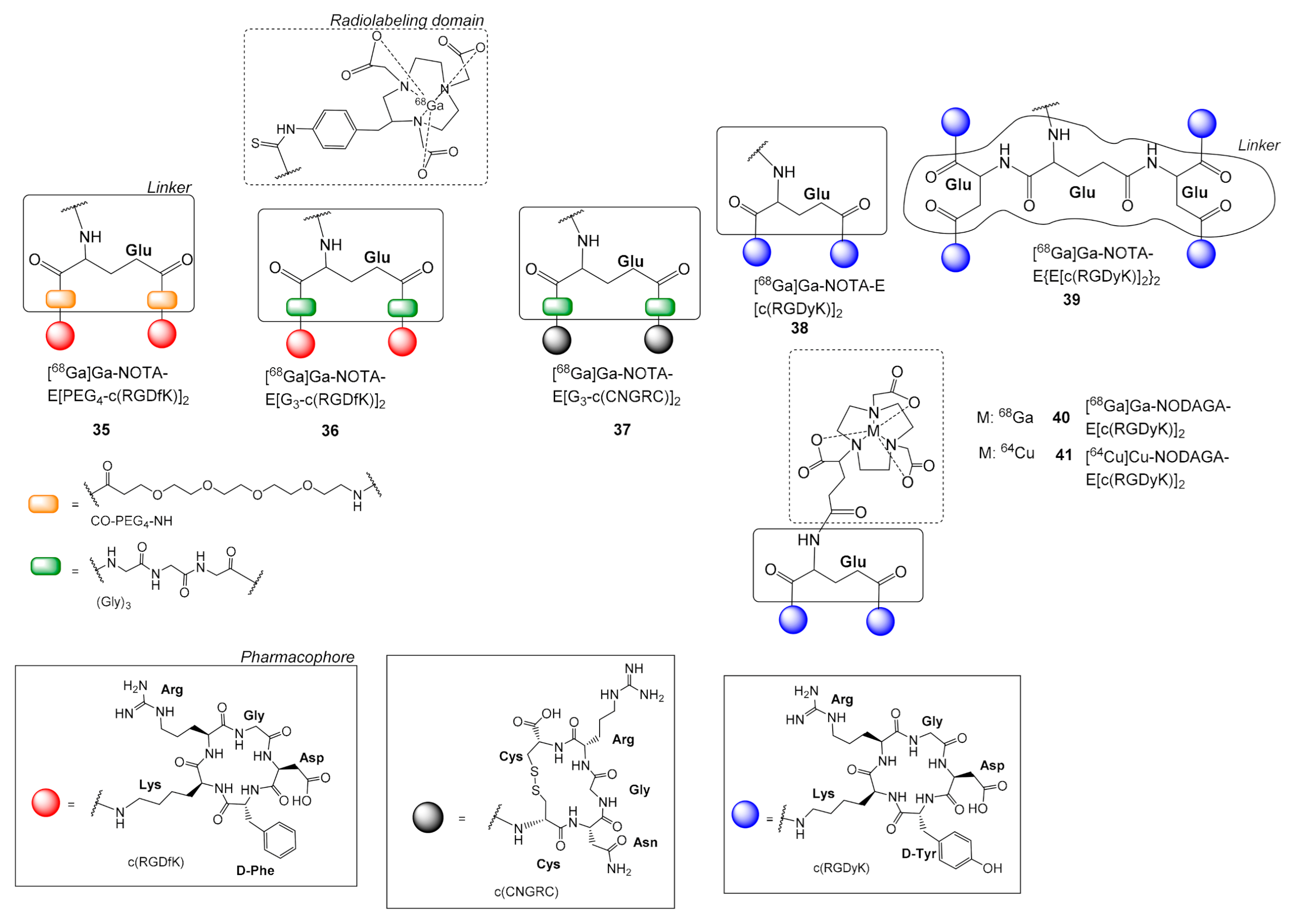
| 35 | [68Ga]Ga-NOTA-E[PEG4-c(RGDfK)]2 | U87MG: MDA-MB-435 | [62] | Figure 11 |
| 36 | [68Ga]Ga-NOTA-E[G3-c(RGDfK)]2 | U87MG, MDA-MB-435, HT1080 fibrosarcoma | [62,64] | |
| 37 | [68Ga]Ga-NOTA-E[G3-c(CNGRC)]2 | HT1080 fibrosarcoma | [64] | |
| 38 | [68Ga]Ga-NOTA-E[c(RGDyK)]2 | U87MG | [66] | |
| 39 | [68Ga]Ga-NOTA-E{E[c(RGDyK)]2}2 | |||
| 40 | [68Ga]Ga-NODAGA-E[c(RGDyK)]2 | U87MG, H727 human neuroendocrine | [67] | |
| 41 | [64Cu]Cu-NODAGA-E[c(RGDyK)]2 | |||
| 42 | [68Ga]Ga-FSC-[E-c(RGDfK)]3 | M21 human melanoma, SK-RC-52 (human renal cell carcinoma), FaDu (human squamous cell carcinoma) | [68,70] | Figure 12 |
| 43 | [68Ga]Ga-FSC-(CH2)-Tz-c(RGDfK) | U87MG, M21 human melanoma | [23] | |
| 44 | [68Ga]Ga-FSC-[(CH2)-Tz-c(RGDfK)]2 | |||
| 45 | [68Ga]Ga-FSC-[(CH2)-Tz-c(RGDfK)]3 | |||
| 46 | [68Ga]Ga-TRAP-PEG4-c(RGDfK)3 | M21 human melanoma, SK-RC-52, FaDu | [69,70] | Figure 13 |
| 47 | [68Ga]Ga-THP-c(RGDfK)3 | SK-RC-52, FaDu | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules 2021, 26, 1792. https://doi.org/10.3390/molecules26061792
Liolios C, Sachpekidis C, Kolocouris A, Dimitrakopoulou-Strauss A, Bouziotis P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules. 2021; 26(6):1792. https://doi.org/10.3390/molecules26061792
Chicago/Turabian StyleLiolios, Christos, Christos Sachpekidis, Antonios Kolocouris, Antonia Dimitrakopoulou-Strauss, and Penelope Bouziotis. 2021. "PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors" Molecules 26, no. 6: 1792. https://doi.org/10.3390/molecules26061792
APA StyleLiolios, C., Sachpekidis, C., Kolocouris, A., Dimitrakopoulou-Strauss, A., & Bouziotis, P. (2021). PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules, 26(6), 1792. https://doi.org/10.3390/molecules26061792








