Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice
Abstract
1. Introduction
2. Results and Discussion
2.1. Changes in Physicochemical Characteristics of the Juice
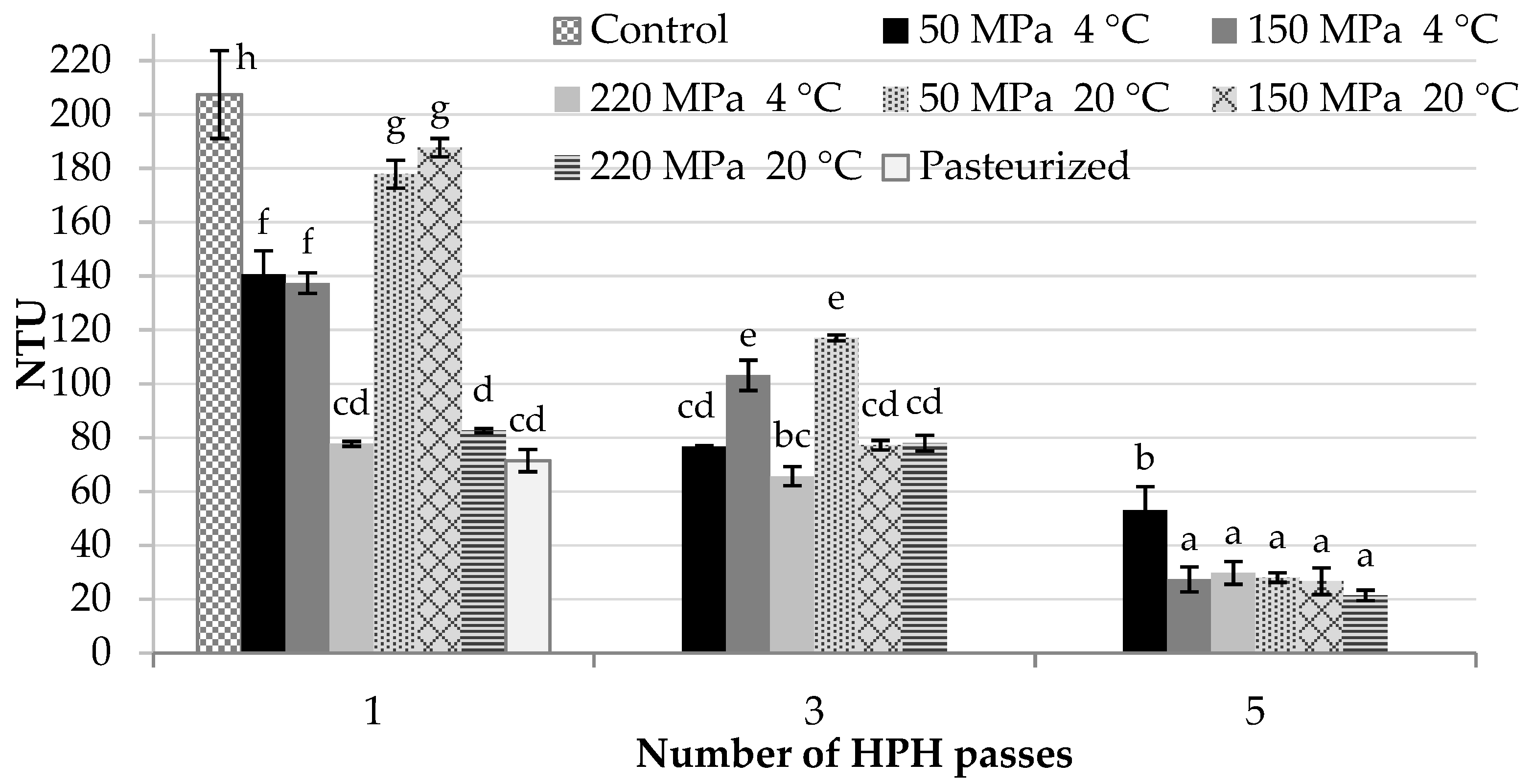
2.2. Nephelometric Turbidity of Juice
2.3. Juice Colour Stability
2.4. Changes in Anthocyanins Content
2.5. Total Vitamin C, Ascorbic Acid, and Dehydroascorbic Acid Stability
2.6. Total Phenolic Content and Antioxidant Capacity
2.7. Principal Component Analysis of Gathered Data
3. Materials and Methods
3.1. Blackcurrant Berries and Juice Production
3.2. HPH Treatment and Thermal Pasteurization of the Juice
3.3. Chemicals and Reagents
3.4. Determination of pH, Titratable Acidity, and Total Soluble Solids
3.5. Colour Analysis
3.6. Nephelometric Turbidity Measurement
3.7. Determination of Total Phenolic Content
3.8. Determination of Total Vitamin C, AA, and DHAA Content
3.9. Determination of Anthocyanin Content
3.10. DPPH-EPR Radical Scavenging Assay
3.11. ORAC Assay
3.12. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.M.; Veteläinen, M. High variability in flavonoid contents and composition between different North-European currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional Diversity among Blackcurrant (Ribes nigrum) Cultivars Originating from European Countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef]
- Laaksonen, O.A.; Mäkilä, L.; Sandell, M.A.; Salminen, J.P.; Liu, P.; Kallio, H.P.; Yang, B. Chemical-Sensory Characteristics and Consumer Responses of Blackcurrant Juices Produced by Different Industrial Processes. Food Bioprocess Technol. 2014, 7, 2877–2888. [Google Scholar] [CrossRef]
- Mäkilä, L.; Laaksonen, O.; Kallio, H.; Yang, B. Effect of processing technologies and storage conditions on stability of black currant juices with special focus on phenolic compounds and sensory properties. Food Chem. 2017, 221, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Georget, E.; Miller, B.; Callanan, M.; Heinz, V.; Mathys, A. (Ultra) High Pressure Homogenization for Continuous High Pressure Sterilization of Pumpable Foods—A Review. Front. Nutr. 2014, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.; Guamis, B. Opportunities for Ultra-High-Pressure Homogenisation (UHPH) for the Food Industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Moscovici Joubran, A.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The effect of pressure level and cycling in high-pressure homogenization on physicochemical, structural and functional properties of filtered and non-filtered strawberry nectar. Innov. Food Sci. Emerg. Technol. 2019, 57. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Atalar, I.; Yilmaz, V.A.; Odabas, H.I.; Gul, O. Application of multi pass high pressure homogenization to improve stability, physical and bioactive properties of rosehip (Rosa canina L.) nectar. Food Chem. 2019, 282, 67–75. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, W.; Ge, Z.; Zhang, L.; Li, C.; Zhang, B.; Zong, W. Comparison of the effects of dynamic high-pressure microfluidization and conventional homogenization on the quality of peach juice. J. Sci. Food Agric. 2019, 99, 5994–6000. [Google Scholar] [CrossRef]
- Aguayo, E.; Tarazona-Díaz, M.P.; Martínez-Sánchez, A.; García-González, A. Influence of moderate high-pressure homogenization on quality of bioactive compounds of functional food supplements. J. Food Qual. 2017, 42, e12997. [Google Scholar] [CrossRef]
- Yildiz, G. Application of ultrasound and high-pressure homogenization against high temperature-short time in peach juice. J. Food Process Eng. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F.; et al. (Ultra) high pressure homogenization potential on the shelf-life and functionality of kiwifruit juice. Front. Microbiol. 2019, 10, 246. [Google Scholar] [CrossRef]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of innovative technologies on the content of vitamin C and its bioavailability from processed fruit and vegetable products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef]
- Koley, T.K.; Nishad, J.; Kaur, C.; Su, Y.; Sethi, S.; Saha, S.; Sen, S.; Bhatt, B.P. Effect of high-pressure microfluidization on nutritional quality of carrot (Daucus carota L.) juice. J. Food Sci. Technol. 2020, 57, 2159–2168. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Guamis-López, B.; Roig-Saguès, A.X. Influence of ultra-high pressure homogenisation on physicochemical and sensorial properties of orange juice in comparison with conventional thermal processing. Int. J. Food Sci. Technol. 2019, 54, 1858–1864. [Google Scholar] [CrossRef]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. Ananassa) juice. LWT Food Sci. Technol. 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Marszałek, K.; Kruszewski, B.; Woźniak, Ł.; Skąpska, S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious). Innov. Food Sci. Emerg. Technol. 2017, 39, 42–48. [Google Scholar] [CrossRef]
- Suárez-Jacobo, Á.; Rüfer, C.E.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011, 127, 447–454. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Cao, J.; Zhou, J. Effects of pressure and multiple passes on the physicochemical and microbial characteristics of lupin-based beverage treated with high-pressure homogenization. J. Food Process. Preserv. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Silva, V.M.; Sato, A.C.K.; Barbosa, G.; Dacanal, G.; Ciro-Velásquez, H.J.; Cunha, R.L. The effect of homogenisation on the stability of pineapple pulp. Int. J. Food Sci. Technol. 2010, 45, 2127–2133. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Thermal pasteurization effects on color of red grapefruit juices. J. Food Sci. 1999, 64, 663–666. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, L.; Almeida, A.C.S.; de Carvalho, C.W.P.; Borguini, R.G.; Ferreira, J.C.S.; Freitas, S.P.; da Matta, V.M. Effect of Processing on Bioactive Compounds, Physicochemical and Rheological Characteristics of Juçara, Banana and Strawberry Smoothie. Plant Foods Hum. Nutr. 2018, 73, 222–227. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef]
- Deutsch, J.C. Spontaneous hydrolysis and dehydration of dehydroascorbic acid in aqueous solution. Anal. Biochem. 1998, 260, 223–229. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Franchi, M.A.; de Massaguer, P.R.; Cristianini, M. Quality of Mango Nectar Processed by High-Pressure Homogenization with Optimized Heat Treatment. J. Food Sci. 2011, 76, 106–110. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, L.; Bi, J.; Yi, J.; Liu, X.; Chen, Q.; Wu, X.; Zhou, M. Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innov. Food Sci. Emerg. Technol. 2016, 36, 320–329. [Google Scholar] [CrossRef]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Gralec, M.; Wawer, I.; Zawada, K. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Goiffon, J.P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV-Vis study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]

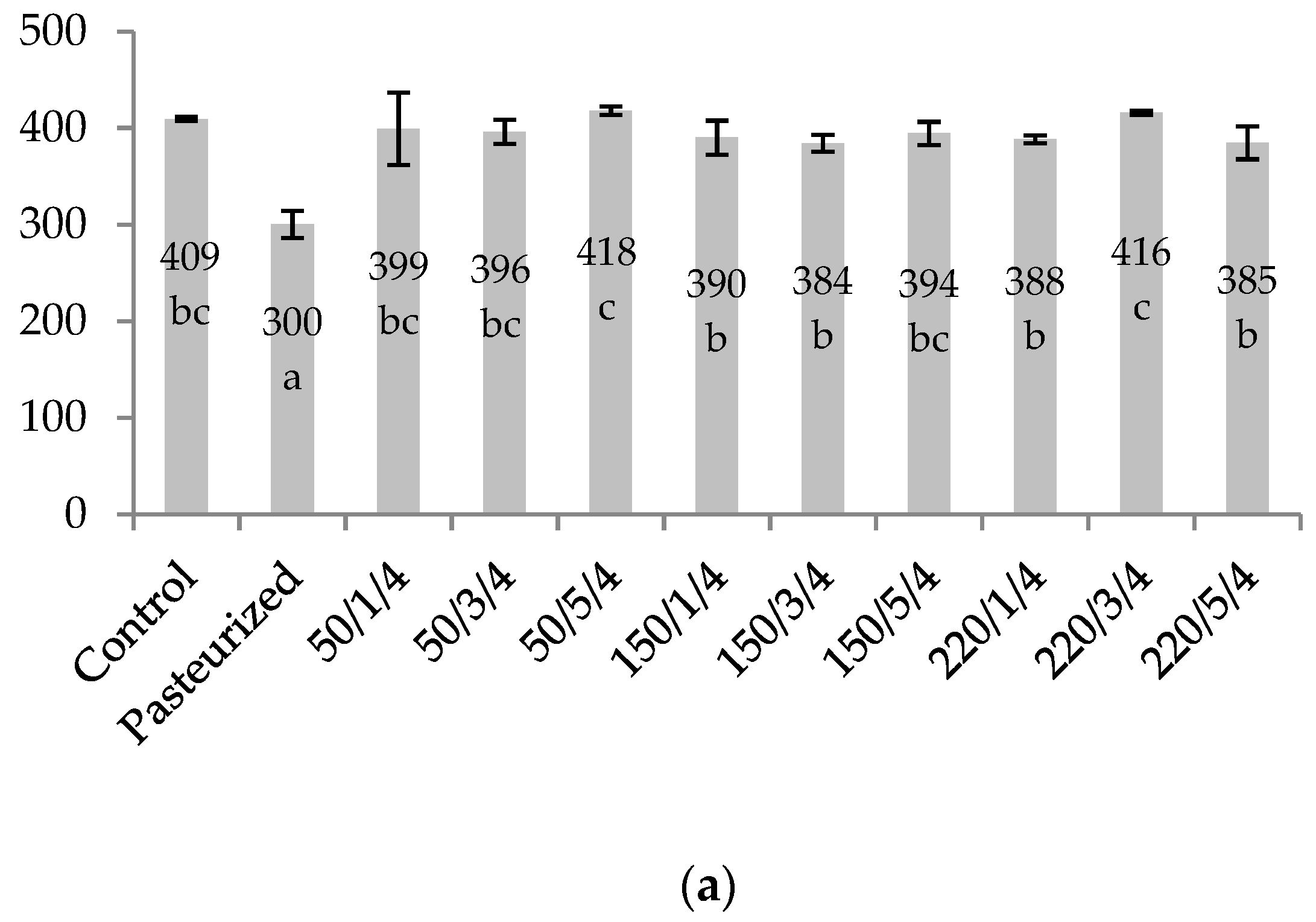


| Treatment | pH | TA (g of Citric Acid /100 mL) | TSS (Brix) | ΔE* | ||
|---|---|---|---|---|---|---|
| Control Sample/Raw Juice | 2.48 ± 0.04 a | 5.05 ± 0.06 a | 15.83 ± 0.05 bc | - | ||
| Pasteurization 90 °C, 1 min | 2.52 ± 0.04 a | 4.94 ± 0.06 a | 15.32 ± 0.05 a | 4.26 ± 0.06 m | ||
| Homogenization | ||||||
| Inlet Temp. °C | Pressure MPa | Number of Passes | ||||
| 4 | 50 | 1 | 2.45 ± 0.04 a | 5.05 ± 0.07 a | 15.70 ± 0.09 bc | 0.49 ± 0.04 a |
| 3 | 2.45 ± 0.05 a | 5.05 ± 0.07 a | 15.70 ± 0.10 bc | 1.38 ± 0.03 de | ||
| 5 | 2.43 ± 0.05 a | 5.10 ± 0.04 a | 15.90 ± 0.14 cdef | 1.78 ± 0.06 fg | ||
| 150 | 1 | 2.47 ± 0.04 a | 5.00 ± 0.03 a | 16.45 ± 0.07 h | 0.89 ± 0.03 bc | |
| 3 | 2.50 ± 0.03 a | 4.95 ± 0.07 a | 16.50 ± 0.05 h | 1.42 ± 0.03 de | ||
| 5 | 2.46 ± 0.04 a | 4.90 ± 0.05 a | 16.40 ± 0.11 h | 1.44 ± 0.02 e | ||
| 220 | 1 | 2.51 ± 0.04 a | 5.05 ± 0.04 a | 15.75 ± 0.07 cd | 0.80 ± 0.06 b | |
| 3 | 2.49 ± 0.03 a | 5.05 ± 0.07 a | 15.85 ± 0.15 cde | 1.45 ± 0.03 e | ||
| 5 | 2.49 ± 0.02 a | 4.95 ± 0.06 a | 15.70 ± 0.12 bc | 2.32 ± 0.03 h | ||
| 20 | 50 | 1 | 2.44 ± 0.01 a | 5.05 ± 0.07 a | 16.20 ± 0.14 fg | 1.71 ± 0.03 f |
| 3 | 2.43 ± 0.02 a | 5.15 ± 0.08 a | 16.15 ± 0.06 efg | 2.42 ± 0.04 h | ||
| 5 | 2.46 ± 0.03 a | 5.10 ± 0.04 a | 16.15 ± 0.07 efg | 2.70 ± 0.05 i | ||
| 150 | 1 | 2.44 ± 0.02 a | 5.15 ± 0.05 a | 16.30 ± 0.08 gh | 0.95 ± 0.02 c | |
| 3 | 2.46 ± 0.04 a | 5.10 ± 0.03 a | 16.45 ± 0.13 h | 1.32 ± 0.02 d | ||
| 5 | 2.50 ± 0.04 a | 4.90 ± 0.07 a | 16.50 ± 0.10 h | 1.89 ± 0.03 g | ||
| 220 | 1 | 2.46 ± 0.01 a | 5.10 ± 0.05 a | 16.05 ± 0.13 defg | 2.85 ± 0.07 j | |
| 3 | 2.46 ± 0.05 a | 5.10 ± 0.06 a | 16.15 ± 0.09 efg | 3.11 ± 0.06 k | ||
| 5 | 2.48 ± 0.02 a | 4.95 ± 0.07 a | 16.25 ± 0.10 gh | 3.33 ± 0.06 l | ||
| Treatment | Del-3-glu 1 | Del-3-rut 2 | Cya-3-glu 3 | Cya-3-rut 4 | ||
|---|---|---|---|---|---|---|
| Control sample/raw juice | 64.3 ± 2.6 e | 167.1 ± 6.5 e | 19.0 ± 1.0 e | 88.7 ± 4.4 f | ||
| Pasteurization 90 °C, 1 min | 48.6 ± 2.8 a | 137.2 ± 3.8 bc | 12.7 ± 1.0 a | 66.8 ± 3.0 a | ||
| Homogenization | ||||||
| Inlet Temp. °C | Pressure MPa | Number of Passes | ||||
| 4 | 50 | 1 | 66.4 ± 1.4 f | 160.6 ± 2.2 e | 19.5 ± 0.5 e | 84.8 ± 1.6 e |
| 3 | 60.1 ± 0.1 c | 151.8 ± 1.0 cd | 17.5 ± 0.1 d | 78.6 ± 1.4 c | ||
| 5 | 58.4 ± 0.2 bc | 148.2 ± 1.7 c | 16.5 ± 0.5 cd | 75.2 ± 0.2 b | ||
| 150 | 1 | 60.4 ± 0.2 c | 155.1 ± 1.6 d | 17.7 ± 0.4 d | 83.6 ± 2.1 d | |
| 3 | 62.3 ± 1.3 d | 146.5 ± 0.4 c | 17.7 ± 1.2 d | 78.8 ± 0.9 c | ||
| 5 | 62.8 ± 0.5 d | 140.4 ± 0.1 bc | 17.8 ± 0.1 d | 82.5 ± 0.2 d | ||
| 220 | 1 | 61.8 ± 1.5 d | 168.5 ± 0.7 f | 17.0 ± 0.7 cd | 81.4 ± 0.3 d | |
| 3 | 57.4 ± 0.9 bc | 155.6 ± 0.6 d | 15.4 ± 0.4 c | 76.6 ± 0.3 c | ||
| 5 | 54.6 ± 1.2 b | 154.8 ± 0.1 d | 14.0 ± 1.0 c | 73.9 ± 0.6 b | ||
| 20 | 50 | 1 | 54.8 ± 2.6 b | 156.8 ± 0.3 d | 15.1 ± 0.8 bc | 74.5 ± 1.7 b |
| 3 | 55.1 ± 0.5 b | 149.9 ± 0.8 c | 15.1 ± 0.3 bc | 75.1 ± 0.3 b | ||
| 5 | 52.6 ± 1.2 a | 142.9 ± 1.6 bc | 14.8 ± 1.4 bc | 72.8 ± 0.8 b | ||
| 150 | 1 | 61.0 ± 2.6 cd | 161.8 ± 2.2 e | 16.5 ± 1.2 cd | 79.8 ± 1.4 cd | |
| 3 | 57.2 ± 1.3 bc | 158.9 ± 0.7 e | 16.9 ± 0.7 cd | 76.7 ± 0.8 c | ||
| 5 | 57.0 ± 0.5 b | 153.8 ± 1.2 cd | 15.7 ± 0.5 c | 74.4 ± 0.3 b | ||
| 220 | 1 | 53.7 ± 1.5 ab | 134.9 ± 0.5 ab | 15.7 ± 0.7 c | 72.2 ± 2.5 b | |
| 3 | 51.9 ± 0.5 a | 131.9 ± 1.9 ab | 15.0 ± 0.1 bc | 68.7 ± 0.1 a | ||
| 5 | 53.3 ± 0.1 a | 124.1 ± 0.4 a | 13.9 ± 0.1 ab | 66.4 ± 1.2 a | ||
| Treatment | Ascorbic Acid | Dehydroascorbic Acid | Total Vitamin C | % Value Relative to Raw Juice | ||
|---|---|---|---|---|---|---|
| Control sample/raw juice | 110.5 ± 4.1 h | 68.1 ± 2.6 efg | 178.6 ± 4.9 g | 100.0 | ||
| Pasteurization 90 °C, 1 min | 57.4 ± 9.2 a | 68.2 ± 2.1 fg | 125.6 ± 7.1 bc | 70.3 | ||
| Homogenization | ||||||
| Inlet Temp. °C | Pressure MPa | Number of Passes | ||||
| 4 | 50 | 1 | 101.2 ± 6.9 fg | 67.7 ± 8.5 efg | 168.8 ± 1.6 fg | 94.5 |
| 3 | 76.3 ± 4.4 cd | 75.2 ± 0.9 gh | 151.6 ± 5.3 d | 84.9 | ||
| 5 | 64.8 ± 3.4 ab | 66.7 ± 2.4 efg | 131.5 ± 5.8 c | 73.6 | ||
| 150 | 1 | 108.8 ± 2.3 gh | 69.6 ± 6.9 g | 168.4 ± 4.5 fg | 94.3 | |
| 3 | 76.0 ± 2.5 cd | 81.3 ± 10.2 h | 147.4 ± 7.7 d | 82.5 | ||
| 5 | 64.2 ± 1.0 ab | 56.5 ± 0.9 bcd | 120.2 ± 1.9 bc | 67.6 | ||
| 220 | 1 | 97.7 ± 2.1 f | 71.5 ± 6.1 gh | 169.27± 4.0 fg | 94.7 | |
| 3 | 68.6 ± 1.6 bc | 75.5 ± 2.0 gh | 144.1 ± 0.4 d | 80.7 | ||
| 5 | 62.3 ± 0.0 ab | 64.3 ± 3.0 def | 126.6 ± 3.0 bc | 70.9 | ||
| 20 | 50 | 1 | 99.5 ± 0.1 f | 66.6 ± 0.2 efg | 166.1 ± 0.4 ef | 93.0 |
| 3 | 77.7 ± 4.1 d | 51.4 ± 6.7 bc | 129.1 ± 10.7 c | 72.3 | ||
| 5 | 75.0 ± 2.5 cd | 45.6 ± 3.3 ab | 120.6 ± 5.8 bc | 67.5 | ||
| 150 | 1 | 87.7 ± 2.7 e | 67.9 ± 1.5 efg | 155.6 ± 4.2 de | 87.1 | |
| 3 | 70.3 ± 4.0 bcd | 57.8 ± 3.2 cde | 128.1 ± 7.3 c | 71.7 | ||
| 5 | 61.6 ± 2.0 ab | 53.8 ± 8.3 bcd | 115.4 ± 6.3 b | 64.6 | ||
| 220 | 1 | 93.0 ± 0.0 ef | 57.1 ± 2.0 cde | 150.1 ± 2.0 d | 84.1 | |
| 3 | 76.4 ± 4.2 cd | 39.3 ± 5.4 a | 115.6 ± 1.2 b | 64.8 | ||
| 5 | 59.2 ± 3.5 a | 40.4 ± 0.2 a | 99.5 ± 3.3 a | 55.7 | ||
| Treatment | DPPH-EPR [µM Tr/L] | % Value Relative to Raw Juice | ORAC [µM Tr/L] | % Value Relative to Raw Juice | ||
|---|---|---|---|---|---|---|
| Control sample/raw juice | 38,128 ± 4229 abc | 100.0 | 72,505 ± 4306 def | 100.0 | ||
| Pasteurization 90 °C, 1 min | 33,304 ± 749 a | 87.3 | 65,342 ± 3793 bc | 90.1 | ||
| Homogenization | ||||||
| Inlet Temp. °C | Pressure MPa | Number of Passes | ||||
| 4 | 50 | 1 | 33,353 ± 2098 a | 87.5 | 69,633 ± 1775 cdef | 96.0 |
| 3 | 32,553 ± 1582 a | 85.3 | 71,766 ± 4189 def | 99.0 | ||
| 5 | 32,669 ± 1134 a | 85.7 | 65,640 ± 1565 bc | 90.5 | ||
| 150 | 1 | 34,956 ± 880 abc | 91.7 | 67,996 ± 3566 cd | 93.8 | |
| 3 | 34,122 ± 1559 ab | 89.5 | 69,972 ± 3548 cdef | 96.5 | ||
| 5 | 32,950 ± 1321 a | 86.4 | 63,099 ± 4955 ab | 87.0 | ||
| 220 | 1 | 35,543 ± 1428 abc | 93.2 | 69,991 ± 3480 cdef | 96.5 | |
| 3 | 34,475 ± 1052 abc | 90.4 | 65,589 ± 4179 bc | 91.6 | ||
| 5 | 34,591 ± 742 abc | 90.7 | 69,169 ± 2742 cde | 95.4 | ||
| 20 | 50 | 1 | 41,175 ± 1038 cde | 108.0 | 74,358 ± 3930 f | 102.6 |
| 3 | 47,908 ± 1445 ef | 125.6 | 82,701 ± 3683 g | 114.1 | ||
| 5 | 50,014 ± 981 g | 131.2 | 100,197 ± 3770 h | 138.2 | ||
| 150 | 1 | 40,812 ± 1206 bcd | 107.0 | 73,085 ± 2434 ef | 100.8 | |
| 3 | 48,880 ± 2011 f | 128.2 | 79,306 ± 2513 g | 109.4 | ||
| 5 | 45,239 ± 1420 def | 118.6 | 81,411 ± 2405 g | 112.3 | ||
| 220 | 1 | 41,199 ± 619 cde | 108.1 | 65,203 ± 2717 bc | 89.9 | |
| 3 | 32,148 ± 404 a | 84.3 | 62,961 ± 4026 ab | 86.8 | ||
| 5 | 33,741 ± 829 a | 88.5 | 59,224 ± 3367 a | 81.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. https://doi.org/10.3390/molecules26061802
Kruszewski B, Zawada K, Karpiński P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules. 2021; 26(6):1802. https://doi.org/10.3390/molecules26061802
Chicago/Turabian StyleKruszewski, Bartosz, Katarzyna Zawada, and Piotr Karpiński. 2021. "Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice" Molecules 26, no. 6: 1802. https://doi.org/10.3390/molecules26061802
APA StyleKruszewski, B., Zawada, K., & Karpiński, P. (2021). Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules, 26(6), 1802. https://doi.org/10.3390/molecules26061802








