Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anti-Helicobacter pylori Activity and Structure-Activity Relationship Studies
2.3. Effects of Thymol and Its Semi-Synthetic Derivatives on AGS Cell Viability
2.4. Pan Assay Interference Compounds (PAINS) and Drug-Likeness Evaluation
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of Thymol Derivatives
3.2.1. General Procedure for the Synthesis of Compounds 2–7 and 9–39
3.2.2. Synthesis of Compound 8
3.3. Characterization Data for Thymol Derivatives 2–39
3.4. Anti-Helicobacter pylori Activity
3.5. Cell Lines and Treatments
3.6. Cell Viability
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Ivanovich Reshetnyak, V.; Igorevich Burmistrov, A.; Veniaminovich Maev, I. Helicobacter pylori: Commensal, symbiont or pathogen? World J. Gastroenterol. 2021, 21, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Sisto, F.; Puca, V.; Carradori, S.; Ronci, M.; Aceto, A.; Muraro, R.; Mincione, G.; Scotti, L. Antimicrobial and Antibiofilm Activities of New Synthesized Silver Ultra-NanoClusters (SUNCs) Against Helicobacter pylori. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Grande, R.; Migdał, P.; Paluch, E.; Gościniak, G. Biofilm formation as a complex result of virulence and adaptive responses of Helicobacter pylori. Pathogens 2020, 9, 1062. [Google Scholar] [CrossRef]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Ahmad, I.Z. Molecular Docking and Dynamics Simulation Analysis of Thymoquinone and Thymol Compounds from Nigella sativa L. that Inhibit Cag A and Vac A Oncoprotein of Helicobacter pylori: Probable Treatment of H. pylori Infections. Med. Chem. (Los. Angeles). 2020, 17, 146–157. [Google Scholar] [CrossRef]
- Bkhaitan, M.M.; Alarjah, M.; Mirza, A.Z.; Abdalla, A.N.; El-Said, H.M.; Faidah, H.S. Preparation and biological evaluation of metronidazole derivatives with monoterpenes and eugenol. Chem. Biol. Drug Des. 2018, 92, 1954–1962. [Google Scholar] [CrossRef]
- Piscione, M.; Mazzone, M.; Di Marcantonio, M.C.; Muraro, R.; Mincione, G. Eradication of Helicobacter pylori and Gastric Cancer: A Controversial Relationship. Front. Microbiol. 2021, 12, 630852. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer activity of thymol: A literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, Y.S.; Kim, E.K.; Hwang, J.W.; Jeong, J.H.; Dong, X.; Lee, J.W.; Moon, S.H.; Jeon, B.T.; Park, P.J. Anticancer effect of thymol on AGS human gastric carcinoma cells. J. Microbiol. Biotechnol. 2015, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Kiziltan, H.S. Effects of thymol, a natural phenolic compound, on human gastric adenocarcinoma cells in vitro. Altern. Ther. Health Med. 2019, 25, 12–21. [Google Scholar] [PubMed]
- Günes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Dadak, A. In vitro hormetic effect investigation of thymol on human fibroblast and gastric adenocarcinoma cells. Molecules 2020, 25, 3270. [Google Scholar] [CrossRef]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Traversi, C.B.; Spano, M.; Sobolev, A.P.; Secci, D.; Di Marcantonio, M.C.; Haloci, E.; Grande, R.; et al. Synthesis and biological evaluation of carvacrol-based derivatives as dual inhibitors of H. pylori strains and AGS cell proliferation. Pharmaceuticals 2020, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Bergonzelli, G.E.; Donnicola, D.; Porta, N.; Corthésy-Theulaz, I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. [Google Scholar] [CrossRef]
- Eftekhar, F.; Nariman, F.; Yousefzadi, M.; Hadian, J.; Ebrahimi, S.N. Anti-Helicobacter pylori activity and essential oil composition of Thymus caramanicus from Iran. Nat. Prod. Commun. 2009, 4, 1139–1142. [Google Scholar] [CrossRef]
- Falsafi, T.; Moradi, P.; Mahboubi, M.; Rahimi, E.; Momtaz, H.; Hamedi, B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015, 22, 173–177. [Google Scholar] [CrossRef]
- Eftekhari, M.; Ardekani, M.R.S.; Amin, M.; Attar, F.; Akbarzadeh, T.; Safavi, M.; Karimpour-Razkenari, E.; Amini, M.; Isman, M.; Khanavi, M. Oliveria decumbens, a bioactive essential oil: Chemical composition and biological activities. Iran. J. Pharm. Res. 2019, 18, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three Decades of P-gp Inhibitors: Skimming Through Several Generations and Scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef] [PubMed]
- Sisto, F.; Scaltrito, M.M.; Russello, G.; Bonomi, A.; Dubini, F. Antimicrobial susceptibility testing of Helicobacter pylori determined by microdilution method using a new medium. Curr. Microbiol. 2009, 58, 559–563. [Google Scholar] [CrossRef]
- Barranco, S.C.; Townsend, C.M.; Casartelli, C.; Macik, B.G.; Burger, N.L.; Boerwinkle, W.R.; Gourley, W.K. Establishment and Characterization of an in Vitro Model System for Human Adenocarcinoma of the Stomach. Cancer Res. 1983, 43, 1703–1709. [Google Scholar]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Ronci, M.; Del Prete, S.; Puca, V.; Carradori, S.; Carginale, V.; Muraro, R.; Mincione, G.; Aceto, A.; Sisto, F.; Supuran, C.T.; et al. Identification and characterization of the α-CA in the outer membrane vesicles produced by Helicobacter pylori. J. Enzyme Inhib. Med. Chem. 2019, 34, 189–195. [Google Scholar] [CrossRef]
| Compound | MIC/MBC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 190 | 23 | 110R | NCTC 11637 | F1 | F40/499 | F4 | F34/497 | |
| 1 (thymol) | 128/128 | 128/128 | 128/128 | 128/128 | 64/64 | 64/64 | 64/64 | 64/64 |
| 2 | ˃128/˃128 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | 128/128 | 128/128 | ˃128/˃128 | ˃128/˃128 |
| 3 | 64/64 | 64/128 | 64/64 | 128/128 | 32/32 | 32/32 | 64/128 | 128/128 |
| 4 | 128/128 | 128/128 | 128/128 | 64/128 | 128/128 | 64/64 | 64/64 | 64/64 |
| 5 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | 128/128 | ˃128/˃128 | ˃128/˃128 | 128/128 |
| 6 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 64/64 |
| 7 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 64/64 | 128/128 | 128/128 |
| 8 | 64/128 | 64/128 | 128/128 | 64/64 | 128/128 | 128/128 | 128/128 | 128/128 |
| 9 | 64/64 | 128/128 | 32/32 | 128/128 | 16/16 | 16/16 | 64/128 | 128/128 |
| 10 | 64/128 | 64/128 | 128/128 | 128/128 | 64/128 | 64/64 | 64/128 | 64/64 |
| 11 | 64/64 | 64/128 | 128/128 | 64/128 | 64/64 | 64/64 | 64/64 | 64/64 |
| 12 | 32/32 | 64/128 | 128/128 | 128/128 | 16/64 | 16/16 | 64/128 | 64/128 |
| 13 | 128/128 | 64/128 | 64/128 | 128/128 | 128/128 | 64/128 | 128/128 | 64/64 |
| 14 | 128/128 | 128/128 | 64/128 | 64/64 | 128/128 | 32/32 | 128/128 | 64/64 |
| 15 | 32/32 | 64/64 | 32/64 | 64/64 | 16/16 | 16/16 | 32/64 | 64/64 |
| 16 | 64/128 | 64/64 | 64/128 | 64/64 | 32/32 | 32/32 | 128/128 | 64/128 |
| 17 | 32/32 | 64/128 | 64/64 | 64/128 | 16/32 | 8/16 | 64/64 | 32/64 |
| 18 | 64/128 | 128/128 | 128/128 | 128/128 | 32/32 | 32/32 | 32/32 | 64/128 |
| 19 | 64/128 | 64/128 | 128/128 | 64/64 | 32/64 | 32/32 | 32/32 | 128/128 |
| 20 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | 64/64 | 64/64 | ˃128/˃128 | 128/128 |
| 21 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | 64/128 | 64/128 | ˃128/˃128 | 128/128 |
| 22 | 128/˃128 | ˃128/˃128 | 128/˃128 | 128/˃128 | 32/64 | 32/64 | ˃128/˃128 | 64/64 |
| 23 | 128/˃128 | 128/˃128 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 | ˃128/˃128 |
| 24 | 16/16 | 16/16 | 16/16 | 16/16 | 16/16 | 4/4 | 32/32 | 32/32 |
| 25 | 32/32 | 32/64 | 64/64 | 64/64 | 16/32 | 8/16 | 32/32 | 32/64 |
| 26 | 64/64 | 64/128 | ˃128/˃128 | 64/128 | 16/64 | 8/16 | 32/32 | 128/128 |
| 27 | 64/128 | 32/32 | 64/64 | 32/32 | 32/32 | 16/16 | 32/32 | 64/128 |
| 28 | 64/128 | 128/128 | 128/128 | 128/128 | 32/32 | 16/16 | 32/32 | 128/128 |
| 29 | 64/128 | 32/64 | 64/128 | 64/64 | 16/32 | 16/32 | 128/128 | 32/32 |
| 30 | 128/128 | 64/128 | 128/128 | 128/128 | 8/8 | 16/16 | 32/32 | 64/64 |
| 31 | 64/64 | 128/128 | 128/128 | 128/128 | 16/64 | 16/16 | 32/64 | 64/128 |
| 32 | 16/32 | 64/128 | 64/128 | 64/128 | 16/16 | 16/64 | 8/16 | 32/32 |
| 33 | 128/128 | 64/128 | 128/128 | 128/128 | 32/32 | 32/32 | 32/32 | 128/128 |
| 34 | 64/64 | 128/128 | 64/64 | 128/128 | 8/16 | 4/8 | 64/64 | 64/64 |
| 35 | 32/32 | 32/32 | 32/64 | 32/32 | 16/32 | 4/4 | 64/128 | 16/32 |
| 36 | 32/64 | 32/32 | 16/16 | 32/32 | 32/32 | 32/32 | 32/32 | 128/128 |
| 37 | ˃128/˃128 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | 128/˃128 | ˃128/˃128 | ˃128/˃128 | 128/˃128 |
| 38 | 32/64 | 32/64 | 32/64 | 32/32 | 8/8 | 4/4 | 8/16 | 16/32 |
| 39 | 16/16 | 32/64 | 32/64 | 16/32 | 16/32 | 16/32 | 32/64 | 16/32 |
| carvacrol | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 32/64 | 64/64 | 16/32 |
| Antibiotic susceptibility | MTZ− CLR− AMX− | MTZ− CLR− AMX− | MTZ+ CLR− AMX− | MTZ+ CLR− AMX− | MTZ− CLR+ AMX− | MTZ+ CLR+ AMX− | MTZ+ CLR+ AMX− | MTZ+ CLR+ AMX− |
| Compound | IC50 (μM) a |
|---|---|
| thymol (1) | 200 ± 6.5 |
| 9 | 100 ± 5.8 |
| 15 | 93.5 ± 7.6 |
| 24 | 365 ± 8.5 |
| 25 | 607 ± 9.5 |
| 26 | 396 ± 8.5 |
| 30 | 613 ± 9.9 |
| 34 | 600 ± 9.0 |
| 35 | na |
| 36 | 574 ± 9.0 |
| 38 | 194 ± 6.0 |
| 39 | na |
| Carvacrol b | 300 ± 6.4 |
| 5-FU | 82.3 ± 5.6 |
| Compound | 9 | 15 | 38 |
|---|---|---|---|
| Molecular weight (MW) b | 206.32 | 240.34 | 316.44 |
| H-bond acceptors (HBA) | 1 | 1 | 1 |
| H-bond donators (HBD) | 0 | 0 | 0 |
| Consensus Log PO/W * | 4.22 | 4.52 | 5.78 |
| Lipinski violations | 0 | 1 | 1 |
| GI absorption | high | high | low |
| P-gp substrate | no | no | no |
| PAINS alerts | no alert | no alert | no alert |
| WLOGP a | 4.30 | 4.55 | 6.21 |
| TPSA (Å2) a,b | 9.23 | 9.23 | 9.23 |
| XLOGP3 b | 5.00 | 5.25 | 6.50 |
| Log S (ESOL) b | −4.24 | −4.87 | −6.12 |
| Fraction Csp3 b | 0.57 | 0.29 | 0.22 |
| N° of rotatable bonds b | 5 | 4 | 5 |
| MLOGP | 3.87 | 4.36 | 5.48 |
| boiled-egg graph | 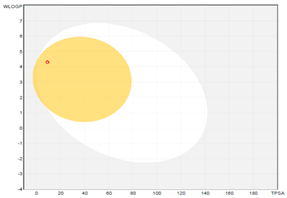 | 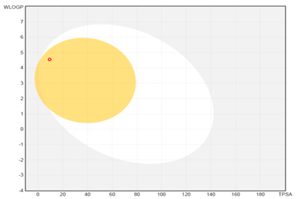 | 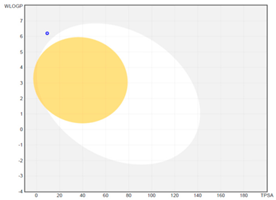 |
| bioavailability radar |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisto, F.; Carradori, S.; Guglielmi, P.; Spano, M.; Secci, D.; Granese, A.; Sobolev, A.P.; Grande, R.; Campestre, C.; Di Marcantonio, M.C.; et al. Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules 2021, 26, 1829. https://doi.org/10.3390/molecules26071829
Sisto F, Carradori S, Guglielmi P, Spano M, Secci D, Granese A, Sobolev AP, Grande R, Campestre C, Di Marcantonio MC, et al. Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules. 2021; 26(7):1829. https://doi.org/10.3390/molecules26071829
Chicago/Turabian StyleSisto, Francesca, Simone Carradori, Paolo Guglielmi, Mattia Spano, Daniela Secci, Arianna Granese, Anatoly P. Sobolev, Rossella Grande, Cristina Campestre, Maria Carmela Di Marcantonio, and et al. 2021. "Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line" Molecules 26, no. 7: 1829. https://doi.org/10.3390/molecules26071829
APA StyleSisto, F., Carradori, S., Guglielmi, P., Spano, M., Secci, D., Granese, A., Sobolev, A. P., Grande, R., Campestre, C., Di Marcantonio, M. C., & Mincione, G. (2021). Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules, 26(7), 1829. https://doi.org/10.3390/molecules26071829














