Four New Gallate Derivatives from Wine-Processed Corni Fructus and Their Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Results and Discussion
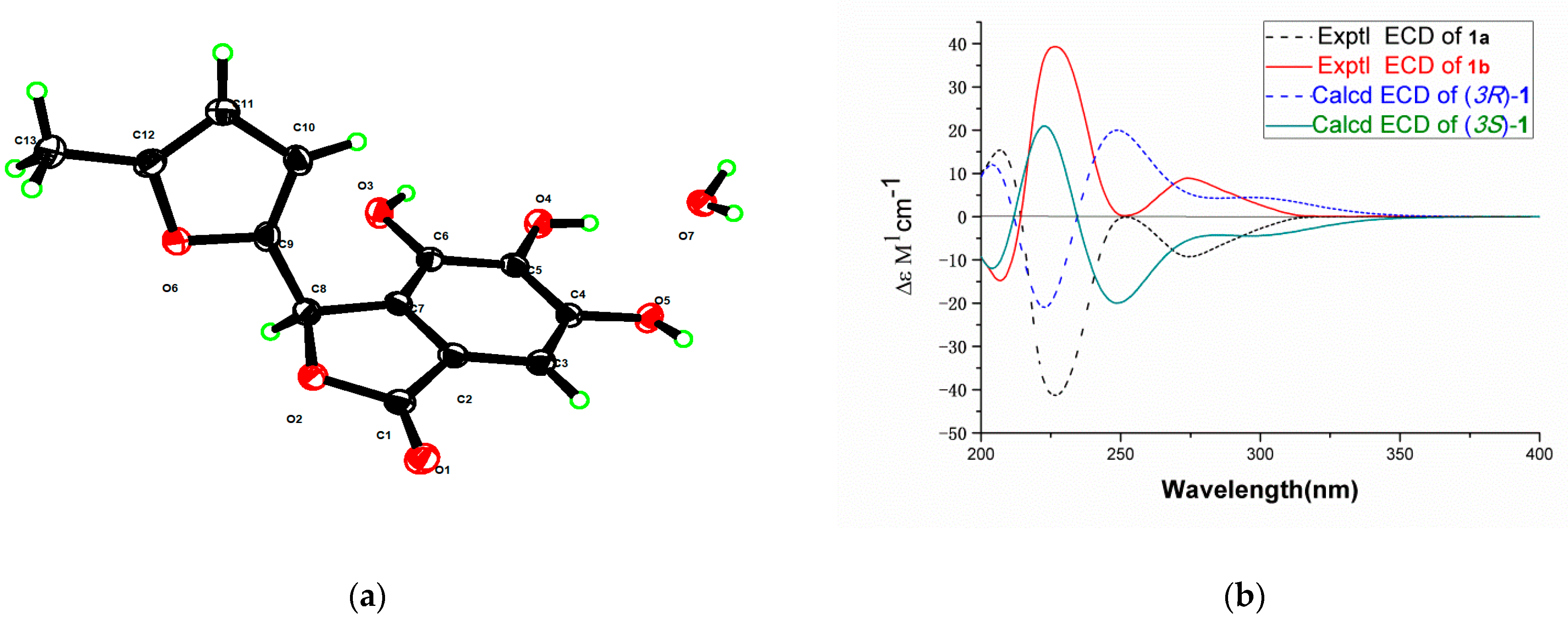
2.1. Structure Elucidation
2.2. Anti-Inflammatory Effects of Compounds 1–3
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. General Experimental Procedures
4.3. Cell Lines, Chemicals, and Biochemicals
4.4. Extraction and Isolation
4.5. Compounds Characterization Data
4.6. Anti-Inflammatory Bioassays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- China Flora Editorial Board (Chinese Academy of Sciences). In Flora of China; Science Press: Beijing, China, 1990; Volume 56, p. 83.
- He, J.; Ye, X.-S.; Wang, X.-X.; Yang, Y.-N.; Zhang, P.-C.; Ma, B.-Z.; Zhang, W.-K.; Xu, J.-K. Four new iridoid glucosides containing the furan ring from the fruit of Cornus officinalis. Fitoter. 2017, 120, 136–141. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 27–28. [Google Scholar]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- Lee, S.H.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Sedoheptulose digallate from Cornus officinalis. Phytochemistry 1989, 28, 3469–3472. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Wang, R.; Shi, Y.-P. Chemical constituents from the fruits of Cornus officinalis. Biochem. Syst. Ecol. 2012, 45, 120–123. [Google Scholar] [CrossRef]
- Ji, L.-L.; Wang, X.; Li, J.-J.; Zhong, X.-J.; Zhang, B.; Juan, J.; Shang, X.-Y. New Iridoid Derivatives from the Fruits of Cornus officinalis and Their Neuroprotective Activities. Mol. 2019, 24, 625. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.-S.; He, J.; Cheng, Y.-C.; Zhang, L.; Qiao, H.-Y.; Pan, X.-G.; Zhang, J.; Liu, S.-N.; Zhang, W.-K.; Xu, J.-K. Cornusides A–O, Bioactive Iridoid Glucoside Dimers from the Fruit of Cornus officinalis. J. Nat. Prod. 2017, 80, 3103–3111. [Google Scholar] [CrossRef]
- Yao, R.-Q.; Zhang, L.; Wang, W.; Li, L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res. Bull. 2009, 79, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.J.; Dai, B.; Li, X.Y.; Yang, L.M.; Xiao, Z.Z.; Shi, L.J. Research Progress in Processing of Corni Fructus with Wine. Chin. J. Mod. Appl. Pharm. 2016, 33, 1604–1608. [Google Scholar] [CrossRef]
- Gong, C.X.; Guo, L.J.; Pan, J.J.; Wu, F.Z. The development of Corni Fructus quality standard considering the effects of processing. Chin. J. Chem. Eng. 2020, 1–23. [Google Scholar] [CrossRef]
- Jia, M.H.; Yu, H.Y.; Yu, M.; Zhang, W.H.; Ju, G.C.; Gao, L.; Zou, M.Z. Qualitative and quantitative analysis of characteristic chemical constituents in wine-processed Corni Fructus by chemometric and UPLC-PDA. Chin. Tradit. Herbal Drugs 2020, 51, 1294–1301. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.-S.; Pan, M.-H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 1–37. [Google Scholar]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Butin, A.V.; Dmitriev, A.S.; Uchuskin, M.G.; Abaev, V.T.; Trushkov, I.V. ChemInform Abstract: Simple and Convenient Synthesis of 4-Unsubstituted-3-(3-oxoalkyl)isocoumarins. Chemin 2008, 39, 1569–1578. [Google Scholar] [CrossRef]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Itoh, A.; Hamada, N. Three isocoumarins and a benzofuran from the cultured lichen mycobionts of Pyrenula sp. Phytochemistry 2004, 65, 3119–3123. [Google Scholar] [CrossRef] [PubMed]
- Jogia, M.; Vakamoce, V.; Weavers, R. Synthesis of Some Furfural and Syringic Acid Derivatives. Aust. J. Chem. 1985, 38, 1009–1016. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin Inhibits LPS-Induced Chemokine and Pro-inflammatory Cytokine Secretion from RAW 264.7 Macrophages via Blocking TLRs/NF-κB Signal Transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef]
- Kamada, T.; Kang, M.-C.; Phan, C.-S.; Zanil, I.I.; Jeon, Y.-J.; Vairappan, C.S. Bioactive Cembranoids from the Soft Coral Genus Sinularia sp. in Borneo. Mar. Drugs 2018, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-B.; Lee, Y.-J.; Park, S.K.; Kim, H.-C.; Bae, H.; Kim, H.M.; Ko, S.-G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Yun, K.-J.; Kim, J.-Y.; Kim, J.-B.; Lee, K.-W.; Jeong, S.-Y.; Park, H.-J.; Jung, H.-J.; Cho, Y.-W.; Yun, K.; Lee, K.-T. Inhibition of LPS-induced no and PGE2 production by asiatic acid via NF-κB inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]

 ) spectra of compounds 1–3.
) spectra of compounds 1–3.

| No. | Compounds 1a and 1b | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 173.2 | - | 163.3 | - | 165.6 |
| 2 | - | 119.1 | ||||
| 3 | 6.34, s | 75.4 | - | 147.8 | 6.92, s | 108.7 |
| 4 | - | 141.4 | 7.19, s | 109.6 | - | 145.5 |
| 5 | - | 141.3 | - | 142.4 | - | 138.6 |
| 6 | - | 149.0 | - | 141.3 | - | 145.5 |
| 7 | 6.85, s | 103.0 | - | 149.5 | 6.92, s | 108.7 |
| 8 | - | 117.5 | 7.25, s | 107.2 | ||
| 9 | - | 127.1 | - | 120.6 | ||
| 10 | - | 114.0 | ||||
| 1′ | 2.36, s | 27.5 | ||||
| 2′ | - | 148.4 | - | 200.3 | - | 151.8 |
| 3′ | 6.22, d (2.8) | 112.2 | 6.70, d (15.8) | 126.9 | 7.52, d (3.7) | 124.3 |
| 4′ | 5.98, d (2.6) | 107.3 | 7.26, d (12.1) | 136.0 | 6.70, d (3.5) | 109.8 |
| 5′ | - | 154.5 | - | 161.4 | ||
| 6′ | 2.24, s | 13.2 | 9.56, s | 178.2 | ||
| 7′ | 4.97, t (5.8) | 64.8 | ||||
| 8′ | 4.37, dd (11.3, 6.4) | 65.7 | ||||
| 4.41, dd (11.3, 5.3) | ||||||
| Sample | c (μM) | NO Release (μM) | NO Inhibition Rate (%) |
|---|---|---|---|
| Control | - | 8.76 ± 0.84 | - |
| Model c | - | 14.23 ± 0.84 | - ** |
| 1 | 25 | 7.89 ± 0.87 | 115.85 ± 2.86 ** |
| 50 | 4.11 ± 1.02 | 184.96 ± 1.73 ** | |
| 2 | 25 | 8.63 ± 0.93 | 102.26 ± 2.48 ** |
| 50 | 3.33 ± 0.64 | 199.09 ± 1.98 ** | |
| 3 | 25 | 11.80 ± 0.56 | 44.38 ± 1.16 ** |
| 50 | 7.06 ± 0.69 | 130.98 ± 1.74 ** | |
| dexamethasone b | 3 | 11.49 ± 0.94 | 50.13±2.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-B.; Feng, Q.-M.; Zhang, L.-X.; Wang, J.; Chi, J.; Chen, S.-Q.; Wang, Z.-M.; Dai, L.-P.; Xu, E.-P. Four New Gallate Derivatives from Wine-Processed Corni Fructus and Their Anti-Inflammatory Activities. Molecules 2021, 26, 1851. https://doi.org/10.3390/molecules26071851
Li H-B, Feng Q-M, Zhang L-X, Wang J, Chi J, Chen S-Q, Wang Z-M, Dai L-P, Xu E-P. Four New Gallate Derivatives from Wine-Processed Corni Fructus and Their Anti-Inflammatory Activities. Molecules. 2021; 26(7):1851. https://doi.org/10.3390/molecules26071851
Chicago/Turabian StyleLi, Hong-Bin, Qing-Mei Feng, Ling-Xia Zhang, Jing Wang, Jun Chi, Sui-Qing Chen, Zhi-Min Wang, Li-Ping Dai, and Er-Ping Xu. 2021. "Four New Gallate Derivatives from Wine-Processed Corni Fructus and Their Anti-Inflammatory Activities" Molecules 26, no. 7: 1851. https://doi.org/10.3390/molecules26071851
APA StyleLi, H.-B., Feng, Q.-M., Zhang, L.-X., Wang, J., Chi, J., Chen, S.-Q., Wang, Z.-M., Dai, L.-P., & Xu, E.-P. (2021). Four New Gallate Derivatives from Wine-Processed Corni Fructus and Their Anti-Inflammatory Activities. Molecules, 26(7), 1851. https://doi.org/10.3390/molecules26071851





