Monitoring and Statistical Analysis of Formation of Organochlorine and Organobromine Compounds in Drinking Water of Different Water Intakes
Abstract
:1. Introduction
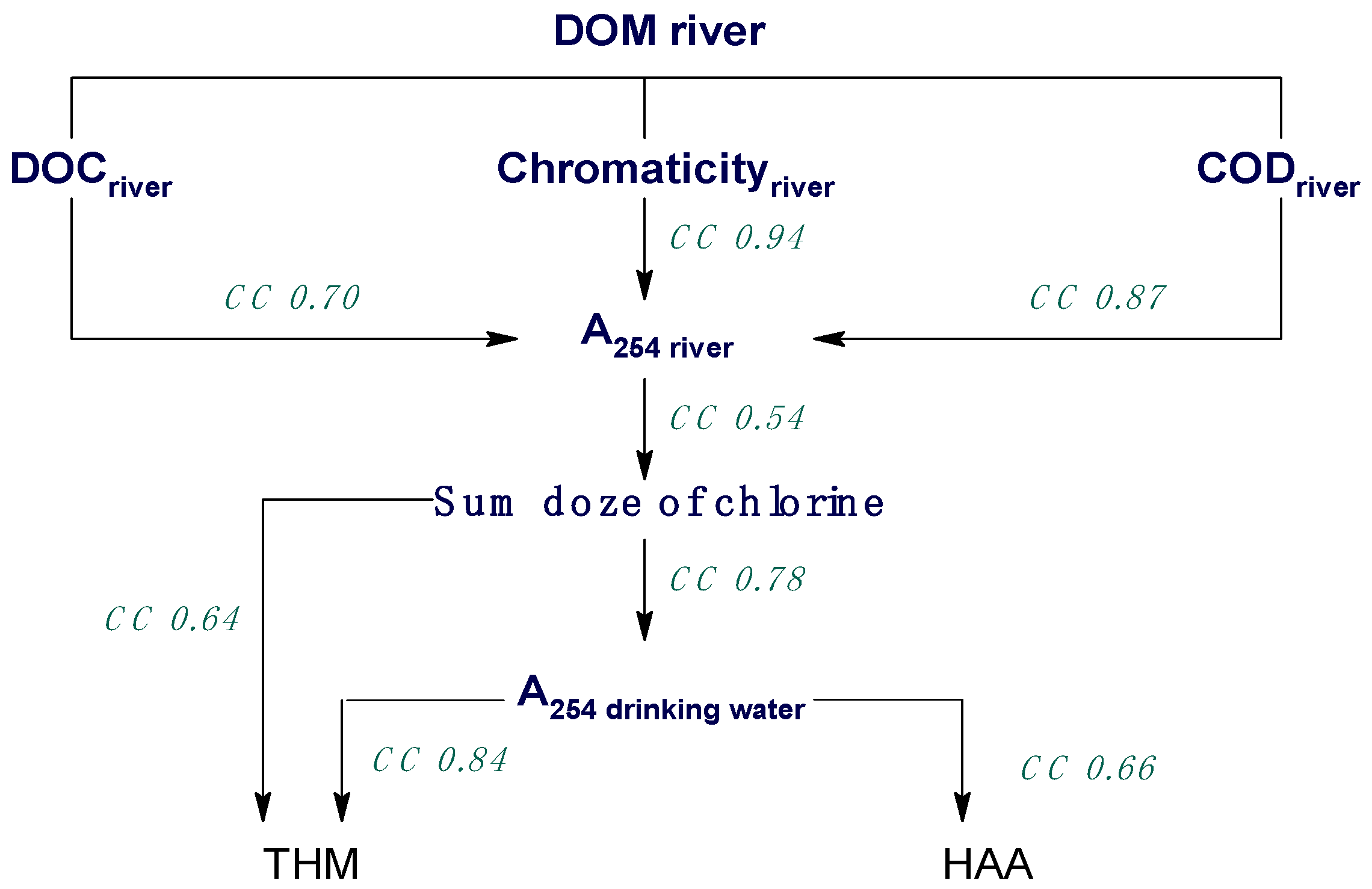
2. Results and Discussion
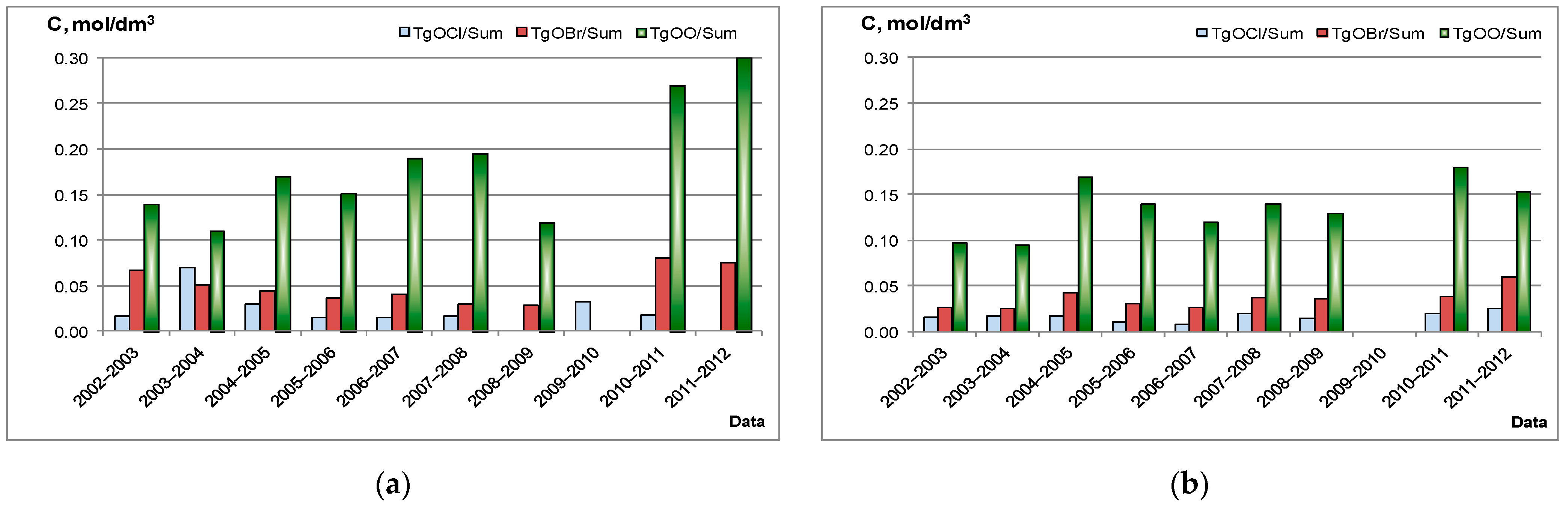
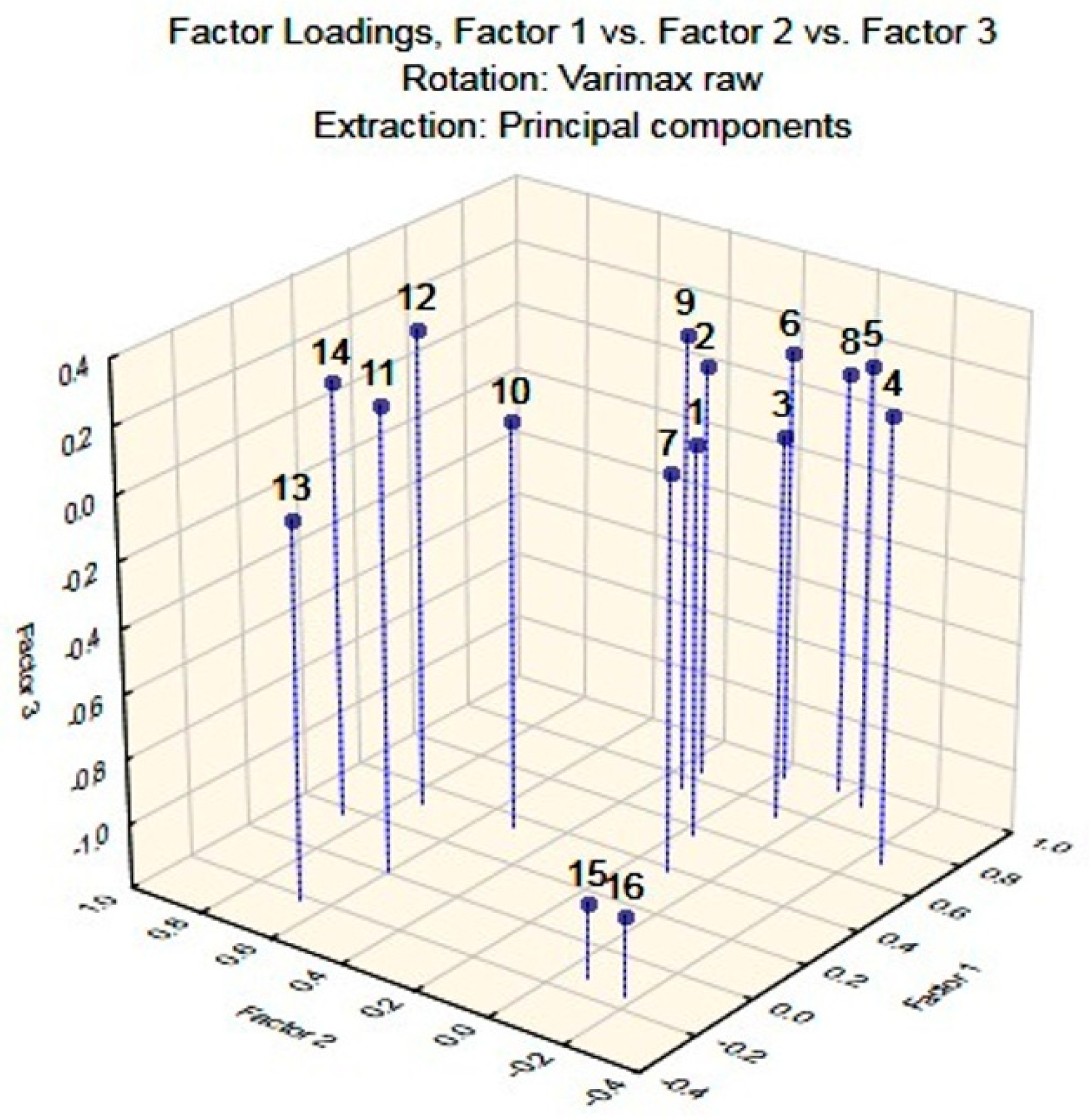
2.1. Chemometric Approaches
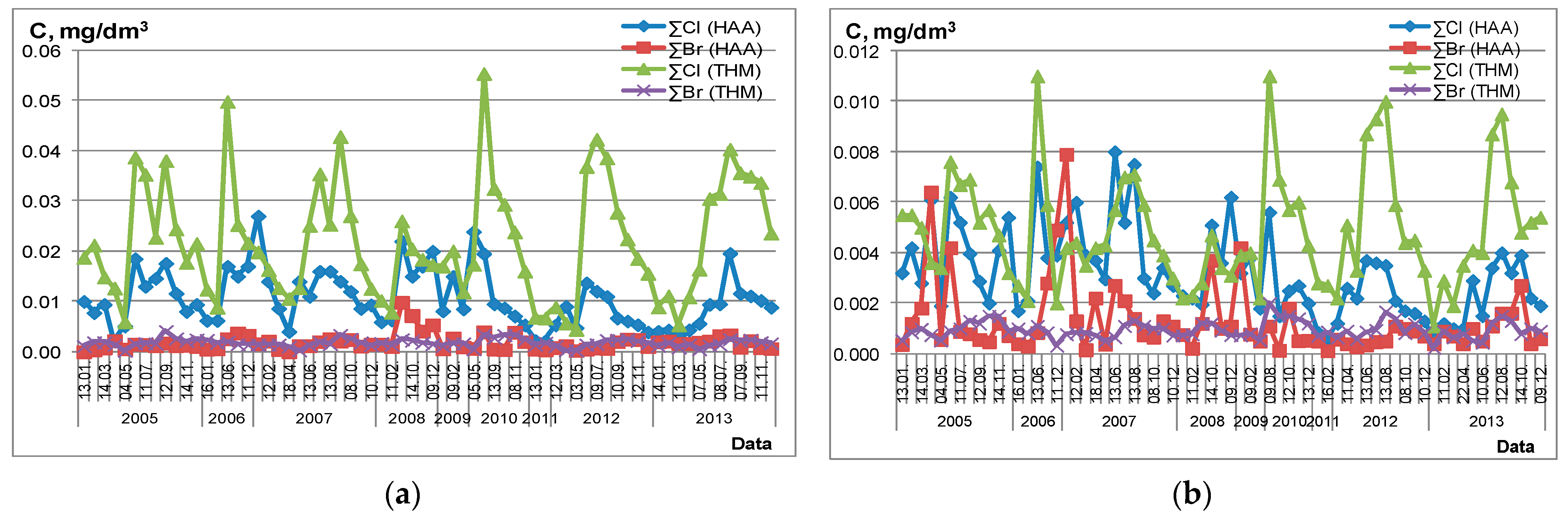
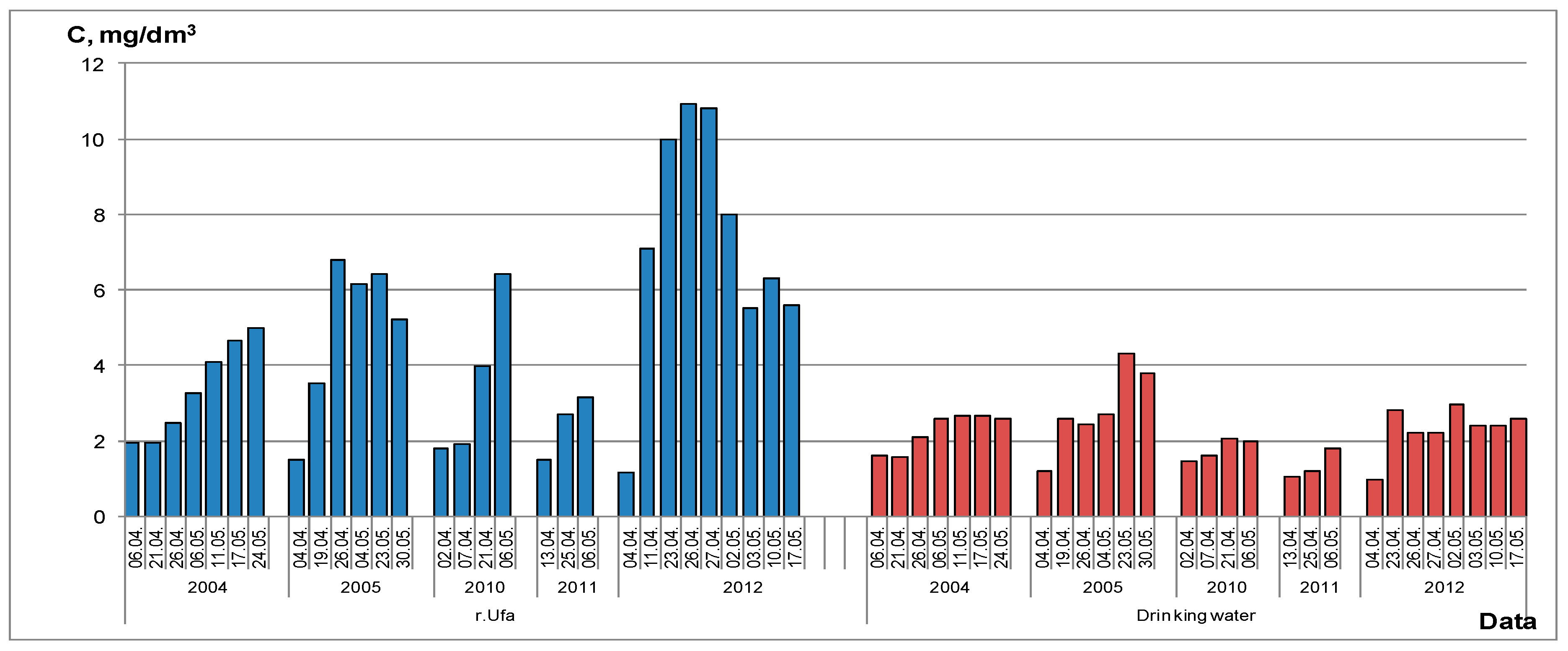
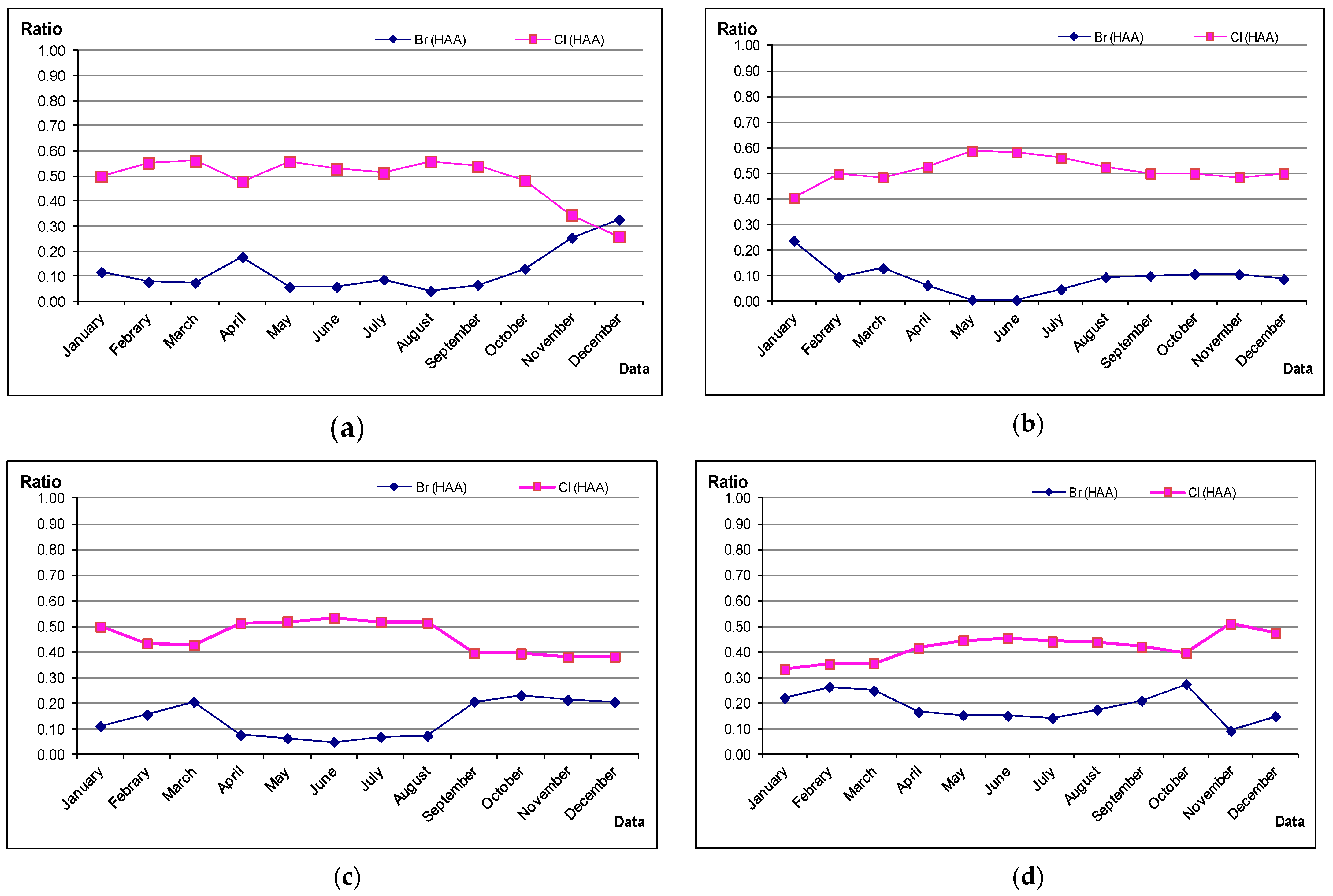
2.2. Seasonal Peculiarities
3. Materials and Methods
3.1. Sampling
- -
- drinking water of the surface water intake (SW). Water comes from the Ufa River and is subjected to multistage purification including UV irradiation, primary chlorination, coagulation with aluminum sulfate, flocculation with polyacrylamide, upholding, fast filtration through filters with baked clay and secondary chlorination;
- -
- drinking water of the infiltration water intake (IW). Water comes from the infiltration wells and is subjected only to the single-stage disinfection with molecular chlorine;
- -
- water of the River Ufa before and after aqueous chlorination in the laboratory with various doses of molecular chlorine;
- -
- water from the joint collector at the infiltration water intake (well depth ~25 m, distance ~120 m from the river).
3.2. Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cutler, D.; Miller, G. The role of public health improvements in health advances: The twentieth-century United States. Demography 2005, 42, 1–22. [Google Scholar] [CrossRef] [PubMed]
- CDC. History of Drinking Water Treatment; US Department of Health & Human Services; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2012.
- Bellar, T.A.; Lichtenberg, J.J.; Kroner, R.C. Occurrences of organohalides in chlorinated drinking waters. J. Am. Water Works Assoc. 1974, 66, 703–706. [Google Scholar] [CrossRef]
- Rook, J.J. Chlorination reactions of fulvic acids in natural waters. Environ. Sci. Technol. 1977, 11, 478–482. [Google Scholar] [CrossRef]
- Manuel, J.; Rodriguez, M.; Levallois, S.; Levallois, P. Behavior of trihalomethanes and haloacetic acids in a drinking water distribution system. Water Res. 2004, 38, 4367–4382. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Zhu, L.; Liu, Z.; He, W.; Han, H. Disinfection by-products and their precursors in a water treatment plant in North China: Seasonal changes and fraction analysis. Sci. Total Environ. 2008, 397, 140–147. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Sung, W.; Reilly-Matthews, B.; O’Day, D.K.; Horrigan, K. Modeling DBP Formation. J. Am. Water Works Assoc. 2005, 92, 53–63. [Google Scholar] [CrossRef]
- Trukhanova, E.V.; Vozhdaeva, M.Y.; Kantor, L.I.; Kantor, E.A. Basic By-products Formation During Chlorination of Water Containing Humic Substances. In Proceedings of the 15th International Humic Substances Society Meeting, Book of Abstracts, Tenerife, Canary Islands, Spain, 27 June–2 July 2010; p. 67. [Google Scholar]
- Richardson, S.D. Disinfection by-products: Formation and occurrence in drinking water. Encycl. Environ. Health 2011, 2, 110–136. [Google Scholar] [CrossRef]
- Richardson, S.D.; Postigo, C. Discovery of New Emerging DBPs by High-Resolution Mass Spectrometry. Compr. Anal. Chem. 2016, 71, 335–356. [Google Scholar] [CrossRef]
- Bull, R.J.; Reckhow, D.A.; Li, X.; Humpage, A.R.; Joll, C.; Hrudey, S.E. Potential carcinogenic hazards of non-regulated disinfection by-products: Haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology 2011, 286, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Proulx, F.; Rodriguez, M.J. Occurrence and spatiotemporal variability of halogenated acetaldehydes in full-scale drinking water systems. Sci. Total Environ. 2019, 323, 1–33. [Google Scholar] [CrossRef]
- Vozhdaeva, M.Y.; Kholova, A.R.; Vagner, E.V.; Kantor, E.A.; Kantor, L.I.; Trukhanova, N.V.; Melnitsky, I.A. The use of results of expanded monitoring research for the integrated assessment of drinking water according to indices of chemical harmlessness. Hyg. Sanit. 2018, 97, 117–124. [Google Scholar] [CrossRef]
- Richardson, S.D.; Temes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef]
- Wong, H.K.M.; Mok, X.J. Fan Natural organic matter and formation of trihalomethanes in two water treatment processes. Desalination 2007, 210, 44–51. [Google Scholar] [CrossRef]
- Zhang, X.; Roger, A. Minear Formation, adsorption and separation of high molecular weight disinfection by-products resulting from chlorination of aquatic humic substances. Water Res. 2006, 40, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Zhao, Q.L.; Xue, S. Reduction of trihalomethane precursors of dissolved organic matter in the secondary effluent by advanced treatment processes. J. Hazard. Mater. 2009, 169, 1012–1021. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D. Environmental Mass Spectrometry: Emerging Contaminants and Current Issues. Anal. Chem. 2012, 84, 747–778. [Google Scholar] [CrossRef]
- Yang, X.; Guo, W.; Shen, Q. Formation of disinfection byproducts from chlo(am)ination of algal organic matter. J. Hazard. Mater. 2011, 197, 378–388. [Google Scholar] [CrossRef]
- Lui, Y.S.; Qiu, J.W.; Zhang, Y.L.; Wong, M.H.; Liang, Y. Algal-derived organic matter as precursors of disinfec-tion by-products and mutagens upon chlorination. Water Res. 2011, 45, 1454–1462. [Google Scholar] [CrossRef]
- Veresmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 7315. [Google Scholar] [CrossRef]
- Lebedev, A.T. Mass spectrometry in the study of mechanisms of aquatic chlorination of organic substrates. Eur. J. Mass Spectrom. 2007, 13, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Popov, M.S.; Latkin, T.B.; Lebedev, A.T. Halogenated fatty amides—A brand new class of disinfection byproducts. Water Res. 2017, 127, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.T.; Bavkon Kralj, M.; Polyakova, O.V.; Detenchuk, E.A.; Pokryshkin, S.A.; Trebse, P. Identification of avobenzone by-products formed by various disinfectants in different types of swimming pool waters. Environ. Int. 2020, 137, 105495. [Google Scholar] [CrossRef]
- Lebedev, A.T. Environmental Mass Spectrometry. Annu. Rev. Anal. Chem. 2013, 6, 163–189. [Google Scholar] [CrossRef]
- Ferretti, E.; Lucentini, L.; Veschetti, E.; Bonadonna, L.; Stammati, A.; Turco, L.; Ottaviani, M. Screening and identification of unknown contaminants in water destined to human consumption: A case study. Microchem. J. 2007, 85, 57–64. [Google Scholar] [CrossRef]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and otherorganic wastewater contaminants in the United States—II Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, J.; Liu, H.; Zhao, X. Characterization of isolated fractions of dissolved organic matter from sewage treatment plant and the related disinfection by-products formation potential. J. Hazard. Mater. 2009, 164, 1433–1438. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Characterization of disinfection byproduct precursors based on hydrophobicity and molecular size. Environ. Sci. Technol. 2007, 41, 3309–3315. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, X.; Zhang, M. Removal of dissolved organic matter in municipal effluent with ozonation, slow sand filtration and nanofiltration as high quality pre-treatment option for artificial groundwater recharge. Chemosphere 2011, 83, 693–699. [Google Scholar] [CrossRef]
- Anett, G.; Annett, R.; Ulf, T.; Frank-Dieter, K. Influence of sorption to dissolved humic substances on transformation reactions of hydrophobic organic compounds in water. I Chlorination of PAHs. Environ. Sci. Technol. 2007, 41, 7003–7009. [Google Scholar] [CrossRef]
- Lekkas, S.T.; Nikolaou, A.; Golfinopoulos, S. Structural investigations of aquatic humic substances from different watersheds. Desalination 2007, 210, 125–137. [Google Scholar] [CrossRef]
- Lowe, J.; Hossain, M.M. Application of ultrafiltration membranes for removal of humic acid from drinking water. Desalination 2008, 218, 343–354. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Suphandag, S.A.; Kerc, A.; Bekbolet, M. Evaluation of adsorption and coagulation characteristics of humic acids preceded by alternative advanced oxidation techniques. Desalination 2007, 210, 183–193. [Google Scholar] [CrossRef]
- Klymenko, N.A.; Kozyatnyk, I.P.; Savchyna, L.A. Removing of fulvic acids by ozonation and biological active carbon filtration. Water Res. 2010, 44, 5316–5322. [Google Scholar] [CrossRef]
- Sun, C.; Yue, Q.; Gao, B.; Mu, R.; Liu, J.; Zhao, Y.; Yang, Z.; Xu, W. Effect of pH and shear force on flocs characteristics for humic acid removal using polyferric aluminum chloride–organic polymer dual-coagulants. Desalination 2011, 281, 243–247. [Google Scholar] [CrossRef]
- Nawrocki, J.; Bilozor, S. Brominated oxidation by-products in drinking water treatment. J. Water SRT AQUA 1997, 46, 304–323. [Google Scholar]
- Sun, Y.; Wu, Q.; Hu, H.; Tian, J. Effect of bromide on the formation of disinfection by-products during wastewater chlorination. Water Res. 2009, 43, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X. Modeling the formation of TOCl, TOBr and TOI during chlor (am) ination of drinking water. Water Res. 2016, 96, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Carbohydrates as trihalomethanes precursors. Influence of pH and the presence of ClL and BrL on trihalomethane formation potential. Water Res. 2008, 42, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Z.; Qiu, Y.; Zhao, J. Effect of ferric and bromide ions on the formation and speciation of disinfection byproducts during chlorination. J. Environ. Sci. 2011, 23, 765–772. [Google Scholar] [CrossRef]
- Inabaa, K.; Doi, T.; Isobe, N.; Yamamoto, T. Formation of bromo-substituted triclosan during chlorination by chlorine in the presence of trace levels of bromide. Water Res. 2006, 40, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, A.I.; Vozhdaeva, M.Y.; Gagarina, L.N.; Tsypysheva, L.G.; Martynenkova, L.N.; Kantor, L.I. Sources of formation of organobromide compounds in drinking water. Post 2. Analysis of environmental objects. In Proceedings of the “V All-Russian Conference” Ecoanalytics-2000” with International Participation, Krasnodar, Russia, 17–23 September 2000; pp. 282–284. [Google Scholar]
- Shaydullina, G.M.; Sinikova, N.A.; Lebedev, A.T. Reaction of ortho-methoxybenzoic acid with the water disinfecting agents ozone, chlorine and sodium hypochlorite. Environ. Chem. Lett. 2005, 3, 1–5. [Google Scholar] [CrossRef]
- Sinikova, N.A.; Shaydullina, G.M.; Lebedev, A.T. Comparison of Chlorine and Sodium Hypochlorite Activity in the Chlorination of Structural Fragments of Humic Substances in Water Using GC–MS. J. Anal. Chem. 2014, 69, 1300–1306. [Google Scholar] [CrossRef]
- Richardson, S.; Plewa, M.; Wagner, E.; Schoeny, R.; DeMarini, D. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Plewa, M.J.; Simmons, J.E.; Richardson, S.D.; Wagner, E.D. Mammalian cell cytotoxicity and genotoxicity of the haloaceticacids, a major class of drinking water disinfection byproducts. Environ. Mol. Mutagen. 2010, 51, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Muellner, M.G.; Richardson, S.D.; Fasano, F.; Buettner, K.M.; Woo, Y.T.; McKague, A.B.; Wagne, R.E.D. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection by-products. Environ. Sci. Technol. 2008, 42, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Snyder, S.A.; Sedlak, D.L. Use of biodegradable dissolved organic carbon (BDOC) to assess the potential for transformation of wastewater-derived contaminants in surface waters. Water Res. 2008, 42, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Shang, C. Evaluation and improvement of total organic bromine analysis with respect to reductive property of activated carbon. Water Res. 2011, 45, 1229–1237. [Google Scholar] [CrossRef]
- Ates, N.; Kitis, M.; Yetis, U. Formation of chlorination by-products in waters with low SUVA—Correlations with SUVA and differential UV Spectroscopy. Water Res. 2007, 41, 4139–4148. [Google Scholar] [CrossRef]
- Liu, B.; Gu, L.; Yu, X.; Yu, G.; Zhang, H.; Xu, J. Dissolved organic nitrogen (DON) profile during back-ishing cycle of drinking water biofiltration. Sci. Total Environ. 2012, 414, 508–514. [Google Scholar] [CrossRef]
- Muller, G. Sense or no-sense of the sum parameter for water soluble “adsorbable organic halogens” (aox) and “absorbed organic halogens” (aox-s18) for the assessment of organohalogens in sludges and sediments. Chemosphere 2003, 52, 371–379. [Google Scholar] [CrossRef]
- Miyuake, Y.; Kato, M.; Urano, K. A method for measuring semi- and non-volatile organic halogens by combustion ion chromatography. J. Chromatogr. 2007, 1139, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Viryus, E.D.; Kapinus, E.N.; Revelsky, I.A.; Borzenko, A.G. Determination of the total content of organochlorine compounds in water based on microfluidic extraction and microculonometric analysis of the extract. Ind. Lab. 2003, 69, 3–6. [Google Scholar]
- Vozhdaeva, M.Y.; Tsypysheva, L.G.; Kantor, L.I.; Kantor, E.A. Effect of chlorination on the composition of limited-volatile organic pollutants of water. J. Appl. Chem. 2004, 7, 952–955. [Google Scholar]
- Rodrigues, P.; Joaquim, C.; Silva, E.; Antunes, M. Factorial analysis of the trihalomethanes formation in water disinfection using chlorine. Anal. Chim. Acta 2007, 595, 266–274. [Google Scholar] [CrossRef]
- Lui, Y.S.; Hong, H.C.; Zheng, G.J.S.; Liang, Y. Fractionated algal organic materials as precursors of disinfection by-products and mutagens upon chlorination. J. Hazard. Mater. 2012, 209–210, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Matucha, M.; Gryndler, M.; Schröder, P.; Forczek, S.T.; Uhlířová, H.; Fuksová, K.; Rohlenová, J. Chloroacetic acids—Degradation intermediates of organic matter in forest soil. Soil Biol. Biochem. 2007, 39, 382–385. [Google Scholar] [CrossRef]
- Chu, W.; Gao, N.; Deng, Y.; Templeton, M.R.; Yin, D. Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresour. Technol. 2011, 102, 11161–11166. [Google Scholar] [CrossRef]
- Pomes, M.L.; Green, W.R.; Thurman, E.M.; Orem, W.H.; Lerch, H.E. DBP formation of aquatic humic substances. J. Am. Water Works Assoc. 1999, 91, 103–115. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Varsegov, I.S.; Popov, M.S.; Lebedev, A.T. Identification of novel disinfection byproducts in pool water: Chlorination of the algaecide benzalkonium chloride. Chemosphere 2020, 239, 124801. [Google Scholar] [CrossRef]
- Doerffel, K. Statistics in Analytical Chemistry; Adler, Y.P., Ed.; Mir: Moscow, Russia, 1994; pp. 159–164. [Google Scholar]
- Yang, X.; Shang, C.; Lee, W.; Westerhoff, P.; Fan, C. Correlations between organic matter properties and DBP formation during chloramination. Watter Res. 2008, 42, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Fabbricinoa, M.; Korshin, G.V. Modelling disinfection by-products formation in bromide-containing waters. J. Hazard. Mater. 2009, 168, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Banowetz, G.M.; Whittaker, G.W.; Dierksen, K.P.; Azevedo, M.D.; Kennedy, A.C.; Griffith Stephen, M.; Steiner Jeffrey, J. Fatty acid methyl ester analysis to identify sources of soil in surface water. J Environ. Qual. 2006, 35, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Vozhdaeva, M.Y.; Wagner, E.V.; Cantor, L.I.; Konstantinov, A.I.; Perminova, I.V.; Cantor, E.A.; Trukhanova, N.V.; Melnitsky, I.A. Effect of Seasonal Dynamics and Chemical Treatment on the Quality of Dis-solved Organic Matter in Water Sources and Potable Water of Ufa. Mosc. Univ. Chem. Bull. 2017, 72, 154–159. [Google Scholar] [CrossRef]
- Romanovskaya, S.L.; Kantor, L.I.; Kantor, E.A.; Khabibullin, R.R. Analysis of the total stiffness in the water source and drinking water. In Proceedings of the Ekvatek-2004, Moscow, Russia, 1–4 June 2004; p. 822. [Google Scholar]
- Turecek, F.; McLafferty, F.W. Interpretation of Mass Spectra, 4th ed.; University Science Books: Mill Valley, CA, USA, 1993. [Google Scholar]
- Vozhdaeva, M.Y.; Tsypysheva, L.G.; Kantor, L.I.; Kantor, E.A. Efficiency of the combination of mass-selective and atomic emission detection in chromatographic analysis of water quality. Mass Spectrom. 2005, 2, 229–236. [Google Scholar]
- Available online: https://statistica.software.informer.com/10.0/ (accessed on 20 October 2020).



| Name | CAS | Characteristic Ions in the Mass Spectra (m/z (Intensity)) |
|---|---|---|
| 1-Bromopropanone-2 | 598-31-2 | 43 (100); 136 (12); 138 (11) |
| 2-Methyl-4-bromobutene-1 | 20038-12-4 | 69 (100); 41 (60); 55 (25)) |
| 1,1-Dimethyl-3-chloropropanol-1 | 1985-88-2 | 59 (100); 43 (71); 107 (25) |
| 2,3-Dichloro-2-methylbutane | 507-45-9 | 77 (100); 41 (51); 76 (33) |
| 1,1,1-Trichloropropanone-2 | 918-00-3 | 43 (100); 125 (11); 127 (7) |
| 2-Methyl-3-bromobutanol-2 | 2588-77-4 | 59 (100); 151 (15); 153 (14) |
| 1,1-Dibromopropanone-2 | 867-54-9 | 43 (100); 173 (64); 216 (44) |
| 1-Bromo-2-methylbutanol-2 | 31736-62-6 | 73 (100); 57 (36); 137 (29) |
| 1,2,3-Trichloropropene-1 | 13116-57-9 | 109 (100); 144 (20); 146 (15) |
| 1,3-Dibromopentane | 42474-20-4 | 69 (100); 149 (77); 151 (74) |
| 1-Bromo-2,3-dichloropropane | 33037-07-9 | 111 (100); 75 (97); 113 (65) |
| 1,4-Dibromopentanol-3 | 159475-15-7 | 137 (100); 139 (92); 57 (63) |
| 1-Bromo-2,4-dimethylbenzene | 583-70-0 | 105 (100); 184 (61); 186 (60) |
| 3,5-Dibromopentanol-2 | 1000288-19-4 | 43 (100); 120 (56); 165 (28) |
| Classes of Disinfection by-Products DBP | Value in Drinking Water | |||||||
|---|---|---|---|---|---|---|---|---|
| Surface Intake | Infiltration Intake | |||||||
| mg/dm3 | % | mole/dm3 | % | mg/dm3 | % | mole/dm3 | % | |
| Trihalomathanes | ||||||||
| ƩC in THM | 0.0025 | 9.6 | 0.00021 | 19.1 | 0.00049 | 7.4 | 0.000041 | 16.4 |
| ƩCl in THM | 0.02189 | 82.9 | 0.00062 | 56.4 | 0.0052 | 78.8 | 0.00015 | 60 |
| ƩBr in THM | 0.00175 | 6.6 | 0.000022 | 2.0 | 0.00087 | 13.2 | 0.000011 | 4.4 |
| ƩTHM | 0.02614 | 0.0011 | 0.0066 | 0.00025 | ||||
| Haloacetic Acids | ||||||||
| ƩC in HAA | 0.0025 | 14.5 | 0.00024 | 24 | 0.00076 | 16.9 | 0.000063 | 25.0 |
| ƩO in HAA | 0.0033 | 19 | 0.00024 | 24 | 0.0010 | 22.2 | 0.000063 | 25.0 |
| ƩCl in HAA | 0.011 | 55 | 0.00031 | 31 | 0.0021 | 46.7 | 0.000059 | 23.3 |
| ƩBr in HAA | 0.0018 | 9 | 0.000023 | 2.3 | 0.0006 | 13.3 | 0.0000075 | 3.0 |
| ƩHAA | 0.019 | 0.0010 | 0.0045 | 0.00025 | ||||
| Halogenated SVOC | ||||||||
| TgOC | 0.0083 | 55.3 | 0.00069 | 75.8 | 0.0063 | 65.0 | 0.00052 | 81.3 |
| TgOO | 0.0025 | 16.7 | 0.00015 | 16.5 | 0.0015 | 15.5 | 0.000093 | 14.5 |
| TgOCl | 0.00069 | 4.6 | 0.000019 | 2.5 | 0.00024 | 2.4 | 0.0000067 | 1.0 |
| TgOBr | 0.0038 | 25.3 | 0.000047 | 5.2 | 0.0017 | 17.1 | 0.000022 | 3.2 |
| ƩSVOC | 0.015 | 0.00091 | 0.0097 | 0.00064 | ||||
| Drinking Water | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum SVOC | TgOC | TgOBr | TgOCl | TgOO | Sum HAA | Br in HAA | Cl in HAA | Sum THM | Br in THM | Cl in THM | DOC | A254 | SUVA | ||
| Drinking water | Sum SVOC | 1.00 | |||||||||||||
| TgOC | 0.99 | 1.00 | |||||||||||||
| TgOBr | 0.92 | 0.83 | 1.00 | ||||||||||||
| TgOCl | 0.72 | 0.69 | 0.80 | 1.00 | |||||||||||
| TgOO | 0.95 | 0.91 | 0.92 | 0.66 | 1.00 | ||||||||||
| Sum HAA | −0.16 | −0.14 | −0.20 | −0.21 | −0.16 | 1.00 | |||||||||
| Br in HAA | −0.18 | −0.17 | −0.18 | −0.13 | −0.18 | 0.60 | 1.00 | ||||||||
| Cl in HAA | −0.12 | −0.11 | −0.18 | −0.21 | −0.16 | 0.98 | 0.45 | 1.00 | |||||||
| Sum THM | −0.25 | −0.24 | −0.29 | −0.03 | −0.31 | 0.55 | 0.22 | 0.55 | 1.00 | ||||||
| Br in THM | −0.20 | −0.22 | −0.28 | −0.08 | −0.28 | 0.22 | 0.26 | 0.17 | 0.56 | 1.00 | |||||
| Cl in THM | −0.24 | −0.24 | −0.29 | −0.03 | −0.31 | 0.56 | 0.21 | 0.55 | 0.99 | 0.52 | 1.00 | ||||
| DOC | 0.03 | 0.03 | 0.2 | 0.08 | 0.02 | 0.16 | −0.13 | 0.16 | 0.40 | −0.15 | 0.43 | 1.00 | |||
| A254 | −0.23 | −0.23 | −0.24 | −0.23 | −0.22 | 0.66 | −0.19 | 0.67 | 0.84 | 0.18 | 0.85 | 0.15 | 1.00 | ||
| SUVA | −0.28 | −0.27 | −0.34 | −0.40 | −0.27 | 0.07 | 0.18 | 0.02 | 0.28 | −0.05 | 0.29 | −0.50 | 0.76 | 1.00 | |
| Tech | I dose of Cl2 | −0.13 | −0.12 | −0.14 | 0.1 | −0.20 | 0.37 | −0.04 | 0.42 | 0.67 | 0.19 | 0.68 | 0.48 | 0.44 | −0.05 |
| II dose of Cl2 | −0.21 | −0.22 | −0.19 | −0.07 | −0.18 | 0.59 | 0.15 | 0.62 | 0.51 | 0.06 | 0.52 | 0.64 | 0.67 | 0.01 | |
| Sum dose of Cl2 | −0.15 | −0.14 | −0.14 | 0.03 | −0.15 | 0.28 | −0.01 | 0.30 | 0.64 | 0.18 | 0.65 | 0.65 | 0.78 | 0.12 | |
| River water | Turbidity | 0.01 | 0.01 | 0.01 | 0.01 | −0.02 | −0.17 | −0.15 | −0.14 | −0.28 | −0.45 | −0.28 | 0.27 | −0.24 | −0.44 |
| Chromaticity | 0.06 | 0.06 | 0.11 | 0.16 | 0.07 | 0.27 | −0.05 | 0.31 | 0.07 | −0.42 | 0.11 | 0.54 | 0.31 | −0.21 | |
| Permang. index | 0.10 | 0.09 | 0.08 | 0.08 | 0.12 | 0.21 | 0.04 | 0.23 | 0.08 | 0.09 | 0.09 | 0.34 | 0.14 | −0.22 | |
| COD | 0.22 | 0.21 | 0.21 | 0.12 | 0.23 | 0.08 | −0.14 | 0.12 | −0.04 | −0.41 | −0.03 | 0.11 | 0.50 | −0.15 | |
| DOC | 0.17 | 0.16 | 0.22 | 0.21 | 0.16 | 0.28 | 0.41 | 0.27 | −0.17 | −0.40 | −0.15 | 0.21 | 0.13 | −0.10 | |
| A254 | −0.27 | −0.31 | −0.19 | −0.08 | −0.25 | 0.34 | −0.09 | 0.39 | 0.39 | −0.43 | 0.42 | 0.25 | 0.51 | −0.03 | |
| SUVA | −0.78 | −0.80 | −0.72 | −0.65 | −0.78 | 0.14 | 0.35 | 0.04 | 0.17 | −0.37 | 0.19 | −0.07 | 0.18 | 0.21 | |
| Drinking Water | |||||||||||||||
| Sum Dose of Cl2 | Turbidity | Chromaticity | Permang. Index | COD | DOC | A254 | SUVA | ||||||||
| Tech | I dose of Cl2 | ||||||||||||||
| II dose of Cl2 | |||||||||||||||
| Sum dose of Cl2 | 1.00 | ||||||||||||||
| River water | Turbidity | 0.09 | 1.00 | ||||||||||||
| Chromaticity | 0.38 | 0.56 | 1.00 | ||||||||||||
| Permang. index | 0.25 | 0.24 | 0.27 | 1.00 | |||||||||||
| COD | 0.09 | 0.22 | 0.39 | 0.20 | 1.00 | ||||||||||
| DOC | −0.12 | 0.24 | 0.45 | 0.06 | 0.21 | 1.00 | |||||||||
| A254 | 0.54 | 0.39 | 0.94 | 0.23 | 0.87 | 0.70 | 1.00 | ||||||||
| SUVA | 0.38 | 0.13 | 0.52 | 0.07 | 0.42 | −0.08 | 0.62 | 1.00 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozhdaeva, M.Y.; Kholova, A.R.; Melnitskiy, I.A.; Beloliptsev, I.I.; Vozhdaeva, Y.S.; Kantor, E.A.; Lebedev, A.T. Monitoring and Statistical Analysis of Formation of Organochlorine and Organobromine Compounds in Drinking Water of Different Water Intakes. Molecules 2021, 26, 1852. https://doi.org/10.3390/molecules26071852
Vozhdaeva MY, Kholova AR, Melnitskiy IA, Beloliptsev II, Vozhdaeva YS, Kantor EA, Lebedev AT. Monitoring and Statistical Analysis of Formation of Organochlorine and Organobromine Compounds in Drinking Water of Different Water Intakes. Molecules. 2021; 26(7):1852. https://doi.org/10.3390/molecules26071852
Chicago/Turabian StyleVozhdaeva, Margarita Yu., Alfiya R. Kholova, Igor A. Melnitskiy, Ilya I. Beloliptsev, Yulia S. Vozhdaeva, Evgeniy A. Kantor, and Albert T. Lebedev. 2021. "Monitoring and Statistical Analysis of Formation of Organochlorine and Organobromine Compounds in Drinking Water of Different Water Intakes" Molecules 26, no. 7: 1852. https://doi.org/10.3390/molecules26071852







