Transcript Regulation of the Recoded Archaeal α-l-Fucosidase In Vivo
Abstract
:1. Introduction
2. Results
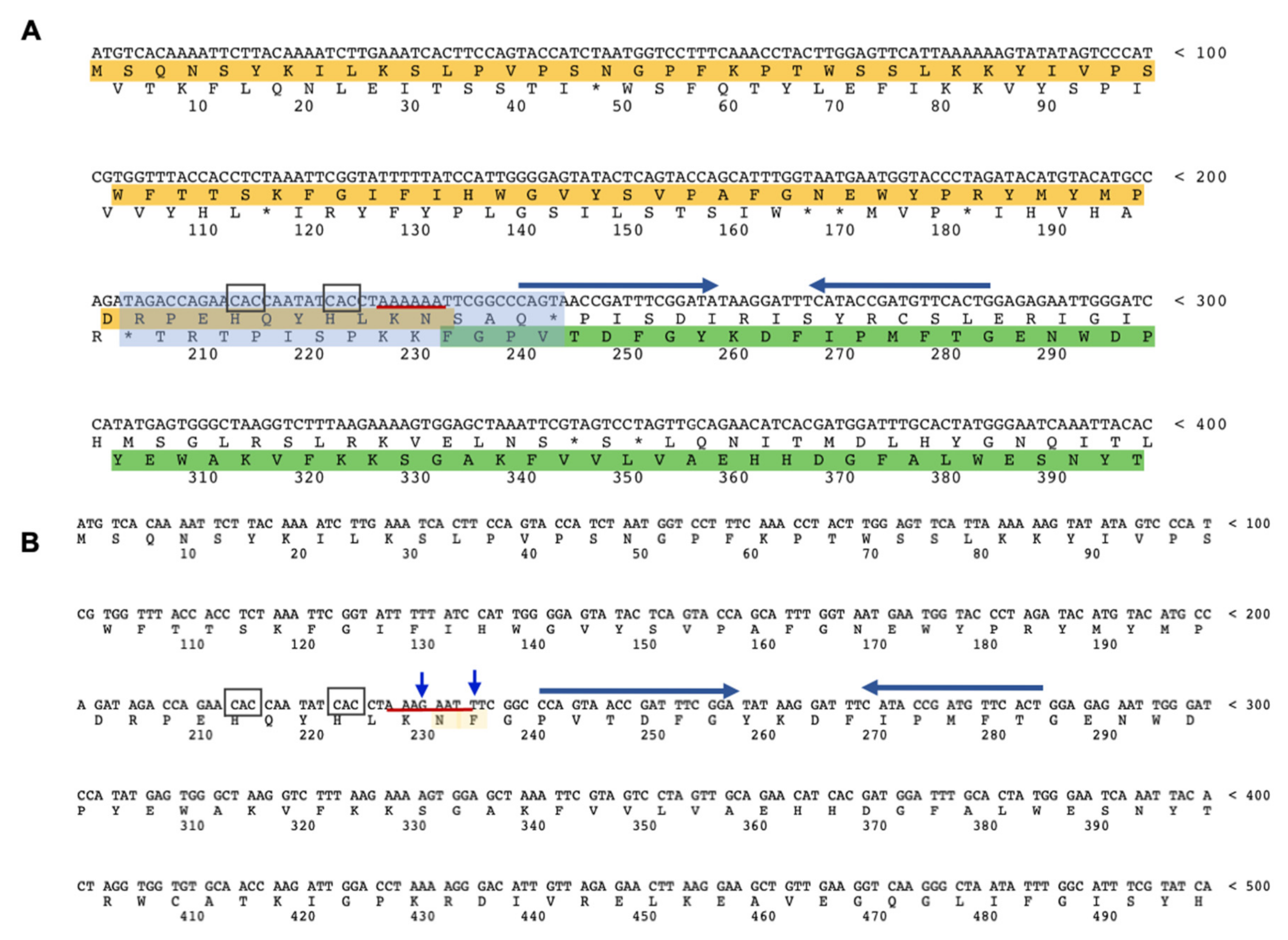
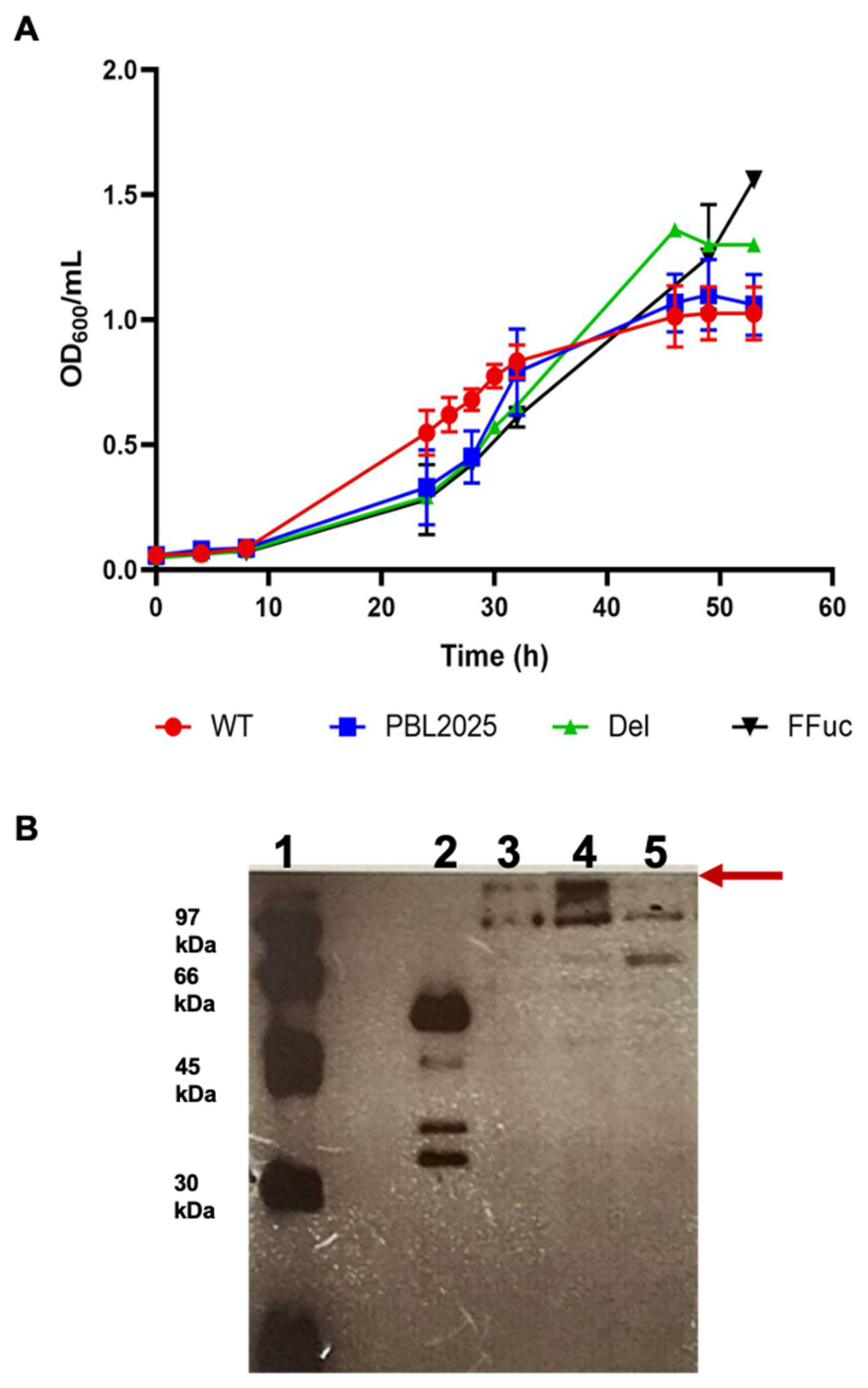
2.1. Knocked-Out and Full-Length fucA S. solfataricus Strains
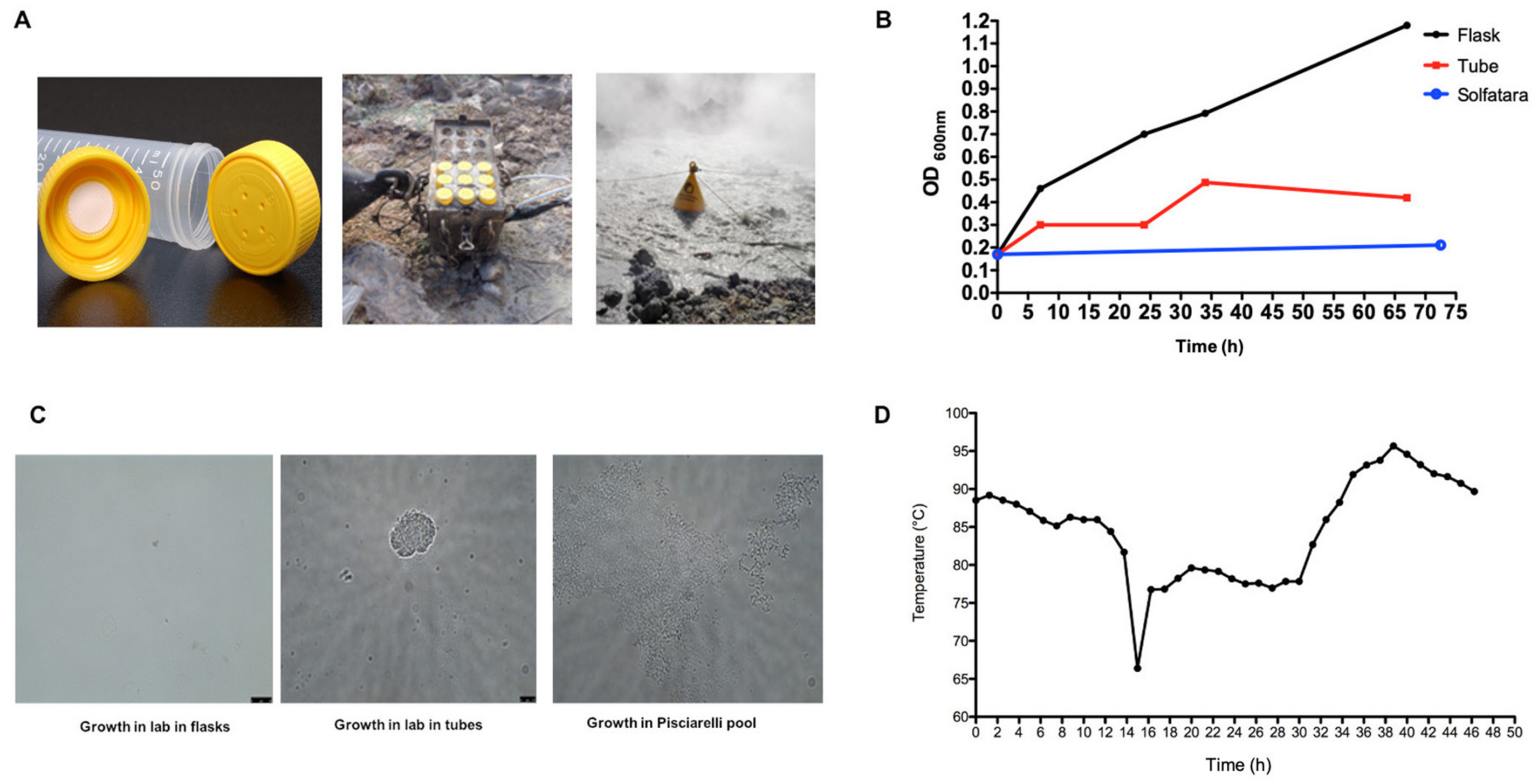
2.2. Environmental Conditions Variation in Pisciarelli Solfatara
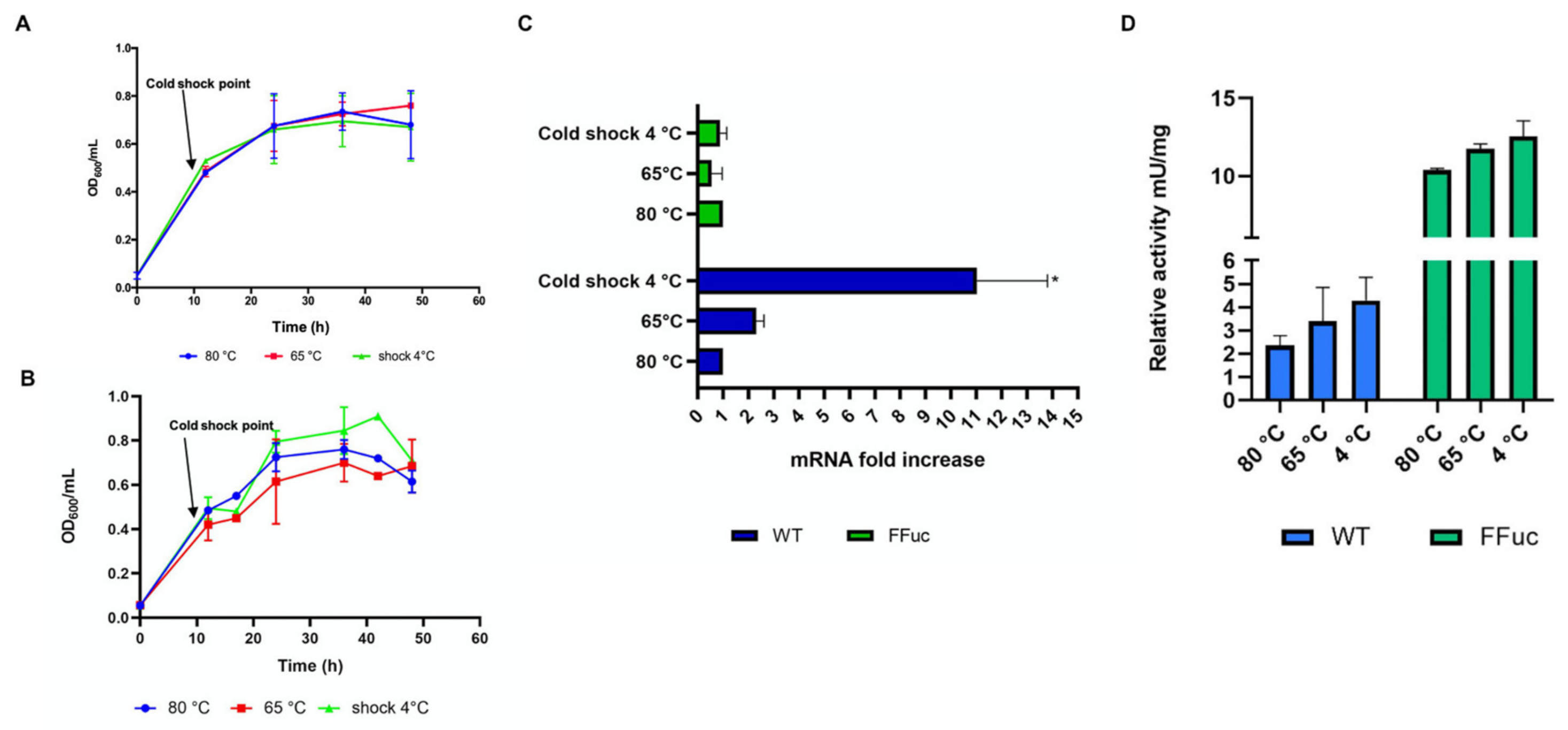
2.3. Transcript and Enzymatic Activity Levels of α-l-Fucosidase in Different Conditions
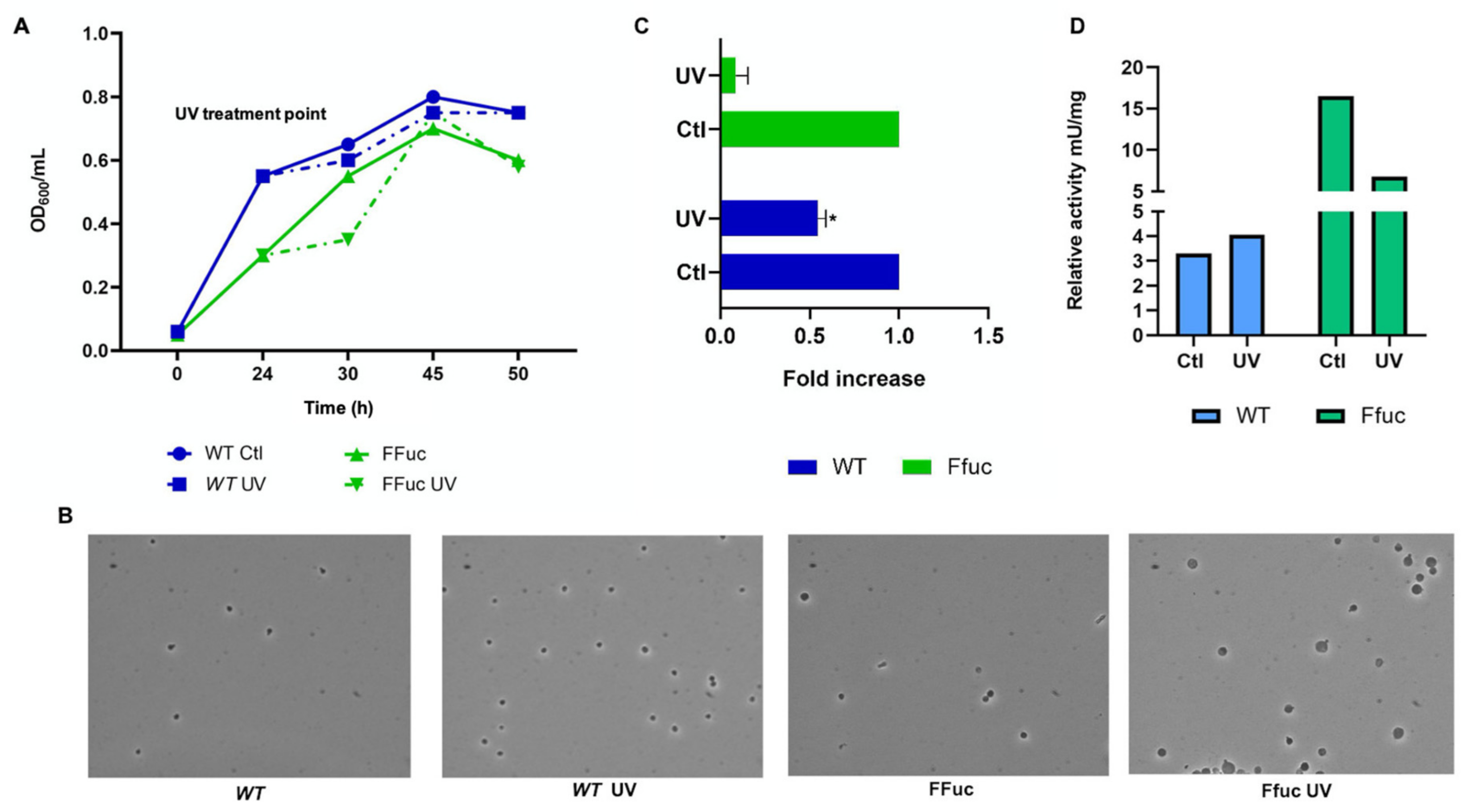
2.3.1. Cold Shock and UV Irradiation
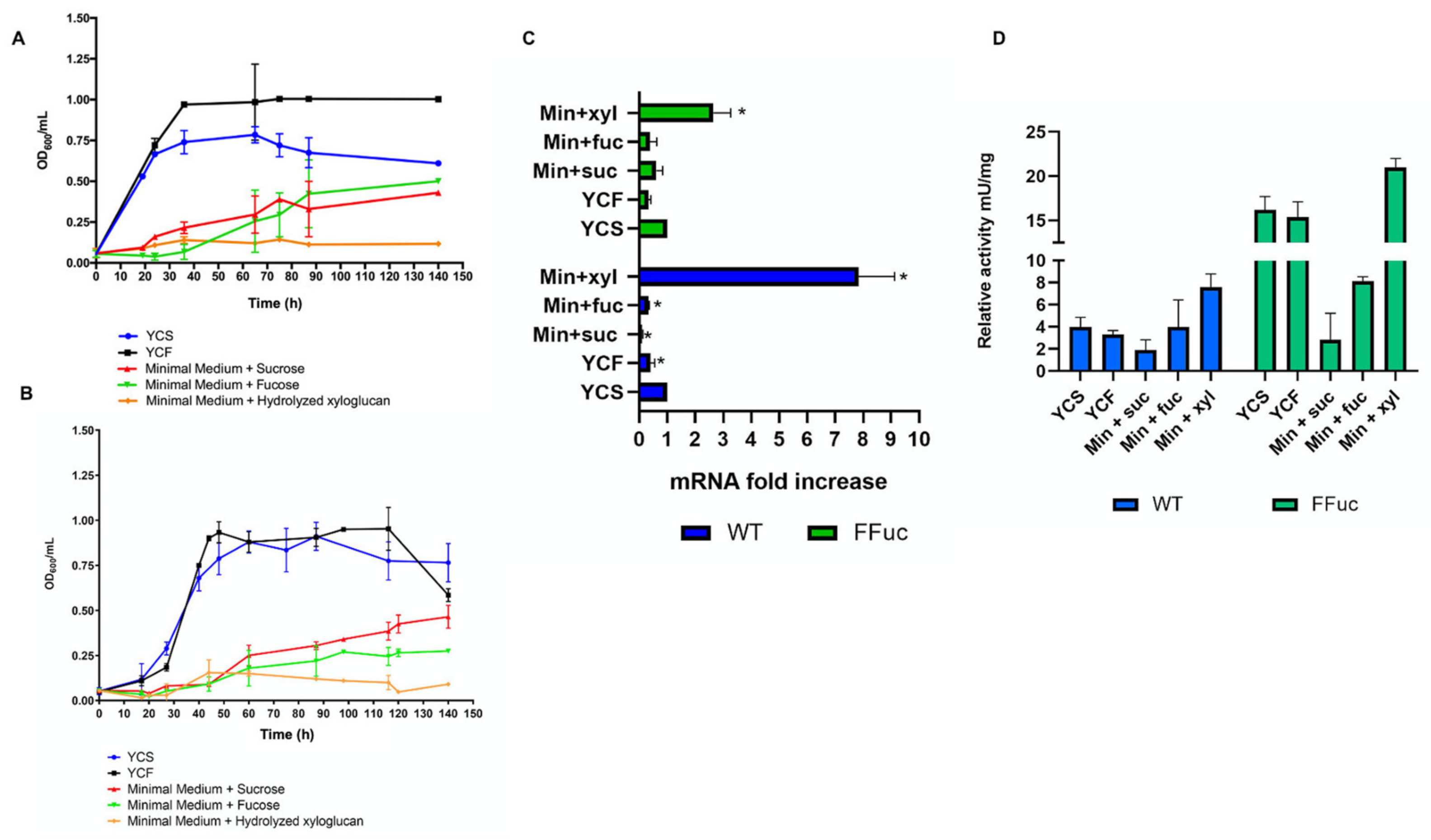
2.3.2. Carbon Sources
3. Discussion
4. Materials and Methods
4.1. Culture Media
4.2. Strains and Growth Conditions
4.3. Growth of S. solfataricus P2 in Pisciarelli Solfatara Pool
4.4. Growths in Different Carbon Sources
4.5. Cold Shock
4.6. UV Irradiation
4.7. RNA Extraction and Real-Time PCR
4.8. Optical Microscopy
4.9. Cell Lysates Preparation
4.10. Western Blot Analyses
4.11. Alpha-L-Fucosidase Activity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Atkins, J.F.; Loughran, G.; Bhatt, P.R.; Firth, A.E.; Baranov, P.V. Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use. Nucleic Acids Res. 2016, 44, 7007–7078. [Google Scholar] [CrossRef] [Green Version]
- Bekaert, M.; Firth, A.E.; Zhang, Y.; Gladyshev, V.N.; Atkins, J.F.; Baranov, P.V. Recode-2: New design, new search tools, and many more genes. Nucleic Acids Res. 2009, 38, D69–D74. [Google Scholar] [CrossRef]
- Gesteland, R.F.; Atkins, J.F. Recoding: Dynamic reprogramming of translation. Annu. Rev. Biochem. 1996, 65, 741–768. [Google Scholar] [CrossRef]
- Farabaugh, P.J. Programmed Translational Frameshifting. Annu. Rev. Genet. 1996, 30, 507–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namy, O.; Rousset, J.-P.; Napthine, S.; Brierley, I. Reprogrammed Genetic Decoding in Cellular Gene Expression. Mol. Cell 2004, 13, 157–168. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Korniy, N.; Klimova, M.; Karki, P.; Peng, B.-Z.; Senyushkina, T.; Belardinelli, R.; Maracci, C.; Wohlgemuth, I.; Samatova, E.; et al. Translational recoding: Canonical translation mechanisms reinterpreted. Nucleic Acids Res. 2020, 48, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, G.; Innes, S.; Minella, O.; Richardson, J.P.; Stansfield, I. Endless possibilities: Translation termination and stop codon recognition. Microbiology 2001, 147, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, P.R.; Jean-François Brugère, J.F.; Atkins, P.W.; O’Toole, G.B. Pyrrolysine in archaea: A 22nd amino acid encoded through a genetic code expansion. Emerg. Top. Life Sci. 2018, 607–618. [Google Scholar]
- Ambrogelly, A.; Palioura, S.; Söll, D. Natural expansion of the genetic code. Nat. Chem. Biol. 2006, 3, 29–35. [Google Scholar] [CrossRef]
- Eswarappa, S.M.; Potdar, A.A.; Koch, W.J.; Fan, Y.; Vasu, K.; Lindner, D.; Willard, B.; Graham, L.M.; DiCorleto, P.E.; Fox, P.L. Programmed Translational Readthrough Generates Antiangiogenic VEGF-Ax. Cell 2014, 157, 1605–1618. [Google Scholar] [CrossRef] [Green Version]
- Keeling, K.M.; Xue, X.; Gunn, G.; Bedwell, D.M. Therapeutics Based on Stop Codon Readthrough. Annu. Rev. Genom. Hum. Genet. 2014, 15, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Plant, E.P.; Rakauskaitė, R.; Taylor, D.R.; Dinman, J.D. Achieving a Golden Mean: Mechanisms by Which Coronaviruses Ensure Synthesis of the Correct Stoichiometric Ratios of Viral Proteins. J. Virol. 2010, 84, 4330–4340. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.A.; Olson, A.N.; Neupane, K.; Munshi, S.; San Emeterio, J.; Pollack, L.; Woodside, M.T.; Dinman, J.D. Structural and functional conservation of the programmed −1 ribosomal frameshift signal of SARS coronavirus 2 (SARS-CoV-2). J. Biol. Chem. 2020, 295, 10741. [Google Scholar] [CrossRef]
- Kurian, L.; Palanimurugan, R.; Gödderz, D.; Dohmen, R.J. Polyamine sensing by nascent ornithine decarboxylase antizyme stimulates decoding of its mRNA. Nat. Cell Biol. 2011, 477, 490–494. [Google Scholar] [CrossRef]
- Rother, M.; Quitzke, V. Selenoprotein synthesis and regulation in Archaea. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2451–2462. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Conte, F.; Rossi, M.; Moracci, M. The α-L-fucosidase from Sulfolobus solfataricus. Extremophiles 2007, 12, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Cobucci-Ponzano, B.; Trincone, A.; Giordano, A.; Rossi, M.; Moracci, M. Identification of an archaeal α-L-fucosidase en-coded by an interrupted gene: Production of a functional enzyme by mutations mimicking programmed −1 frameshifting. J. Biol. Chem. 2003, 278, 14622–14631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobucci-Ponzano, B.; Trincone, A.; Giordano, A.; Rossi, M.; Moracci, M. Identification of the catalytic nucleophile of the family 29 α-L-fucosidase from Sulfolobussolfataricus via chemical rescue of an inactive mutant. Biochemistry 2003, 42, 9525–9531. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Mazzone, M.; Rossi, M.; Moracci, M. Probing the Catalytically Essential Residues of the α-L-Fucosidase from the Hyperthermophilic ArchaeonSulfolobus solfataricus. Biochemestry 2005, 44, 6331–6342. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Conte, F.; Benelli, D.; Londei, P.; Flagiello, A.; Monti, M.; Pucci, P.; Rossi, M.; Moracci, M. The gene of an archaeal α-L-fucosidase is expressed by translational frameshifting. Nucleic Acids Res. 2006, 34, 4258–4268. [Google Scholar] [CrossRef] [Green Version]
- Cobucci-Ponzano, B.; Rossi, M.; Moracci, M. Recoding in Archaea. Mol. Microbiol. 2004, 55, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Zuccotti, S.; Cobucci-Ponzano, B.; Mazzone, M.; Rossi, M.; Moracci, M.; Petoukhov, M.V.; Svergun, D.I.; Bolognesi, M. Structural characterization of the nonameric assembly of an Archaeal alpha-L-fucosidase by synchrotron small angle X-rays cattering. Biochem. Biophys. Res. Commun. 2004, 320, 176–182. [Google Scholar] [CrossRef]
- Pietilä, M.K.; Laurinmäki, P.; Russell, D.A.; Ko, C.-C.; Jacobs-Sera, D.; Butcher, S.J.; Bamford, D.H.; Hendrix, R.W. Insights into Head-Tailed Viruses Infecting Extremely Halophilic Archaea. J. Virol. 2013, 87, 3248–3260. [Google Scholar] [CrossRef] [Green Version]
- Senčilo, A.; Jacobs-Sera, D.; Russell, D.A.; Ko, C.-C.; Bowman, C.A.; Atanasova, N.S.; Österlund, E.; Oksanen, H.M.; Bamford, D.H.; Hatfull, G.F.; et al. Snapshot of haloarchaeal tailed virus genomes. RNA Biol. 2013, 10, 803–816. [Google Scholar] [CrossRef]
- Antonov, I.; Coakley, A.; Atkins, J.F.; Baranov, P.V.; Borodovsky, M. Identification of the nature of reading frame transitions observed in prokaryotic genomes. Nucleic Acids Res. 2013, 41, 6514–6530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobucci-Ponzano, B.; Guzzini, L.; Benelli, D.; Londei, P.; Perrodou, E.; Lecompte, O.; Tran, D.; Sun, J.; Wei, J.; Mathur, E.J.; et al. Functional Characterization and High-Throughput Proteomic Analysis of Interrupted Genes in the ArchaeonSulfolobus solfataricus. J. Proteome Res. 2010, 9, 2496–2507. [Google Scholar] [CrossRef]
- Ling, J.; O’Donoghue, P.; Söll, D. Genetic code flexibility in microorganisms: Novel mechanisms and impact on physiology. Nat. Rev. Genet. 2015, 13, 707–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogna, S.; McLeod, T.; Petric, M. The Meaning of NMD: Translate or Perish. Trends Genet. 2016, 32, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Celik, A.; He, F.; Jacobson, A. NMD monitors translational fidelity 24/7. Curr. Genet. 2017, 63, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Clouet-D’Orval, B.; Batista, M.; Bouvier, M.; Quentin, Y.; Fichant, G.; Marchfelder, A.; Maier, L.-K. Insights into RNA-processing pathways and associated RNA-degrading enzymes in Archaea. FEMS Microbiol. Rev. 2018, 42, 579–613. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Gambacorta, A.; Bu’Lock, J.D. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus aci-docaldarius. J. Gen. Microbiol. 1975, 86, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Van Wolferen, M.; Wagner, A.; van der Does, C.; Albers, S.V. The archaeal Ced system imports DNA. Proc. Natl. Acad. Sci. USA 2016, 13, 2496–2501. [Google Scholar] [CrossRef] [Green Version]
- Fröls, S.; Ajon, M.; Wagner, M.; Teichmann, D.; Zolghadr, B.; Folea, M.; Boekema, E.J.; Driessen, A.J.M.; Schleper, C.; Albers, S.V. UV inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili for-mation. Mol. Microbiol. 2008, 70, 938–952. [Google Scholar] [CrossRef] [Green Version]
- Barak, Z.; Gallant, J.; Lindsley, D.; Kwieciszewki, B.; Heidel, D. Enhanced Ribosome Frameshifting in Stationary Phase Cells. J. Mol. Biol. 1996, 263, 140–148. [Google Scholar] [CrossRef]
- Wenthzel, A.-M.K.; Stancek, M.; Isaksson, L.A. Growth phase dependent stop codon readthrough and shift of translation reading frame in Escherichia coli. FEBS Lett. 1998, 421, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Götz, D.; Paytubi, S.; Munro, S.; Lundgren, M.; Bernander, R.; White, M.F. Responses of hyperthermophilic crenarchaea to UV irradiation. Genome Biol. 2007, 8, R220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfsmeier, M.L.; Laughery, M.F.; Haseltine, C.A. Repair of DNA Double-Strand Breaks following UV Damage in Three Sulfolobussolfataricus Strains. J. Bacteriol. 2010, 192, 4954–4962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Donato, P.; Romano, I.; Mastascusa, V.; Poli, A.; Orlando, P.; Pugliese, M.; Nicolaus, B. Survival and Adaptation of the Thermophilic Species Geobacillus thermantarcticus in Simulated Spatial Conditions. Orig. Life Evol. Biosph. 2017, 48, 141–158. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strazzulli, A.; Cobucci-Ponzano, B.; Iacono, R.; Giglio, R.; Maurelli, L.; Curci, N.; Schiano-di-Cola, C.; Santangelo, A.; Contursi, P.; Lombard, V.; et al. Discovery of hyperstable carbohy-drate-active enzymes through metagenomics of extreme environments. FEBS J. 2020, 287, 1116–1137. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D. Thermophilic Microorganisms and Life at High Temperatures. Ascomycete Syst. 1978, 465. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Moracci, M.; Di Lauro, B.; Ciaramella, M.; D’Avino, R.; Rossi, M. Ionic network at the C-terminus of the beta-glycosidase from the hyperthermophilic archaeon Sulfolobus solfataricus: Functional role in the quaternary structure thermal stabilization. Proteins Struct. Funct. Genet. 2002, 48, 98–106. [Google Scholar] [CrossRef]
- Iacono, R.; Strazzulli, A.; Maurelli, L.; Curci, N.; Casillo, A.; Corsaro, M.M.; Moracci, M.; Cobucci-Ponzano, B. GlcNAc De-N-Acetylase from the hyperthermophilic archaeon Sulfolobus solfataricus. Appl. Environ. Microbiol. 2019, 85, 01879. [Google Scholar]
- Cobucci-Ponzano, B.; Strazzulli, A.; Iacono, R.; Masturzo, G.; Giglio, R.; Rossi, M.; Moracci, M. Novel thermophilic hem-icellulases for the conversion of lignocellulose for second generation biorefineries. Enzym. Microb. Technol. 2015, 78, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.C.; Cobucci-Ponzano, B.; Carpentieri, A.; Henrissat, B.; Rossi, M.; Amoresano, A.; Moracci, M. The identification and molecular characterization of the first archaeal bifunctional exo-β-glucosidase/N-acetyl-β-glucosaminidase demonstrate that family GH116 is made of three functionally distinct subfamilies. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 367–377. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Conte, F.; Strazzulli, A.; Capasso, C.; Fiume, I.; Pocsfalvi, G.; Rossi, M.; Moracci, M. The molecular characterization of a novel GH38 α-mannosidase from the crenarchaeon Sulfolobus solfataricus revealed its ability in de-mannosylating glycoproteins. Biochimestry 2010, 92, 1895–1907. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Aurilia, V.; Riccio, G.; Henrissat, B.; Coutinho, P.M.; Strazzulli, A.; Padula, A.; Corsaro, M.M.; Pieretti, G.; Pocsfalvi, G.; et al. A new archaeal β-glycosidase from Sulfolobus solfataricus: Seeding a novel retaining β-glycan- specific glycoside hydrolase family along with the human non-lysosomal gluco-sylceramidase GBA. J. Biol. Chem. 2010, 285, 20691–20703. [Google Scholar] [CrossRef] [Green Version]
- Ausili, A.; Cobucci-Ponzano, B.; Di Lauro, B.; D’Avino, R.; Perugino, G.; Bertoli, E.; Scirè, A.; Rossi, M.; Tanfani, F.; Moracci, M. A comparative infrared spectroscopic study of glycoside hydrolases from extremophilic archaea revealed different molecular mechanisms of adaptation to high temperatures. Proteins Struct. Funct. Bioinform. 2007, 67, 991–1001. [Google Scholar] [CrossRef]
- Moracci, M.; Ponzano, B.C.; Trincone, A.; Fusco, S.; De Rosa, M.; van der Oost, J.; Sensen, C.W.; Charlebois, R.L.; Rossi, M. Identification and Molecular Characterization of the First α-Xylosidase from an Archaeon. J. Biol. Chem. 2000, 275, 22082–22089. [Google Scholar] [CrossRef] [Green Version]
- Belew, A.T.; Meskauskas, A.; Musalgaonkar, S.; Advani, V.M.; Sulima, S.O.; Kasprzak, W.K.; Shapiro, B.A.; Dinman, J.D. Ribosomal frameshifting in the CCR5 mRNA is regulated by miRNAs and the NMD pathway. Nat. Cell Biol. 2014, 512, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.K.; Wright, P.C. Identification and characterization of the Sulfolobus solfataricus P2 proteome. J. Proteome Res. 2005, 4, 1789–1798. [Google Scholar] [CrossRef]
- Raimondeau, E.; Bufton, J.C.; Schaffitzel, C. New insights into the interplay between the translation machinery and nonsense-mediated mRNA decay factors. Biochem. Soc. Trans. 2018, 46, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Blanco, D.A.; Shell, S.S. Regulation of mRNA Stability During Bacterial Stress Responses. Front. Microbiol. 2020, 11, 2111. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Copeland, P.R.; Driscoll, N.M. Polysome distribution of phospholipid hydroperoxide glutathione peroxidase mRNA: Evidence for a block in elongation at the UGA/selenocysteine codon. RNA 2000, 6, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.W.; Berry, M.J. Selenocysteine codons decrease polysome association on endogenous selenoprotein mRNAs. Genes Cells 2001, 6, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.P.; Copeland, P.R. Selenocysteine incorporation: A trump card in the game of mRNA decay. Biochimestry 2015, 114, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Rubianes, D.; Valdivia, E.R.; Revilla, G.; Zarra, I.; Sampedro, J. Xyloglucan exoglycosidases in the monocot model Brachypo-dium distachyon and the conservation of xyloglucan disassembly in angiosperms. Plant Mol. Biol. 2019, 100, 495–509. [Google Scholar] [CrossRef]
- Larsbrink, J.; Thompson, A.J.; Lundqvist, M.; Gardner, J.G.; Davies, G.J.; Brumer, H. A complex gene locus enables xyloglucan utilization in the model saprophyteCellvibrio japonicus. Mol. Microbiol. 2014, 94, 418–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curci, N.; Strazzulli, A.; Iacono, R.; De Lise, F.; Maurelli, L.; Di Fenza, M.; Cobucci-Ponzano, B.; Moracci, M. Xyloglucan Oligosaccharides Hydrolysis by Exo-Acting Glycoside Hydrolases from Hyperthermophilic Microorganism Saccharolobus solfataricus. Int. J. Mol. Sci. 2021, 22, 3325. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol. 1972, 84, 54–68. [Google Scholar] [CrossRef]
- McCarthy, S.; Gradnigo, J.; Johnson, T.; Payne, S.; Lipzen, A.; Martin, J.; Schackwitz, W.; Moriyama, E.; Blum, P. Complete Genome Sequence of Sulfolobus solfataricus Strain 98/2 and Evolved Derivatives. Genome Announc. 2015, 28, e00549-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koerdt, A.; Jachlewski, S.; Ghosh, A.; Wingender, J.; Siebers, B.; Albers, S.-V. Complementation of Sulfolobus solfataricus PBL2025 with an α-mannosidase: Effects on surface attachment and biofilm formation. Extremophiles 2011, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Onofri, S.; Balucani, N.; Barone, V.; Benedetti, P.; Billi, D.; Balbi, A.; Brucato, J.R.; Cobucci-Ponzano, B.; Costanzo, G.; Rocca, N.; et al. OPPS Project Tea the Italian National Project of Astrobiology-Life in Space-Origin, Presence, Persistence of Life in Space, from Molecules to Extremophiles. Astrobiology 2020, 20, 580–582. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lise, F.; Iacono, R.; Strazzulli, A.; Giglio, R.; Curci, N.; Maurelli, L.; Avino, R.; Carandente, A.; Caliro, S.; Tortora, A.; et al. Transcript Regulation of the Recoded Archaeal α-l-Fucosidase In Vivo. Molecules 2021, 26, 1861. https://doi.org/10.3390/molecules26071861
De Lise F, Iacono R, Strazzulli A, Giglio R, Curci N, Maurelli L, Avino R, Carandente A, Caliro S, Tortora A, et al. Transcript Regulation of the Recoded Archaeal α-l-Fucosidase In Vivo. Molecules. 2021; 26(7):1861. https://doi.org/10.3390/molecules26071861
Chicago/Turabian StyleDe Lise, Federica, Roberta Iacono, Andrea Strazzulli, Rosa Giglio, Nicola Curci, Luisa Maurelli, Rosario Avino, Antonio Carandente, Stefano Caliro, Alessandra Tortora, and et al. 2021. "Transcript Regulation of the Recoded Archaeal α-l-Fucosidase In Vivo" Molecules 26, no. 7: 1861. https://doi.org/10.3390/molecules26071861
APA StyleDe Lise, F., Iacono, R., Strazzulli, A., Giglio, R., Curci, N., Maurelli, L., Avino, R., Carandente, A., Caliro, S., Tortora, A., Lorenzini, F., Di Donato, P., Moracci, M., & Cobucci-Ponzano, B. (2021). Transcript Regulation of the Recoded Archaeal α-l-Fucosidase In Vivo. Molecules, 26(7), 1861. https://doi.org/10.3390/molecules26071861








