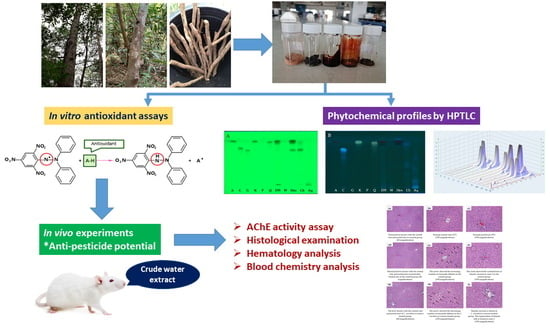
In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats
Abstract
1. Introduction
2. Results
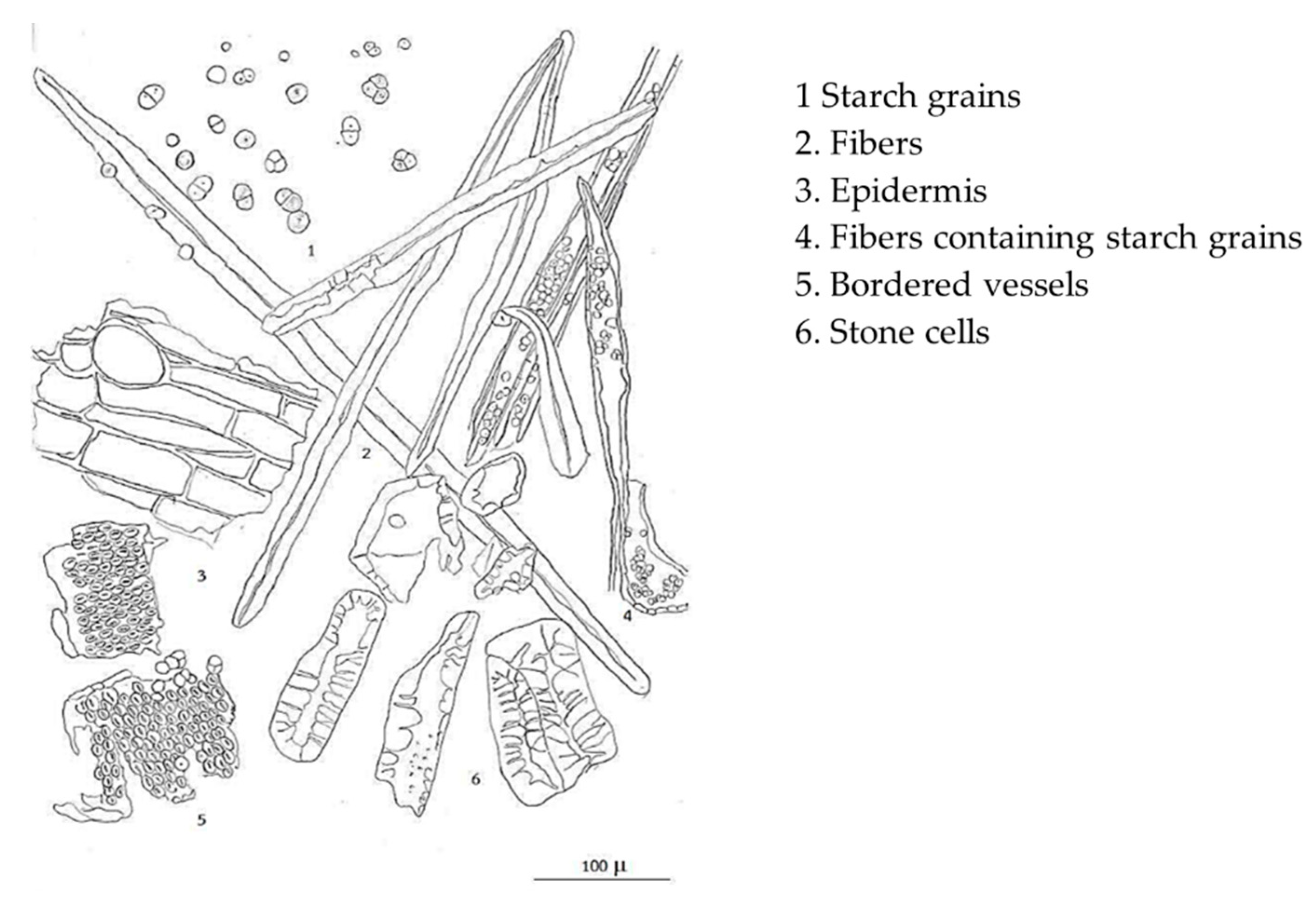
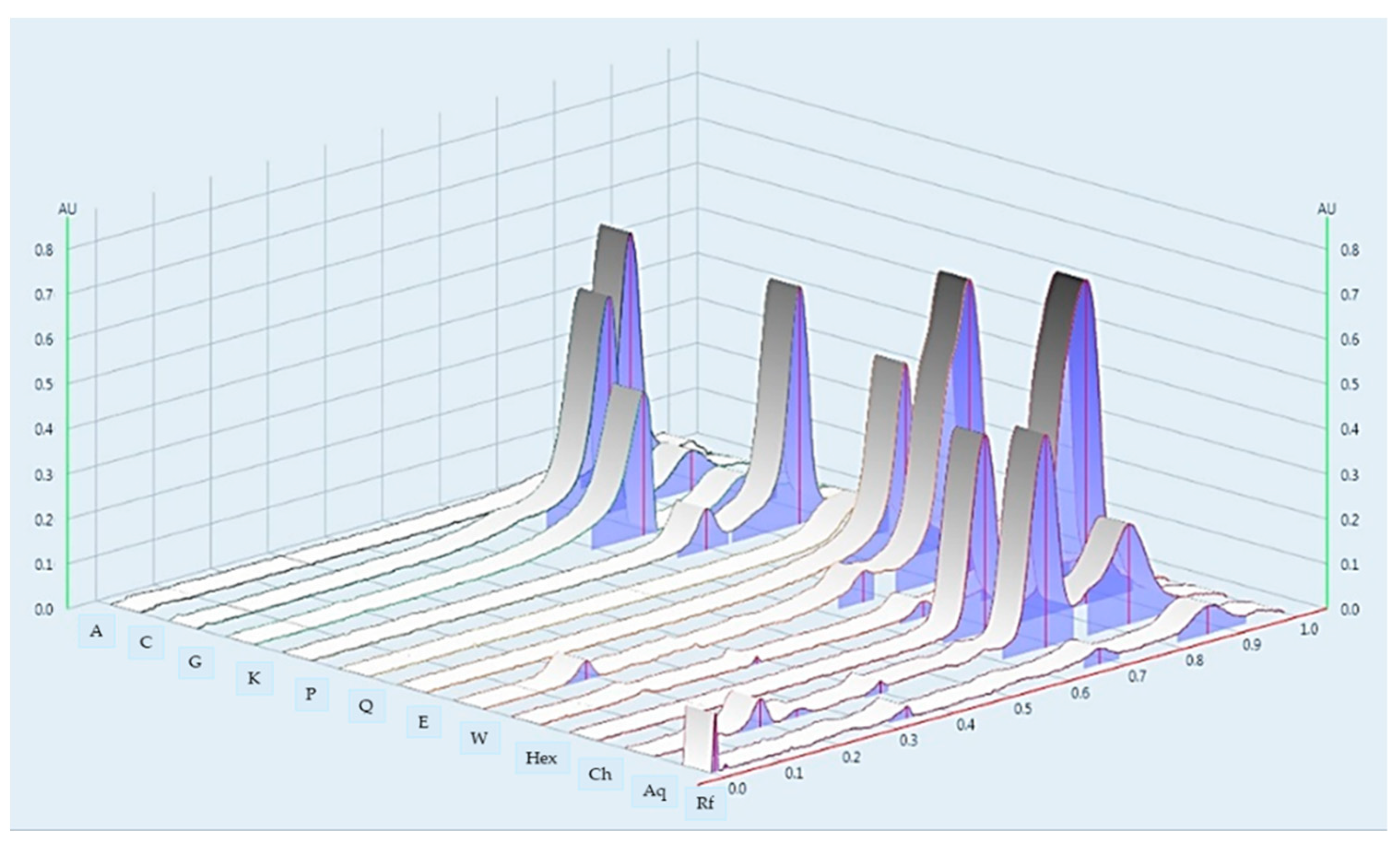
2.1. Microscopic Character and Chemical Pattern of the Extract of L. martabanica
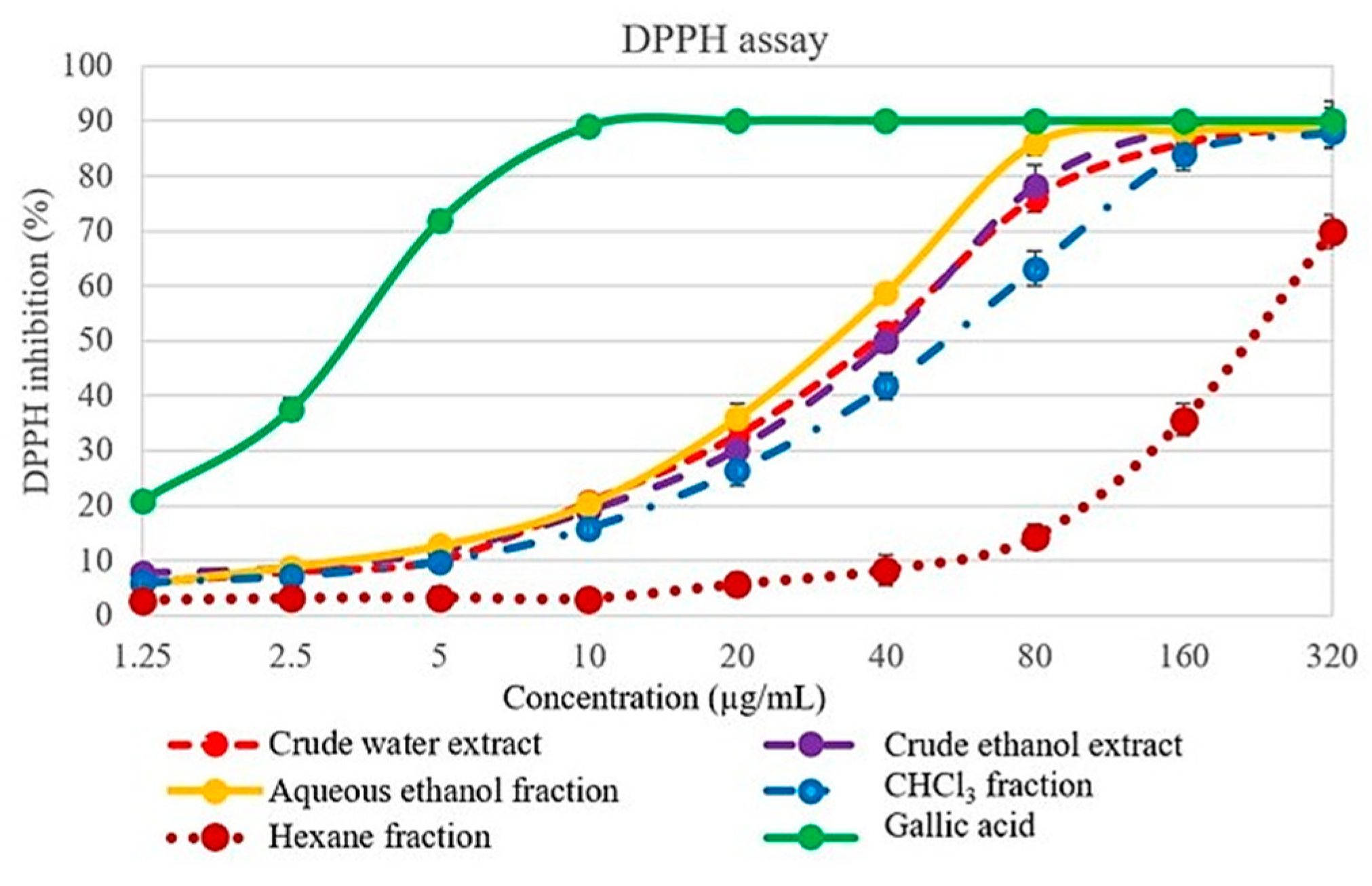
2.2. 2,2′-diphenyl-1-picrylhydrazyl (DPPH) Scavenging Activity
2.3. Superoxide Radical Scavenging Activity
2.4. 2,2′-Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Radical Scavenging Activity
2.5. Ferric Reducing Antioxidant Power (FRAP)
2.6. Total Phenolic Content (TPC)
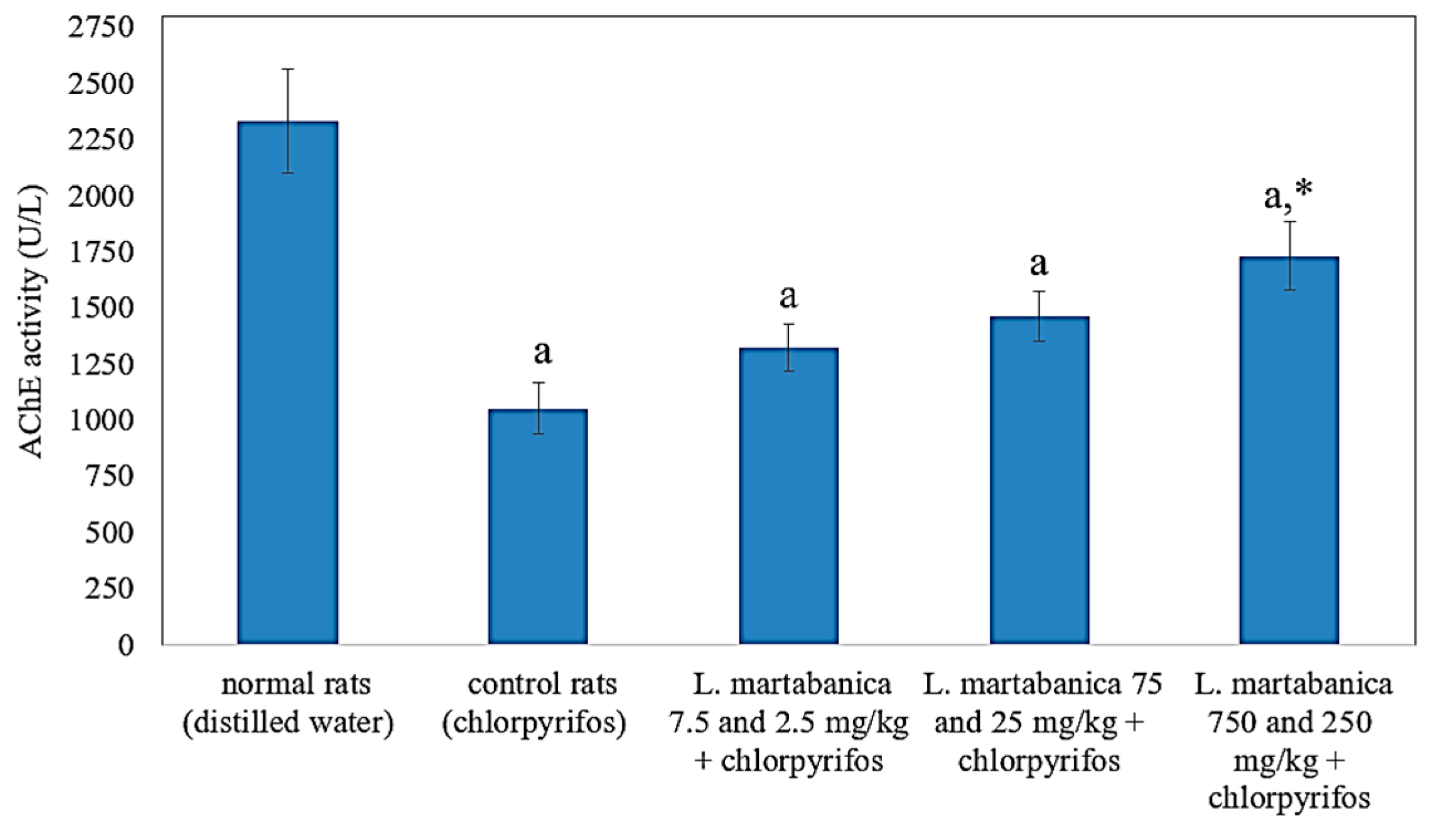
2.7. Anti-Pesticide Potential
2.8. Observation of Behavioral Change and Toxicological Signs
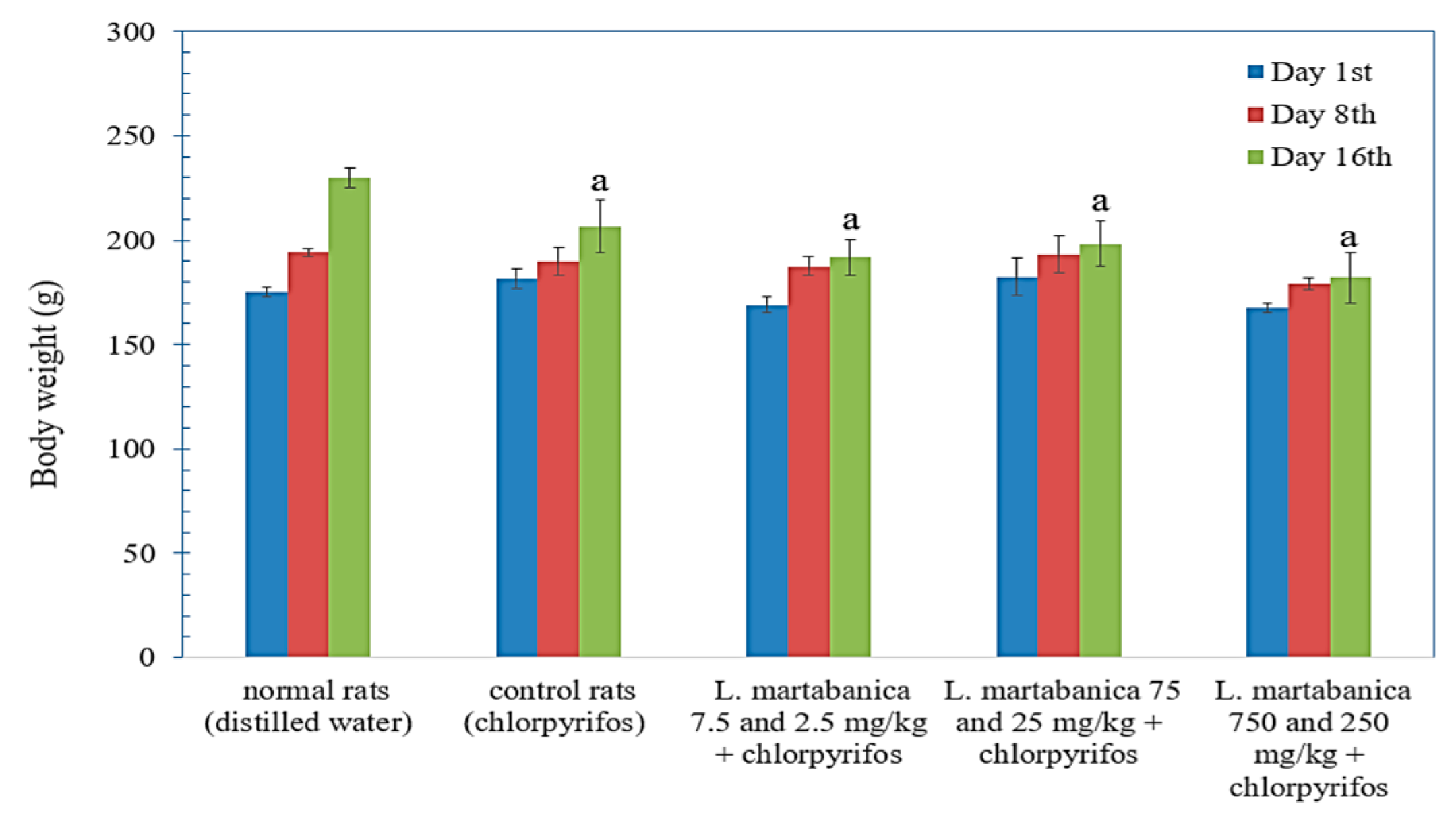
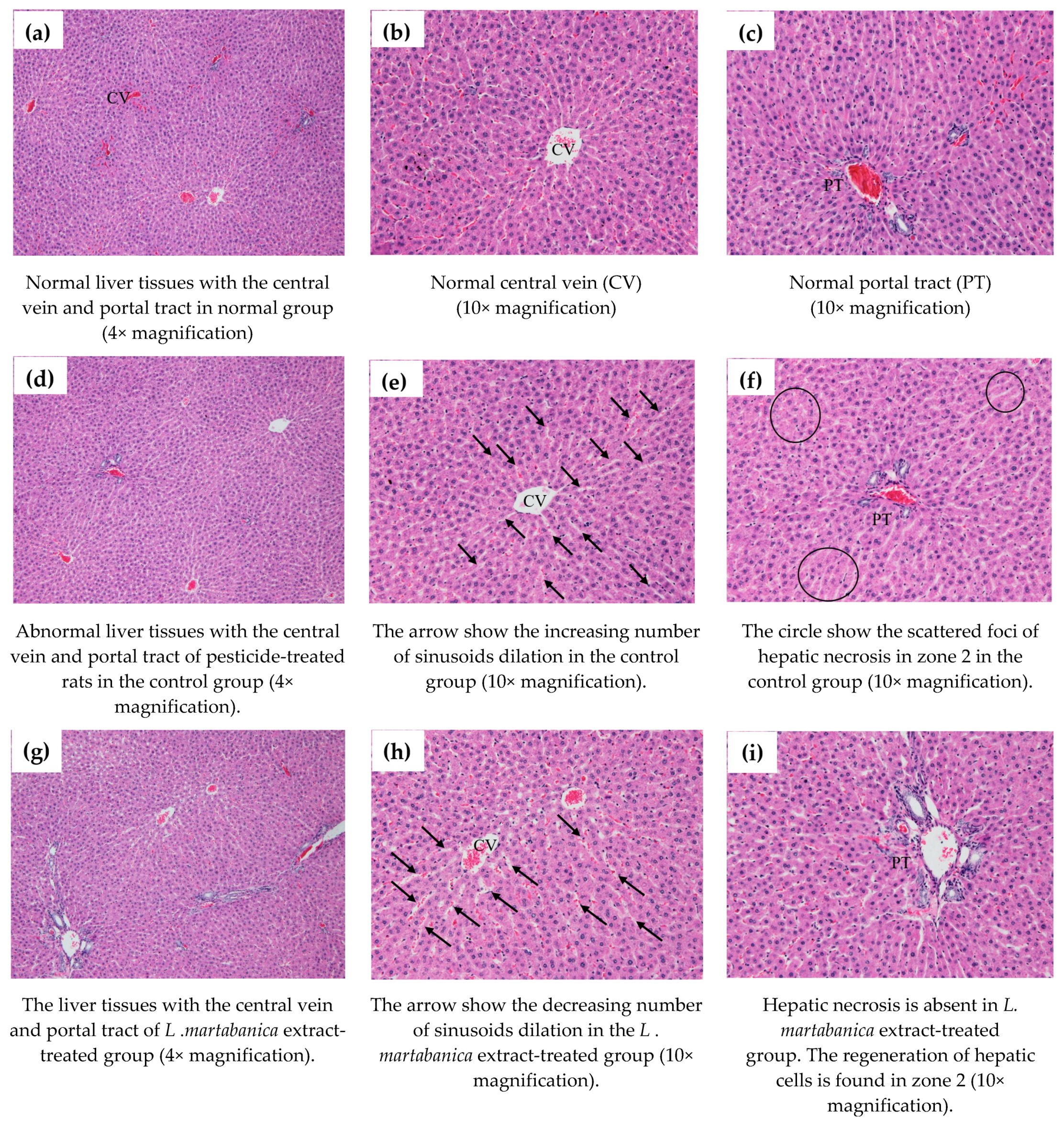
2.9. Body Weight Change, Organs Weight, and Histological Examination Results
2.10. Hematology Analysis
2.11. Blood Chemistry Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of L. martabanica (Root)
4.3. Chemical Profile by High Performance Thin Layer Chromatography
4.4. DPPH Assay
4.5. Superoxide Radical Assay
4.6. 2,2′-Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
4.7. Ferric Reducing Antioxidant Power (FRAP) Assay
4.8. Total Phenolics Content
4.9. Anti-Pesticide Potential
4.9.1. Animals
4.9.2. Experimental Groups
4.10. Assay of AChE Activity
4.11. Observation of Behavioral Change and Toxicological Signs
4.12. Body Weight Change, Internal Organ Weight, and Histopathological Studies
4.13. Hematology Analysis
4.14. Blood Chemistry Analysis
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ngearnsaengsaruay, C.; Middleton, D.J.; Chayamarit, K. A revision of the genus Litsea Lam. (Lauraceae) in Thailand. Thai Forest Bull. Bot. 2011, 40–119. [Google Scholar]
- Kamle, M.; Mahato, D.K.; Lee, K.E.; Bajpai, V.K.; Gajurel, P.R.; Gu, K.S.; Kumar, P. Ethnopharmacological Properties and Medicinal Uses of Litsea cubeba. Plants 2019, 8, 150. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wen, Z.-Q.; Li, B.-T.; Zhang, H.-B.; Yang, J.-H. Ethnobotany, phytochemistry, and pharmacology of the genus Litsea: An update. J. Ethnopharmacol. 2016, 181, 66–107. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-G.; Zhao, Y.; Li, G.-H.; Chen, B.-J.; Wang, X.-N.; Zhou, H.-L.; Lou, H.-X.; Ren, D.-M.; Shen, T. The genus Litsea in traditiona Chinese medicine: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 256–264. [Google Scholar] [CrossRef] [PubMed]
- The Highland Research and Development Institute. Local Plant Utilization Guide; The Highland Research and Development Institute (Public Organization): Chiang Mai, Thailand, 2018; p. 118. [Google Scholar]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.A.; Bradberry, S.M. Organophosphate and Carbamate Insecticide. In Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer: Cham, Switzerland; Available online: https://doi.org/10.1007/978-3-319-17900-1_52 (accessed on 28 March 2021).
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Gupta, R.C.; Aschner, M. Anticholinesterase Toxicity and Oxidative Stress. Sci. World J. 2006, 6, 683173. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef]
- Mecdad, A.A.; Ahmed, M.H.; ElHalwagy, M.E.A.; Afify, M.M.M. A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-sprayers. Egypt. J. Forensic Sci. 2011, 1, 93–98. [Google Scholar] [CrossRef]
- Soltaninejad, K.; Abdollahi, M. Current opinion on the science of organophosphate pesticides and toxic stress: A systematic review. Med. Sci. Monit. 2009, 15, Ra75–Ra90. [Google Scholar]
- Akhgari, M.; Abdollahi, M.; Kebryaeezadeh, A.; Hosseini, R.; Sabzevari, O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, Ra141–Ra147. [Google Scholar] [PubMed]
- Weichenthal, S.; Moase, C.; Chan, P. A review of pesticide exposure and cancer incidence in the Agricultural Health Study cohort. Environ. Health. Perspect. 2010, 118, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Al-Eryani, L.; Wahlang, B.; Falkner, K.C.; Guardiola, J.J.; Clair, H.B.; Prough, R.A.; Cave, M. Identification of Environmental Chemicals Associated with the Development of Toxicant-associated Fatty Liver Disease in Rodents. Toxicol. Pathol. 2015, 43, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Leikin, J.B.; Davis, A.; Klodd, D.A.; Thunder, T.; Kelafant, G.A.; Paquette, D.L.; Rothe, M.J.; Rubin, R. Part IV. Occupational liver disease. Dis. Mon. 2000, 46, 295–310. [Google Scholar] [CrossRef]
- Ranjbar, A.; Solhi, H.; Mashayekhi, F.J.; Susanabdi, A.; Rezaie, A.; Abdollahi, M. Oxidative stress in acute human poisoning with organophosphorus insecticides; a case control study. Environ. Toxicol. Pharmacol. 2005, 20, 88–91. [Google Scholar] [CrossRef]
- Nurulain, S.M.; Szegi, P.; Tekes, K.; Naqvi, S.N. Antioxidants in organophosphorus compounds poisoning. Arh. Hig. Rada. Toksikol. 2013, 64, 169–177. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Jalili, C.; Farzaei, M.; Roshankhah, S.; Salahshoor, M. Resveratrol attenuates malathion-induced liver damage by reducing oxidative stress. J. Lab. Physicians. 2019, 11, 212–219. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Eroğlu, S.; Pandir, D.; Uzun, F.G.; Bas, H. Protective role of vitamins C and E in dichlorvos-induced oxidative stress in human erythrocytes in vitro. Biol. Res. 2013, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ambali, S.F.; Idris, S.B.; Onukak, C.; Shittu, M.u.; Ayo, J.O. Ameliorative effects of vitamin C on short-term sensorimotor and cognitive changes induced by acute chlorpyrifos exposure in Wistar rats. Toxicol. Ind. Health. 2010, 26, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Department of Medical Sciences, Ministry of Public Health, Thailand. THP; Office of Notional Buddishm Press: Bangkok, Thailand, 2018.
- He, Q.; Su, G.; Liu, K.; Zhang, F.; Jiang, Y.; Gao, J.; Liu, L.; Jiang, Z.; Jin, M.; Xie, H. Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method. PLoS ONE 2017, 12, e0189837. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food. Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, X.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y. Comparative Studies on Bioactive Constituents in Hawk Tea Infusions with Different Maturity Degree and Their Antioxidant Activities. Sci. World J. 2014, 2014, 838165. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Choi, E.-M.; Lee, J.H. Antioxidant activity of Litsea cubeba. Fitoterapia 2005, 76, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Choudhary, A.S.; Sharma, M.C.; Dobhal, M.P. Chemical Constituents of Plants from the Genus Litsea. Chem. Biodivers. 2011, 8, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015, 8, 621. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Pourreza, N. Phenolic compounds as potential antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149–150. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nunes, X.P.; Silva, F.S.; Almeida, J.R.G.D.S.; Barbosa Filho, J.M.; de Lima, J.T.; de Araújo Ribeiro, L.A.; Júnior, L.J.Q. Biological Oxidations and Antioxidant Activity of Natural Products. In Phytochemicals as Nutraceuticals-Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Shangai, China, 2012; pp. 1–20. [Google Scholar] [CrossRef]
- Singh, N.; Lawana, V.; Luo, J.; Phong, P.; Abdalla, A.; Palanisamy, B.; Rokad, D.; Sarkar, S.; Jin, H.; Anantharam, V.; et al. Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events. Neurobiol. Dis. 2018, 117, 82–113. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Wellington, K.; Jarvis, B. Silymarin: A Review of its Clinical Properties in the Management of Hepatic Disorders. BioDrugs 2001, 15, 465–489. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Permender, R.; Hema, C.; Sushila, R.; Dharmender, R.; Vikash Kumar and Kanchan, K. Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review. Inflamm. Allergy Drug Targets. 2009, 8, 229–235. [Google Scholar] [CrossRef]
- Clegg, D.J.; van Gemert, M. Determination of the reference dose for chlorpyrifos: Proceedings of an expert panel. J. Toxicol. Environ. Health. B Crit. Rev. 1999, 2, 211–255. [Google Scholar] [CrossRef] [PubMed]
- Adeneye, A.A.; Ajagbonna, O.P.; Adeleke, T.I.; Bello, S.O. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J. Ethnopharmacol. 2006, 105, 374–379. [Google Scholar] [CrossRef]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar] [PubMed]
- Mary Anna, T.; Glade, W.; Robin, A.; Terry, C. Veterinary Hematology and Clinical Chemistry, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 61–401. [Google Scholar]
- Kazmi, F.; Shakoori, A.; Hafeez, M.A.; Ali, S. Short term effects of chlorpyrifos on hematology and biochemical components of blood of the Sprague-Dawley rat. Pak. J. Zool. 2003, 35, 237–243. [Google Scholar]
- El-Deeb, A.E.A.; Abd El-Aleem, I.M.; Sherin, S.G. Harmful effect of some insecticides on vital parameters of albino rats. J. Egypt. Soc. Toxicol. 2007, 36, 53–60. [Google Scholar]
- Rifai, N.; Horvath, A.R.; Wittwer, C. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 1348–1397. [Google Scholar]
- Bishop, M.L.; Fody, E.P.; Schoeff, L.E. Clinical Chemistry: Principles, Techniques, and Correlations, 7th ed.; Wolters Kluwer Health/Hippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 260–297. [Google Scholar]
- Al-Attar, A.M.; Elnaggar, M.H.R.; Almalki, E.A. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J. Biol. Sci. 2017, 24, 1162–1171. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Alternat. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Phull, A.-R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I.-u. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Agrawal, P.; Bang, B.H.; Park, Y.-H. Phytochemical analysis, antioxidant and antilipid peroxidation effects of a medicinal plant, Adhatoda vasica. Front. Life Sci. 2015, 8, 305–312. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components--an experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Bolanos de la Torre, A.A.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food. Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Abd-El-hameed, S.Y.; Mohamed, A.A.; Thabet, H.Z. Potential Ameliorative Role Of Silymarin Against Methyl Parathion-Induced Oxidative Stress And Hepato-Renal Toxicity In Albino Rats. MJFMCT 2009, 17, 15–40. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]



| Test | Result |
|---|---|
| Foreign matter (%w/w) | Not found |
| Ethanol extractive content (%w/w) | 33.0534 ± 0.06 |
| Water extractive content (%w/w) | 24.1200 ± 0.04 |
| Loss on drying (%v/w) | 9.6867 ± 0.02 |
| Total ash (%w/w) | 1.4645 ± 0.01 |
| Acid-insoluble ash (%w/w) | 0.1578 ± 0.00 |
| Chemical composition | phenolics, flavonoids, terpenes |
| Test Samples | IC50 (µg/mL) | |
|---|---|---|
| DPPH | Superoxide Radical Scavenging | |
| Gallic acid | 2.7 ± 0.01 | 23.8 ± 3.9 |
| Crude water extract | 42.8 ± 4.1 | 118.6 ± 10.4 |
| Crude ethanol extract | 44.2 ± 2.3 | 259.3 ± 28.9 |
| Hexane fraction | 233.8 ± 21.7 | 593.5 ± 9.7 |
| CHCl3 fraction | 57.0 ± 1.6 | 417.7 ± 10.1 |
| Aqueous ethanol fraction | 32.4 ± 1.5 | 58.9 ± 5.2 |
| Test Samples | ABTS | FRAP | TPC |
|---|---|---|---|
| (TE mg/g Extract) | (mM Fe (II)/g Extract) | GAE (mg/g Extract) | |
| Crude water extract | 78.2 ± 1.4 | 368.9 ± 23.4 | 42.2 ± 4.5 |
| Crude ethanol extract | 188.0 ± 0.9 | 1376.2 ± 60.4 | 147.9 ± 2.8 |
| Hexane fraction | 98.8 ± 10.9 | 275.4 ± 39.5 | 56.5 ± 4.9 |
| CHCl3 fraction | 163.4 ± 8.8 | 1554.1 ± 23.9 | 173.1 ± 0.4 |
| Aqueous ethanol fraction | 71.1 ± 10.6 | 418.6 ± 31.0 | 39.3 ± 5.1 |
| Group | Liver Weight (g) |
|---|---|
| Normal rats | 10.7 ± 0.58 |
| Control rats (chlorpyrifos) | 11.89 ± 0.65 |
| L. martabanica extract + chlopyrifos | |
| 7.5 and 2.5 mg/kg | 12.37 ± 0.96 |
| 75 and 25 mg/kg | 9.47 ± 0.90 * |
| 750 and 250 mg/kg | 11.21 ± 0.70 |
| Parameters | Normal Rats | Control Rats | L. martabanica. Water Extract (mg/kg) | ||
|---|---|---|---|---|---|
| 7.5 and 2.5 | 75 and 25 | 750 and 250 | |||
| RBC (×106/µL) | 7.0 ± 0.13 | 5.8 ± 0.80 | 7.5 ± 2.45 | 7.0 ± 0.22 | 6.9 ± 0.23 |
| HGB (g/dL) | 13.6 ± 0.22 | 12.5 ± 0.41 | 11.5 ± 1.02 | 13.7 ± 0.41 | 13.4 ± 0.43 |
| HCT (%) | 41.3 ± 0.87 | 36.5 ± 3.88 | 38.6 ± 0.64 | 41.7 ± 1.62 | 41.3 ± 1.20 |
| MCV (fL) | 59.4 ± 0.28 | 64.3 ± 2.92 a | 59.2 ± 0.44 * | 59.2 ± 0.56 * | 59.2 ± 0.43 * |
| MCH (pg) | 19.6 ± 0.12 | 25.8 ± 4.20 a | 19.3 ± 0.13 * | 19.5 ± 0.11 * | 19.2 ± 0.18 * |
| MCHC (g/dL) | 33.0 ± 0.17 | 39.2 ± 4.38 a | 32.7 ± 0.22 * | 32.9 ± 0.33 * | 32.4 ± 0.19 * |
| PLT (×105/µL) | 7.53 ± 0.34 | 8.78 ± 1.04 a | 6.76 ± 0.14 * | 7.91 ± 0.50 | 7.25 ± 0.19 |
| WBC (×103 cells/µL) | 2.84 ± 0.49 | 2.87 ± 0.72 | 1.68 ± 0.31 a,* | 2.72 ± 1.32 | 2.18 ± 0.37 |
| Nu (cells/µL) | 0.39 ± 0.06 | 0.45 ± 0.21 | 0.30 ± 0.06 | 0.31 ± 0.14 | 0.16 ± 0.05 a,* |
| Lymph (cells/µL) | 2.26 ± 0.38 | 2.24 ± 0.45 | 3.00 ± 1.92 | 2.30 ± 1.12 | 0.97 ± 0.30 a,* |
| Mono (cells/µL) | 0.15 ± 0.04 | 0.15 ± 0.05 | 0.09 ± 0.02 | 0.09 ± 0.06 | 0.05 ± 0.02 |
| E (cells/µL) | 0.00 ± 0.01 | 0.00 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.00 |
| Ba (cells/µL) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Parameters | Normal Rats | Control Rats | L. martabanica. Water Extract (mg/kg) | ||
|---|---|---|---|---|---|
| 7.5 and 2.5 | 75 and 25 | 750 and 250 | |||
| BUN (mg/dL) | 18.4 ± 0.74 | 20.1 ± 1.02 | 15.6 ± 0.91 * | 16.6 ± 1.47 | 19.9 ± 2.38 |
| Cr (mg/dL) | 0.66 ± 0.01 | 0.79 ± 0.02 | 0.67 ± 0.01 | 0.57 ± 0.10 * | 0.72 ± 0.04 |
| TP (g/dL) | 5.9 ± 0.07 | 6.9 ± 0.45 a | 5.8 ± 0.11 * | 6.1 ± 0.23 | 6.1 ± 0.40 |
| ALB (g/dL) | 2.9 ± 0.04 | 3.4 ± 0.24 a | 2.9 ± 0.02 * | 3.1 ± 0.14 | 3.0 ± 0.19 |
| TB (mg/dL) | 0.09 ± 0.01 | 0.16 ± 0.03 a | 0.10 ± 0.01 * | 0.10 ± 0.02 * | 0.08 ± 0.02 * |
| DB (mg/dL) | 0.03 ± 0.01 | 0.09 ± 0.01 a | 0.03 ± 0.00 * | 0.05 ± 0.00 * | 0.04 ± 0.01 * |
| AST (U/L) | 85 ± 5.57 | 133 ± 9.20 a | 85 ± 4.24 * | 92 ± 9.16 * | 92 ± 6.70 * |
| ALT (U/L) | 30 ± 7.65 | 57 ± 5.26 a | 45 ± 3.35 * | 41 ± 5.33 * | 38 ± 2.55 * |
| ALP (U/L) | 165 ± 9.26 | 214 ± 19.69 a | 180 ± 11.99 * | 137 ± 10.46 * | 174 ± 14.86 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunnaja, P.; Chansakaow, S.; Wittayapraparat, A.; Yusuk, P.; Sireeratawong, S. In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats. Molecules 2021, 26, 1906. https://doi.org/10.3390/molecules26071906
Kunnaja P, Chansakaow S, Wittayapraparat A, Yusuk P, Sireeratawong S. In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats. Molecules. 2021; 26(7):1906. https://doi.org/10.3390/molecules26071906
Chicago/Turabian StyleKunnaja, Phraepakaporn, Sunee Chansakaow, Absorn Wittayapraparat, Pedcharada Yusuk, and Seewaboon Sireeratawong. 2021. "In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats" Molecules 26, no. 7: 1906. https://doi.org/10.3390/molecules26071906
APA StyleKunnaja, P., Chansakaow, S., Wittayapraparat, A., Yusuk, P., & Sireeratawong, S. (2021). In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats. Molecules, 26(7), 1906. https://doi.org/10.3390/molecules26071906