Structures and Bioactivities of Steroidal Saponins Isolated from the Genera Dracaena and Sansevieria
Abstract
1. Introduction
2. General Aspects of Steroidal Saponins Isolated from Dracaena and Sansevieria Species
2.1. Glycosidic Moieties of Dracaena and Sansevieria Saponins
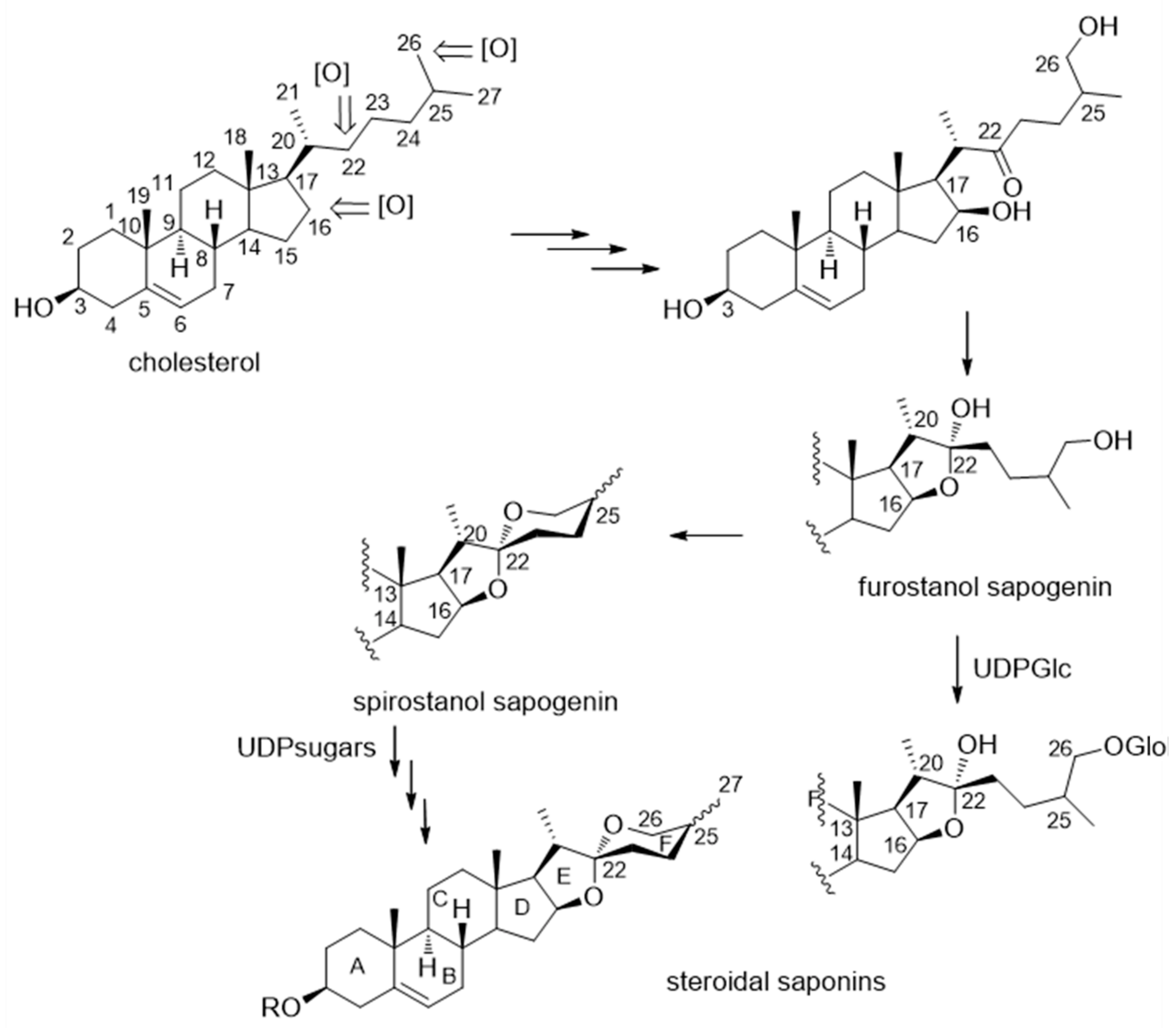
2.2. Isolation and Structure Determination
2.3. NMR Spectra of Dracaena and Sansevieria Saponins
2.3.1. Type of Parent Skeleton and Stereochemistry at C-22
2.3.2. Stereochemistry at C-25
2.3.3. Olefinic Protons and Carbons
2.3.4. Sugar Units
3. Steroidal Saponins Isolated from Dracaena and Sansevieria Species
4. Biological Activities
4.1. Hemolytic Properties
4.2. Antiinflammatory Activity
4.3. Antimicrobial Activity
| Bioactivity | Saponin (Ref.) | Description |
|---|---|---|
| Haemolytic effects | 152 [69] | No haemolytic effects and inhibition of the capillary permeability activity |
| Anti-inflammatory activity | 7 [51] | Anti-inflammatory activity on carrageenan-induced paw edema (maximum inhibitory activity of 71.22%) |
| 46 [51] | Anti-inflammatory activity on carrageenan-induced paw edema (maximum inhibitory activity of 80.57%) | |
| 97 [51] | Anti-inflammatory activity on carrageenan-induced paw edema (maximum inhibitory activity of 66.19%) | |
| Anti-neutrophilic inflammatory activity | 37 [58] | Inhibitory activity against formyl-L-methionyl-L-leucyl-L-phenylalanine-induced superoxide anion generation (IC50 = 18.55 ± 0.23 μM) and elastase release by human neutrophils (IC50 = 1.74 ± 0.25 μM) |
| 83 [58] | Inhibitory activity against formyl-L-methionyl-L-leucyl-L-phenylalanine-induced superoxide anion generation (IC50 = 26.39 ± 1.63 μM) and elastase release by human neutrophils (IC50 = 3.94 ± 0.19 μM) | |
| Antimicrobial activity | 25 [57] | Antimicrobial activities against Cryptococcus neoformans (MIC = 1 μg/mL) and Candida albicans (MIC = 2 μg/mL) |
| 28 [48] | Antifungal activity against Cryptococcus neoformans (IC50 = 20.0 μg/mL) | |
| 32 [62] | Antibacterial activity against Staphylococcus aureus | |
| 35 [48] | Antifungal activity against Cryptococcus neoformans (IC50 = 9.5 μg/mL) | |
| 57 [57] | Antimicrobial activities against Cryptococcus neoformans (MIC = 1–2 μg/mL) and Candida albicans (MIC = 2 μg/mL) | |
| 84 [57] | Antimicrobial activities against Cryptococcus neoformans (MIC = 1–2 μg/mL) | |
| 88 [57] | Antimicrobial activities against Cryptococcus neoformans (MIC = 1–2 μg/mL) and Candida albicans (MIC = 2 μg/mL) | |
| 91 [57] | Antimicrobial activities against Cryptococcus neoformans (MIC = 2 μg/mL) and Candida albicans (MIC = 4–8 μg/mL) | |
| Molluscicidal activity | 97 [66] | At the concentration of 5–6 ppm, spiroconazole A caused 100% mortality of the snails Bulinus globosus, B. forskalii, Biomphalaria pfeifferi, B. glabrata, and Lymnaea natalensis within 24 h. Other two related (unidentified) saponins were lethal with LC50 values in the range of 10–25 ppm |
4.4. Molluscicidal Activity
4.5. Cell Antiproliferative/Cytotoxic Activity
| Drug (Ref.) | Cell Line IC50 (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Promye- locytic leukemia HL-60 | |||||||||
| cisplatin [32] | 1.40 ± 0.08 | ||||||||
| etoposide [32] | 0.38 ± 0.06 | ||||||||
| 1 [32] | 9.34 ± 2.93 | ||||||||
| 4 [32] | 7.38 ± 0.78 | ||||||||
| 11 [32] | 7.85 ± 0.43 | ||||||||
| 32 [32] | 17.3 ± 2.99 | ||||||||
| 34 [32] | 12.3 ± 2.56 | ||||||||
| 46 [32] | 20 | ||||||||
| 59 [32] | 9.45 ± 2.22 | ||||||||
| 73 [32] | 4.45 ± 0.39 | ||||||||
| 80 [32] | 11.3 ± 1.21 | ||||||||
| 97 [32] | 6.36 ± 0.14 | ||||||||
| 98 [32] | 7.64 ± 0.59 | ||||||||
| 100[32] | >20 | ||||||||
| 108 [32] | 6.00 ± 1.22 | ||||||||
| 111 [32] | 0.47 ± 0.04 | ||||||||
| 112 [32] | 0.38 ± 0.04 | ||||||||
| 114 [32] | 2.73 ± 0.42 | ||||||||
| 115 [32] | 1.66 ± 0.20 | ||||||||
| 118 [32] | 0.74 ± 0.05 | ||||||||
| 21 [28] | 9.7 ± 2.7 | ||||||||
| 85 [28] | 3.7 ± 0 | ||||||||
| 85 [55] | 4.0 ± 0.4 | ||||||||
| 88 [28] | 2.0 ± 0.9 | ||||||||
| 88 [55] | 2.3 ± 0.8 | ||||||||
| 155 [28] | 7.2 ± 2.3 | ||||||||
| 157 [28] | 7.3 ± 3.7 | ||||||||
| 49 [29] | 9 ± 4 | ||||||||
| etoposide [29] | 0.2 | ||||||||
| 25 [53] | 1.8 | ||||||||
| 53 [53] | 2.5 | ||||||||
| etoposide [53] | 0.5 | ||||||||
| 124 [55] | 2.6 ± 0.9 |
Epi-dermoid carcinoma A-431 | HeLa (derived from cervical cancer cells) | Colo rectal cancer HCT116 | Hepato cyte carcinoma HepG2 | Breast carci noma MCF7 | Myelo genous leuke mia K-562 | Hepatoma BEL-7402 | Gastric cancer SGC-7901 |
| 49 [29] | 16.1 | ||||||||
| 109 [33] | 26.5 | ||||||||
| 110 [33] | 26.5 | ||||||||
| 33 [47] | 29.6 ± 1.4 | ||||||||
| 38 [47] | 16.9 ± 1.4 | 15.5 ± 2.8 | 18.3 ± 1.4 | ||||||
| 77 [47] | 8.3 ± 2.3 | 10.7 ± 2.3 | 4.8 ± 2.3 | ||||||
| 146 [47] | 24.5 ± 1 | 18.6 ± 1 | 20.6 ± 1 | ||||||
| doxorubicin HCl [47] | 22.4 ± 1.7 | 3.4 ± 5.1 | 1.7 ± 1.7 | ||||||
| 58 [45] | 4.77 | 6.44 | 5.61 | ||||||
| 87 [45] | 1.27 | 4.72 | 2.88 | ||||||
| 97 [45] | 5.09 | 1.13 | 3.39 | ||||||
| paclitaxel [45] | 5.98 | 3.75 | 1.88 | ||||||
| Fibro-sarcoma HT-1080 | Murine colon carcinoma 26-L5 | MelanomaB16-BL6 | |||||||
| 17 [26] | 5.3 | 4.2 | |||||||
| 50 [26] | 27.7 | ||||||||
| 51 [26] | 21.6 | ||||||||
| 53 [26] | 3.8 | 30.2 | 20.9 | ||||||
| 72 [26] | 11.1 | 28.4 | |||||||
| 111 [26] | 0.6 | 22.1 | 11.9 | ||||||
| 112 [26] | 0.2 | 26.6 | 9.7 | ||||||
| 113 [26] | 0.3 | 27.7 | 11.8 | ||||||
| 140 [26] | 21.8 | ||||||||
| 5-fluoro uracil [26] | 1.5 | 0.5 | 0.6 | ||||||
| doxorubicin HCl [26] | 0.2 | 0.1 | 0.2 | Lympho cytic leukemia P388 | Pancreas Carci noma BXPC-3 | CNS glioblastoma SF268 | Lung NCI-H460 | Colon carcinoma KM20L2 | |
| 25 [57] | 2.1 | 2.5 | 2.8 | 2.5 | 2.5 | 2.4 | |||
| 57 [57] | 1.8 | 1.3 | 1.3 | 1.5 | 0.5 | 0.5 | |||
| 84 [57] | 2.0 | 1.1 | 0.7 | 0.8 | 0.3 | 0.3 | |||
| 88 [57] | 1.7 | 1.3 | 1.8 | 1.4 | 1.8 | 1.8 | |||
| 91 [57] | 3.0 | 2.0 | 1.6 | 1.5 | 1.4 | 0.6 | |||
| Prostate carcinoma DU-145 | Lung carcinomaA549 | T-cell leukemia Jurkat | Ovarian cancer Skov-3 | Epithelial colorectal adenocarcinoma CaCo-2 | Colon cancer SW480 | Mouse mam mary cancer EMT6 | |||
| 25 [57] | 2. 2 | ||||||||
| 57 [57] | 1.1 | ||||||||
| 84 [57] | 0.5 | ||||||||
| 88 [57] | 1.8 | ||||||||
| 91 [57] | 1.3 | ||||||||
| 6 [50] | 24.51 ± 0.17 | ||||||||
| 25 [50] | 2.91 ± 0.75 | 2.85 ± 0.16 | 7.87 ± 0.12 | 3.47 ± 0.44 | |||||
| 26 [50] | 30.07 ± 2.49 | 18.98 ± 1.16 | |||||||
| 88 [50] | 0.48 ± 0.17 | 1.96 ± 0.44 | 2.19 ± 0.99 | 2.97 ± 0.24 | |||||
| 161 [50] | 4.94 ± 0.27 | 4.53 ± 0.31 | 6.6 ± 0.4 | 15.2 ± 0.3 | |||||
| doxorubicin [49,50] | 2,1 ± 1.5 | 0.1 ± 0.07 | 1.5 ± 0.15 | 4.3 ± 1.9 | 1.47 | 9.21 | |||
| 158 [49] | 14.3 | 8.6 | |||||||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F.; et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Lu, P.-L.; Morden, C.W. Phylogenetic relationships among dracaenoid genera (Asparagaceae: Nolinoideae) inferred from chloroplast DNAloci. Syst. Bot. 2014, 39, 90–104. [Google Scholar] [CrossRef]
- Takawira-Nyenya, R.; Mucina, L.; Cardinal-Mcteague, W.M.; Thiele, K.R. Sansevieria (Asparagaceae, nolinoideae) is a herbaceous clade within Dracaena: Inference from non-coding plastid and nuclear DNA sequence data. Phytotaxa 2018, 376, 254–276. [Google Scholar] [CrossRef]
- Maděra, P.; Forrest, A.; Hanáček, P.; Vahalík, P.; Gebauer, R.; Plichta, R.; Jupa, R.; Van Rensburg, J.J.; Morris, M.; Nadezhdina, N.; et al. What we know and what we do not know about dragon trees? Forests 2020, 11, 236. [Google Scholar] [CrossRef]
- WFO. An Online Flora of All Known Plants. Published on the Internet. 2020. Available online: http://www.worldfloraonline.org (accessed on 26 April 2020).
- Tandu, K.R. Dracaena nitens: A new source of diosgenin. Planta Med. 1988, 54, 85. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Kitaoka, M.; Taki, M.; Takaishi, S.; Boriboon, M.; Akiyama, T. Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity. Planta Med. 1997, 63, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, J.-N.; Fan, B.; Chen, X.-N.; Pang, D.-R.; Zheng, J.; Zhang, Q.; Zhao, Y.-F.; Xiao, W.; Tu, P.-F.; et al. Phenolic constituents, pharmacological activities, quality control, and metabolism od Dracaena species: A review. J. Ethnopharmacol. 2019, 244, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, J.; Wang, H.; Chen, H.; Mei, W. Dragon’s blood from Dracaena cambodiana in China: Applied history and induction techniques toward formation mechanism. Forests 2020, 11, 372. [Google Scholar] [CrossRef]
- Fan, J.-Y.; Yi, T.; Sze-To, C.-M.; Zhu, L.; Peng, W.-L.; Zhang, Y.-Z.; Zhao, Z.-Z.; Chen, H.-B. A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon’s blood”. Molecules 2014, 19, 10650–10669. [Google Scholar] [CrossRef]
- Machala, M.; Kubínová, R.; Hořavová, P.; Suchý, V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother. Res. 2001, 15, 114–118. [Google Scholar] [CrossRef]
- Silva, B.M.; Santos, R.P.; Mendes, L.S.; de Pinho, P.G.; Valentão, P.; Andrade, P.B.; Pereira, J.A.; Carvalho, M. Dracaena draco L. fruit: Phytochemical and antioxidant activity assessment. Food. Res. Int. 2011, 44, 2182–2189. [Google Scholar] [CrossRef]
- Mbugua, P.K.; Moore, D.M. Taxonomic studies of the genus Sansevieria (Dracaenaceae). In The Biodiversity of African Plants; Van der Maesen, L.J.G., Van der Burgt, M., Van Medenbach de Rooy, J.M., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 489–492. [Google Scholar]
- Takawira, R.; Nordal, I. The genus of Sansevieria (family Dracaenaceae) in Zimbabwe. Acta Hortic. 2002, 572, 189–198. [Google Scholar] [CrossRef]
- Andhare, R.N.; Raut, M.K.; Naik, S.R. Evaluation of antiallergic and anti-anaphylactic activity of ethanolic extract of Sanseveiria trifasciata leaves (EEST) in rodents. J. Ethnopharmacol. 2012, 142, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Bero, J.; Ganfon, H.; Jonville, M.-C.; Frédérich, M.; Gbaguidi, F.; DeMol, P.; Moudachirou, M.; Quetin-Leclercq, J. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J. Ethnopharmacol. 2009, 122, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.-J.R. Medicinal plants used to treat snakebite in Central America: Review and assessment of scientific evidence. J. Ethnopharmacol. 2017, 199, 240–256. [Google Scholar] [CrossRef]
- Lekawatana, S.; Suwannamek, B. Ornamental plants in Thailand. Acta Hortic. 2017, 1167, 11–16. [Google Scholar] [CrossRef]
- Saxena, P.; Ghosh, C. Ornamental plants as sinks and bioindicators. Environ. Technol. 2013, 34, 3059–3067. [Google Scholar] [CrossRef]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and stilbenoids of the genera Dracaena and Sansevieria: Structures and bioactivities. Molecules 2020, 25, 2608. [Google Scholar] [CrossRef] [PubMed]
- Simmons-Boyce, J.L.; Tinto, W.F. Steroidal saponins and sapogenins from the agavaceae family. Nat. Prod. Commun. 2007, 2, 99–114. [Google Scholar] [CrossRef]
- Sahu, N.P.; Banerjee, S.; Mondal, N.B.; Mandal, D. Steroidal Saponins. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer: Vienna, Austria, 2008; Volume 89, pp. 45–141. [Google Scholar]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef]
- Tran, Q.L.; Tezuka, Y.; Banskota, A.H.; Tran, Q.K.; Saiki, I.; Kadota, S. New spirostanol steroids and steroidal saponins from roots and rhizomes of Dracaena angustifolia and their antiproliferative activity. J. Nat. Prod. 2001, 64, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Kougan, G.B.; Miyamoto, T.; Tanaka, C.; Paululat, T.; Mirjolet, J.-F.; Duchamp, O.; Sondengam, B.L.; Lacaille-Dubois, M.-A. Steroidal saponins from two species of Dracaena. J. Nat. Prod. 2010, 73, 1266–1270. [Google Scholar] [CrossRef]
- González, A.G.; Hernández, J.C.; León, F.; Padrón, J.I.; Estévez, F.; Quintana, J.; Bermejo, J. Steroidal saponins from the bark of Dracaena draco and their cytotoxic activities. J. Nat. Prod. 2003, 66, 793–798. [Google Scholar] [CrossRef]
- Hernández, J.C.; León, F.; Estévez, F.; Quintana, J.; Bermejo, J. A homo-isoflavonoid and a cytotoxic saponin from Dracaena draco. Chem. Biodivers. 2006, 3, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Moharram, F.A.; EI-Shenawy, S.M. Antinociceptive and anti-inflammatory steroidal saponins from Dracaena ombet. Planta Med. 2007, 73, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Mimaki, Y.; Sashida, Y. Steroidal saponins from Dracaena surculosa. J. Nat. Prod. 2000, 63, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Z.; Wu, H.; Yokosuka, A.; Mimaki, Y. Steroidal glycosides from the underground parts of Dracaena thalioides and their cytotoxic activity. Phytochemistry 2014, 107, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Tanaka, C.; Jie, B.; Tapondjou, L.A.; Miyamoto, T. Trifasciatosides A–J, steroidal saponins from Sansevieria trifasciata. Chem. Pharm. Bull. 2016, 64, 1347–1355. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Pathak, A.K. NMR spectroscopy of steroidal sapogenins and steroidal saponins: An update. Magn. Reson. Chem. 1995, 33, 923–953. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Bunsawansong, P.; Morris, G.A. Dependence of the 1H NMR chemical shifts of ring F resonances on the orientation of the 27-methyl group of spirostane-type steroidal sapogenins. Phytochemistry 1998, 47, 2479–2496. [Google Scholar] [CrossRef]
- Agrawal, P.K. Spectral assignments and reference data: 25R/25S stereochemistry of spirostane-type steroidal sapogenins and steroidal saponins via chemical shift of geminal protons of ring-F. Magn. Reson. Chem. 2003, 41, 965–968. [Google Scholar] [CrossRef]
- Agrawal, P.K. Dependence of 1H NMR chemical shifts of geminal protons of glycosyloxy methylene (H2-26) on the orientation of the 27-methyl group of furostane-type steroidal saponins. Magn. Reson. Chem. 2004, 42, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. Assigning stereodiversity of the 27-Me group of furostane-type steroidal saponins via NMR chemical shifts. Steroids 2005, 70, 715–724. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Mandal, D.; Banerjee, S.; Mondal, N.B.; Chakravarty, A.K.; Sahu, N.P. Steroidal saponins from the fruits of Asparagus racemosus. Phytochemistry 2006, 67, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Claridge, T.D.W. High-Resolution NMR Techniques in Organic Chemistry, 3rd ed.; Elsevier Science: Oxford, UK, 2016. [Google Scholar]
- Kessler, H.; Gehrke, M.; Griesinger, C. Two-dimensional NMR spectroscopy: Background and overview of the experiments. Angew. Chem. Int. Ed. Engl. 1988, 27, 490–536. [Google Scholar] [CrossRef]
- Bross-Walch, N.; Kühn, T.; Moskau, D.; Zerbe, O. Strategies and tools for structure determination of natural products using modern methods of NMR spectroscopy. Chem. Biodivers. 2005, 2, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Zuo, W.-J.; Wang, H.; Zhao, Y.-X.; Guo, Z.-K.; Luo, Y.; Li, X.-N.; Dai, H.-F.; Mei, W.-L. Steroidal saponins from dragon’s blood of Dracaena cambodiana. Fitoterapia 2014, 94, 94–101. [Google Scholar] [CrossRef]
- Rezgui, A.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Lacaille-Dubois, M.-A. Spirostane-type saponins from Dracaena fragrans « Yellow Coast ». Nat. Prod. Commun. 2015, 10, 37–38. [Google Scholar] [CrossRef]
- Raslan, M.A.; Melek, F.R.; Said, A.A.; Elshamy, A.I.; Umeyama, A.; Mounier, M.M. New cytotoxic dihydrochalcone and steroidal saponins from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem. Lett. 2017, 22, 39–43. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.-J.; Li, X.-C.; Jacob, M.R.; Yang, C.-R. Steroidal saponins from fresh stems of Dracaena angustifolia. J. Nat. Prod. 2010, 73, 1524–1528. [Google Scholar] [CrossRef]
- Rezgui, A.; Mitaine-Offer, A.-C.; Pertuit, D.; Miyamoto, T.; Tanaka, C.; Delemasure, S.; Dutartre, P.; Lacaille-Dubois, M.-A. Steroidal saponins from Dracaena marginata. Nat. Prod. Commun. 2013, 8, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Dzoyem, J.P.; Nono, R.N.; Kauhl, U.; Sandjo, L.P.; Tapondjou, L.A.; Bakowsky, U.; Opatz, T. Cytotoxicity of secondary metabolites from Dracaena viridiflora Engl & Krause and their semisynthetic analogues. Rec. Nat. Prod. 2017, 11, 421–430. [Google Scholar]
- Tapondjou, L.A.; Ponou, K.B.; Teponno, R.B.; Mbiantcha, M.; Djoukeng, J.D.; Nguelefack, T.B.; Watcho, P.; Cadenas, A.G.; Park, H.-J. In vivo anti-inflammatory effect of a new steroidal saponin, mannioside A, and its derivatives isolated from Dracaena mannii. Arch. Pharm. Res. 2008, 31, 653. [Google Scholar] [CrossRef]
- Yokosuka, A.; Mimaki, Y.; Sashida, Y. Four new 3,5-cyclosteroidal saponins from Dracaena surculosa. Chem. Pharm. Bull. 2002, 50, 992–995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mimaki, Y.; Kuroda, M.; Ide, A.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the aerial parts of Dracaena draco and their cytostatic activity on HL-60 cells. Phytochemistry 1999, 50, 805–813. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Tanaka, C.; Gabriel, A.F.; Tapondjou, L.A.; Miyamoto, T. Sappanin-type homoisoflavonoids from Sansevieria trifasciata Prain. Phytochem. Lett. 2015, 12, 262–266. [Google Scholar] [CrossRef]
- Hernández, J.C.; León, F.; Quintana, J.; Estévez, F.; Bermejo, J. Icogenin, a new cytotoxic steroidal saponin isolated from Dracaena draco. Bioorg. Med. Chem. 2004, 12, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, C.-R.; Zhang, Y.-J. New C27 steroidal bisdesmosides from the fresh stems of Dracaena cambodiana. Helv. Chim. Acta 2010, 93, 302–308. [Google Scholar] [CrossRef]
- Pettit, G.R.; Zhang, Q.; Pinilla, V.; Hoffmann, H.; Knight, J.C.; Doubek, D.L.; Chapuis, J.-C.; Pettit, R.K.; Schmidt, J.M. Antineoplastic agents. 534. Isolation and structure of Sansevistatins 1 and 2 from the African Sansevieria ehrenbergii,1. J. Nat. Prod. 2005, 68, 729–733. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lin, M.-K.; Hwang, S.-Y.; Hwang, T.-L.; Kuo, Y.-H.; Chang, C.-I.; Ou, C.-Y.; Kuo, Y.-H. Two anti-inflammatory steroidal saponins from Dracaena angustifolia Roxb. Molecules 2013, 18, 8752–8763. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Takaashi, Y.; Sashida, Y. Steroidal saponins from the stems of Dracaena concinna. Phytochemistry 1998, 47, 1351–1356. [Google Scholar] [CrossRef]
- Said, A.; Aboutabl, E.A.; Melek, F.R.; Abdel Jaleel, G.A.R.; Raslan, M. Steroidal saponins and homoisoflavanone from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem. Lett. 2015, 12, 113–118. [Google Scholar] [CrossRef]
- Zheng, Q.-A.; Zhang, Y.-J.; Li, H.-Z.; Yang, C.-R. Steroidal saponins from fresh stem of Dracaena cochinchinensis. Steroids 2004, 69, 111–119. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.-Y.; Zuo, W.-J.; Wang, H.; Mei, W.-L.; Dai, H.-F. A new steroidal saponin from dragon’s blood of Dracaena cambodiana. J. Asian Nat. Prod. Res. 2014, 17, 409–414. [Google Scholar] [CrossRef]
- Mimaki, Y.; Inoue, T.; Kuroda, M.; Sashida, Y. Steroidal saponins from Sansevieria trifasciata. Phytochemistry 1996, 43, 1325–1331. [Google Scholar] [CrossRef]
- Tchegnitegni, B.T.; Teponno, R.B.; Jenett-Siems, K.; Melzig, M.F.; Miyamoto, T.; Tapondjou, L.A. A dihydrochalcone derivative and further steroidal saponins from Sansevieria trifasciata Prain. Z. Nat. C 2017, 72, 477–482. [Google Scholar] [CrossRef]
- Okunji, C.O.; Okeke, C.N.; Gugnani, H.C.; Iwu, M.M. An antifungal spirostanol saponin from fruit pulp of Dracaena mannii. Int. J. Crude Drug Res. 1990, 28, 193–199. [Google Scholar] [CrossRef]
- Okunji, C.O.; Iwu, M.M.; Hostettmann, K. Molluscicidal saponins from the fruit pulp of Dracaena mannii. Int. J. Pharmacogn. 1991, 29, 66–70. [Google Scholar] [CrossRef]
- Yokosuka, A.; Sekiguchi, A.; Mimaki, Y. Chemical constituents of the leaves of Dracaena thalioides. Nat. Prod. Commun. 2013, 8, 315–318. [Google Scholar] [CrossRef]
- Reddy, K.S.; Shekhani, M.S.; Berry, D.E.; Lynn, D.G.; Hecht, S.M. Afromontoside. A new cytotoxic principle from Dracaena afromontana. J. Chem. Soc. Perkin Trans. I 1984, 987–992. [Google Scholar] [CrossRef]
- da Silva Antunes, A.; da Silva, B.P.; Parente, J.P.; Valente, A.P. A new bioactive steroidal saponin from Sansevieria cylindrica. Phytother. Res. 2003, 17, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.A.; Yang, C.R. Dracaenoside A and B, new C-22 steroidal lactone glycosides from the stem of Dracaena cochinchinensis. Chin. Chem. Lett. 2003, 14, 1261–1264. [Google Scholar]
- Mimaki, Y.; Inoue, T.; Kuroda, M.; Sashida, Y. Pregnane glycosides from Sansevieria trifasciata. Phytochemistry 1997, 44, 107–111. [Google Scholar] [CrossRef]
- Zheng, Q.-A.; Yang, C.-R. Pregnane glycosides from Dracaena cochinchinensis. J. Asian Nat. Prod. Res. 2003, 5, 291–296. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]
- Min, H.-Y.; Pei, H.; Hyun, S.Y.; Boo, H.-J.; Jang, H.-J.; Cho, J.; Kim, J.H.; Son, J.; Lee, H.-Y. Potent anticancer effect of the natural steroidal saponin gracillin is produced by inhibiting glycolysis and oxidative phosphorylation-mediated bioenergetics. Cancers 2020, 12, 913. [Google Scholar] [CrossRef]
- Crotti, A.E.M.; Carollo, C.A.; Gobbo-Neto, L.; Santos, M.D.; Gates, P.J.; Lopes, N.P. LC-hyphenated techniques: Uses in the structural elucidation of low and high molecular weight compounds. In Modern Biotechnology in Medicinal Chemistry and Industry; Taft, C.A., Ed.; Research Signpost: Kerala, India, 2006; Volume 61, pp. 99–141. [Google Scholar]
- Mendonça, S.C.; Simas, R.C.; Simas, D.L.R.; Leitão, S.G.; Leitão, G.G. Mass spectrometry as a tool for the dereplication of saponins from Ampelozizyphus amazonicus Ducke bark and wood. Phytochem. Anal. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]












| 1-OH | 3- | 6- | 12- | 15- | 16- | 24- | 26- | 2′- | 3′- | 4′- | 4″- | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spirostanol monoglycosides | Arap 3 Xylp 1 | Glcp 3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Spirostanol diglycosides | Arap 19 Glcp 3 Fucp 3 Xylp 1 | Galp1 Glcp 15 | Glcp 1 | -- | -- | -- | Glcp 3 | -- | Glcp 1 Rhap 28 | Rhap 1 Xylp 1 | Galp 1 Rhap 6 | -- |
| Spirostanol triglycosides | Arap 23 Fucp 2 Glcp 3 Xylp 1 | Glcp 32 | -- | -- | -- | -- | Arap 1 Fucp 5 Glcp 1 | -- | Rhap 56 Glcp 1 | Galp 1 Glcp 8 Rhap 9 Xylp 21 | Apif 1 Arap 1 Rhap 7 Xylp 1 | Galp 3 |
| Spirostanol tetraglycosides | Arap 13 Fucp 1 | -- | -- | -- | -- | -- | Arap 1 Fucp 10 Glcp 2 Rhap 1 | -- | Rhap 13 Xylp 1 | Rhap 1 Xylp 13 | -- | -- |
| Furostanol diglycosides | Fucp 2 Glcp 2 | -- | -- | -- | -- | -- | -- | Glcp 5 | Glcp 1 | -- | -- | -- |
| Furostanol triglycosides | Arap 5 | Arap 2 Fucp 4 Glcp 7 | -- | -- | -- | -- | -- | Glcp 15 Rhap 1 | Rhap 15 | Glcp 1 | Rhap 4 | -- |
| Furostanol tetraglycosides | Arap 6 Fucp 1 Glcp 4 Xylp 1 | Glcp 18 | -- | Rhap 1 | Rhap 1 | -- | -- | Glcp 30 | Rhap 29 | Glcp 6 Xylp 13 | Rhap 10 | -- |
| Cholestane derivatives | Arap 1 Rhap 1 | Glcp 4 | Glcp 1 | Glcp 1 | Rhap 3 | Rhap 2 | -- | -- | ||||
| Pregnanes and lactones 165-167 | Arap 3 Glcp 2 | Glcp 6 | Glcp 1 | Rhap 11 | Glcp 2 Rhap 2 Xylp 3 | Rhap 2 |
| Number | Compound Name | Plant | References |
|---|---|---|---|
| 1 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-α-L-arabinopyranoside | D. angustifolia | [26] |
| D. fragrans (D. deisteliana) | [27] | ||
| D. thalioides | [32] | ||
| 2 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-(4-O-sulfo)-α-L-arabinopyranoside (cambodianoside F) | D. cambodiana | [45] |
| D. fragrans (D. deisteliana) | [46] | ||
| 3 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 3-O-β-D-glucopyranoside | S. cylindrica | [47] |
| 4 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-β-D-xylopyranoside | D. thalioides | [32] |
| 5 | (22R,25S)-Spirost-5-ene-1β,3β,7β-triol 1-O-(4-O-sulfo)-α-L-arabinopyranoside (angudracanoside E) | D. angustifolia | [48] |
| 6 | (22R,25R)-Spirost-5-en-3β-ol 3-O-β-D-glucopyranoside (trillin) | D. marginata D. viridiflora | [49] 50] |
| 7 | (22R,25R)-Spirost-5-ene-3β,17α-diol 3-O-β-D-glucopyranoside (pennogenin 3-O-β-D-glucopyranoside or floribundasaponin A) | D. arborea D. draco D. mannii | [27] [29] [51] |
| Number | Compound Name | Plant | References |
|---|---|---|---|
| 8 | (24S,25R)-24-O-β-D-Glucopyranosyl-3α,5α-cyclospirostane-1β,6β,24-triol 1-O-β-D-fucopyranoside | D. sarculosa | [52] |
| 9 | (24S,25R)-24-O-β-D-Glucopyranosyl-3α,5α-cyclospirostane-1β,6β,24-triol 1-O-β-D-glucopyranoside | D. sarculosa | [52] |
| 10 | (24S,25R)-24-O-β-D-Glucopyranosyl-spirost-5-ene-1β,3β,24-triol 1-O-β-D-fucopyranoside (surculoside B) | D. sarculosa | [31] |
| 11 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. angustifolia | [26] |
| D. cambodiana | [45] | ||
| D. draco | [29,53] | ||
| D. fragrans (D. deisteliana) | [27] | ||
| D. thalioides | [32] | ||
| S. trifasciata (D. trifasciata) | [54] | ||
| 12 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside (angudracanoside B | D. angustifolia | [48] |
| D. cambodiana | [45] | ||
| D. fragrans (D. deisteliana) | [46] | ||
| 13 | (22R)-Spirosta-5,25(27)-diene-1β,3β,7β-triol 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside (angudracanoside C) | D. angustifolia | [48] |
| 14 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. draco | [53] |
| 15 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. draco | [29,53] |
| 16 | (22R,24S)-Spirosta-5,25(27)-diene-1β,3β,24-triol 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside (angudracanoside D) | D. angustifolia | [48] |
| 17 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside (draconin B) | D. angustifolia | [26] |
| D. draco | [28,29,53,55] | ||
| 18 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-(2-O-acetyl)-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (draconin C) | D. draco | [28,29] |
| 19 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-(4-O-acetyl)-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (draconin C) | D. draco | [28,29,53] |
| 20 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-(2,3-di-O-acetyl)-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (draconin B) | D. draco | [28,29] |
| 21 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (draconin A) | D. draco | [28,29] |
| 22 | (22R,25R)-5β-Spirostan-3-β-ol (smilagenin) 3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | D. ombet | [30] |
| 23 | (22R,25S)-5α-Spirostan-3-β-ol-12-one 3-O-β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (terreside B) a | D. angustifolia | [48] |
| 24 | (2 2S,23S,25R)-5α-Spirostane-3β,6α,23-triol-3,6-di-O-β-D-glucopyranoside (cantalasaponin-1) | D. cambodiana | [56] |
| 25 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (prosapogenin A) | D. draco | [53] |
| D. fragrans (D. deisteliana) | [46] | ||
| D. viridiflora | [49] | ||
| S. ehrenbergii | [57] | ||
| 26 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (prosapogenin B) | D. draco D. viridiflora | [28,55] [50] |
| 27 | (22R,25R)-Spirost-5-en-3β-ol 3-O-(3-O-sulfo)-α-L-rhamnosyl-(1→4)-β-D-glucopyranoside (deistelianoside A) | D. fragrans (D. deisteliana) | [27] |
| 28 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (alliospiroside A) | D. angustifolia D. concinna D. marginata S. cylindrica | [48,58] [59] [49] [60] |
| 29 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside | D. angustifolia | [48] |
| D. fragrans (D. deisteliana) | [46] | ||
| 30 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside | D. marginata | [49] |
| 31 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside | D. surculosa | [31] |
| 32 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | D. marginata D. thalioides | [49] [32] |
| 33 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | S. cylindrica | [47] |
| 34 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-xilopyranoside | D. thalioides | [32] |
| 35 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 1-O-β-D-xylopyranosyl-(1→3)-α-L-arabinopyranoside (angudracanoside F) | D. angustifolia | [48] |
| 36 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 3-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside | D. concinna | [59] |
| 37 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (drangustoside B) | D. angustifolia | [58] |
| 38 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | S. cylindrica | [47] |
| 39 | (22R,25S)-Spirost-5-ene-1β,3β-diol-7-one 1-O-α-L-rhamnopyranosyl-(1→2)-4-O-sulfo-α-L-arabinopyranoside (angudracanoside A) | D. angustifolia | [48] |
| 40 | (22R,24S,25R)-Spirost-5-ene-1β,3β,24-triol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (alliospiroside C) | D. marginata | [49] |
| 41 | (22R,24S,25S)-Spirost-5-ene-1β,3β,24-triol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. marginata | [49] |
| 42 | (22R,25R)-Spirost-5-ene-3β,7α-diol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (sansevierin A) | S. ehrenbergii | [57] |
| 43 | (14R,22R,25R and 14R,22R,25S)-Spirost-5-ene-3β,14-diol 3-O-α-L-rhamnoyranosyl-(1→2)-β-D-glucopyranoside (dracaenoside E) | D. cochinchinensis | [61] |
| 44 | (14R,22R,25R and 14R,22R,25S)-Spirost-5-ene-3β,14-diol 3-O-α-L-rhamnoyranosyl-(1→4)-β-D-glucopyranoside (dracaenoside E) | D. cochinchinensis | [61] |
| 45 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | D. draco D. surculosa | [29] [31] |
| 46 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside (mannioside A) | D. arborea D. mannii D. thalioides | [27] [51] [32] |
| 47 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | D. draco | [29] |
| Number | Compound Name | Plant | References |
|---|---|---|---|
| 48 | (22R,24S,25R)-1-O-β-D-Fucopyranosyl-spirost-5-ene-1β,3β,24-triol 3-O-β-D-apiofuranosyl-(1→4)-β-D-glucopyranoside (surculoside A) | D. sarculosa | [31] |
| 49 | (22S,23S,24S)-24-O-β-D-Arabinopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetrol 1-O-[(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranoside (icodeside) | D. draco | [29] |
| 50 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (namonin C) | D. angustifolia | [26] |
| D. cambodiana | [26,62] | ||
| 51 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-[(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranoside (namonin D) | D. angustifolia | [26] |
| D. cambodiana | [56] | ||
| 52 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-[3,4-O-diacetyl)-α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranoside (cambodracanoside A) | D. cambodiana | [56] |
| 53 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-[(2,3,4-O-triacetyl)-α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranoside | D. angustifolia D. draco | [26] [28,53] |
| 54 | (22S,23S,24S,25R)-24-O-β-D-Fucopyranosyl-spirost-5-ene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (cambodracanoside B) | D. cambodiana | [56] |
| 55 | (22R,24S,25R)-24-O-β-D-Glucopyranosyl-spirost-5-en-1b,3β,24-triol 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside (surculoside C) | D. sarculosa | [31] |
| 56 | (22R)-Spirosta-5,25(27)-dien-3β-ol 3-O-α-L-rhamnoyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside I) | D. cochinchinensis | [61] |
| 57 | (22R)-Spirosta-5,25(27)-dien-3β-ol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (sansevistatin 1) | S. ehrenbergii | [57] |
| 58 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. cambodiana | [45] |
| D. fragrans (D. deisteliana) | [27] | ||
| D. thalioides | [32] | ||
| S. trifasciata (D. trifasciata) | [33] | ||
| 59 | (22R)-Spirosta-5,25(27)-diene-1β,3β-diol (neoruscogenin) 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside B) | S. trifasciata (D. trifasciata) | [33] |
| 60 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. draco | [53] |
| S. trifasciata (D. trifasciata) | [63] | ||
| 61 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-(2-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside K) | S. trifasciata (D. trifasciata) | [64] |
| 62 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-(3-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside L) | S. trifasciata (D. trifasciata) | [64] |
| 63 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33,63] |
| 64 | (22S,23S)-Spirosta-5,25(27)-diene-1β,3β,23-triol 1-O-(2,3-di-O-acetyl)- α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [63] |
| 65 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. cambodiana | [62] |
| S. trifasciata (D. trifasciata) | [33,63] | ||
| 66 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside M) | S. trifasciata (D. trifasciata) | [64] |
| 67 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(3-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside N) | S. trifasciata (D. trifasciata) | [64] |
| 68 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33,63] |
| 69 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33,63] |
| 70 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,4-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside G) | S. trifasciata (D. trifasciata) | [33] |
| 71 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(3,4-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside H) | S. trifasciata (D. trifasciata) | [33] |
| 72 | (22S,23S,24S)-Spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. angustifolia | [26] |
| D. thalioides | [32] | ||
| S. trifasciata (D. trifasciata) | [63] | ||
| 73 | (22R,25R)-5β-Spirostan-3-β-ol (smilagenin) 3-O-β-D-galactopyranosyl-(1‴→4″)-β-D-galactopyranosyl-(1″→3′)-β-D-glucopyranoside | D. ombet | [30] |
| 74 | (22R,25R)-5β-spirostan-3-β-ol (smilagenin) 3-O-β-D-galactopyranosyl-(1‴→4″)-β-D-glucopyranosyl-(1′’→3′)-β-D-glucopyranoside | D. ombet | [30] |
| 75 | (22R,25R)-5α-Spirostan-3-β-ol-12-one 3-O-β-D-galactopyranosyl-(1‴→4″)-β-D-glucopyranosyl-(1″→2′)-β-D-glucopyranoside (terreside A) a | D. angustifolia | [48] |
| 76 | (22R,25R)-Spirosta-3β,5α,6β-triol 3-O-b-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (cambodianoside G) | D. cambodiana | [62] |
| 77 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. angustifolia | [48] |
| D. marginata | [49] | ||
| D. thalioides | [32] | ||
| S. cylindrica | [47] | ||
| S. trifasciata (D. trifasciata) | [33] | ||
| 78 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside C) | S. trifasciata (D. trifasciata) | [33] |
| 79 | (22R,25S)-Spirost-5-ene-1b,3β-diol [(S)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside D) | D. cambodiana | [45] |
| S. trifasciata (D. trifasciata) | [33] | ||
| 80 | (22R,25R)-Spirost-5-ene-1β,3β-diol [(R)-ruscogenin] 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-xylopyranoside | D. thalioides | [32] |
| 81 | (22R,24S,25R)-Spirost-5-ene-1β,3β,24-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. marginata | [49] |
| 82 | (22S,23S,24S,25S)-Spirost-ene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. concinna | [59] |
| 83 | (22R,25S)-Spirost-5-ene-1β,3β-diol [(S)-ruscogenin] 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (drangustoside A) | D. angustifolia | [58] |
| 84 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-arabinopyranosyl-(1→4)]-β-D-glucopyranoside (sansevistatin 2) | S. ehrenbergii | [57] |
| 85 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (gracillin) | D. concinna D. draco D. viridiflora | [,59] [28] [50] |
| 86 | (22R,25R and 22R,25S)-Spirost-5-en-3β-ol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | D. cochinchinensis | [61] |
| 87 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside | D. cambodiana | [45] |
| 88 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dioscin) | D. concinna | [59] |
| D. draco | [28,29,55] | ||
| D. viridiflora | [50] | ||
| S. ehrenbergii | [57] | ||
| 89 | (22R,25R and 22R,25S)-Spirost-5-en-3β-ol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | D. cochinchinensis | [61] |
| 90 | (22S,25S)-Spirost-5-en-3β-ol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (borassoside E) | D. marginata | [49] |
| 91 | (22R,25R)-Spirost-5-en-3β-ol (diosgenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→4)]-β-D-glucopyranoside | S. ehrenbergii | [57] |
| 92 | (14R,22R,25R and 14R,25S)-Spirost-5-ene-3β,14-diol 3-O-α-L-rhamnoyranosyl-(1→2)-[β-D-glucopyranosyl-(1→)]-β-D-glucopyranoside (dracaenoside H) | D. cochinchinensis | [61] |
| 93 | (14R,22R,25R and 14R,22R,25S)-Spirost-5-ene-3β,14-diol 3-O-α-L-rhamnoyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dracaenoside G) | D. cochinchinensis | [61] |
| 94 | (14R,22R,24S,25R)-Spirost-5-en-3β,14,24-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside L) | D. cochinchinensis | [61] |
| 95 | (14R,22R,24S,25R)-Spirost-5-en-3β,14,24-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dracaenoside K) | D. cochinchinensis | [61] |
| 96 | (14R,22R,25S)-Spirost-5-ene-3β,14,27-triol 3-O-α-L-rhamnopyranosyl--(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside J) | D. cochinchinensis | [61] |
| 97 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (spiroconazole A) | D. arborea | [27] |
| D. cambodiana | [45] | ||
| D. mannii | [51,65,66] | ||
| D. thalioides | [32] | ||
| 98 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-(4-O-acetyl)-β-D-glucopyranoside | D. thalioides | [32] |
| 99 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-(6-O-acetyl)-β-D-glucopyranoside (arboreasaponin A) | D. arborea | [27] |
| 100 | (17S,22R,25R)-Spirost-5-ene-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-(4,6-O-diacetyl)-β-D-glucopyranoside | D. thalioides | [32] |
| 101 | (17S,22R,25R)-Spirost-5-en-3β,17-diol (pennogenin) 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (pennogenin 3-O-β-chacotrioside) | D. draco D. surculosa | [29] [31] |
| 102 | (17S,22R,24R,25S)-Spirost-5-ene-3β,17,24-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (arboreasaponin B) | D. arborea | [27] |
| 103 | (22R,24S,25S)-Spirost-5-ene-3β,24,27-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (cambodianoside B) | D. cambodiana | [45] |
| 104 | (22R,25S)-Spirost-5-ene-3β,27-diol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside (cambodianoside C) | D. cambodiana | [45] |
| Number | Compound Name | Source | References |
|---|---|---|---|
| 105 | (22S,23S,24S)-24-O-α-L-Arabinopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-β-D-xylopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-fucopyranoside (deistelianoside B) | D. fragrans (D. deisteliana) | [27] |
| 106 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-O-α-L-arabinopyranoside | D. cambodiana | [62] |
| 107 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. cambodiana | [62] |
| S. trifasciata (D. trifasciata) | [33,63] | ||
| 108 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. thalioides | [32] |
| S. trifasciata (D. trifasciata) | [33,63] | ||
| 109 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,4-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside I) | S. trifasciata (D. trifasciata) | [33] |
| 110 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(3,4-di-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (trifasciatoside J) | S. trifasciata (D. trifasciata) | [33] |
| 111 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. angustifolia | [26] |
| D. thalioides | [32] | ||
| S. trifasciata (D. trifasciata) | [33,63] | ||
| 112 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[(3-O-acetyl)-β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (namonin A) | D. angustifolia | [26] |
| D. thalioides | [32] | ||
| 113 | (22S,23S,24S)-24-O-β-D-Fucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[(4-O-acetyl)-β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside (namonin B) | D. angustifolia | [26] |
| 114 | (22S,23S,24S)-24-O-β-D-Glucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. thalioides | [32] |
| S. trifasciata (D. trifasciata) | [63] | ||
| 115 | (22S,23S,24S)-24-O-β-D-Glucopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[(3-O-acetyl)-β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. thalioides | [32] |
| 116 | (22S,23S,24S)-24-O-α-L-Rhamnopyranosyl-spirosta-5,25(27)-diene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-O-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [63] |
| 117 | (22S,23S,24S,25S)-24-O-β-D-Fucopyranosyl-spirost-5-ene-1β,3β,23,24-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-O-α-L-arabinopyranoside | D. concinna | [59] |
| 118 | (22S,23S,24S,25S)-24-O-β-D-Fucopyranosyl-spirost-5-ene-1β,3β,23,24-tetraol 1-O-(2,3,4-tri-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-O-α-L-arabinopyranoside | D. thalioides | [32] |
| Number | Compound Name | Source | References |
|---|---|---|---|
| 119 | (22S,25S)-Furost-5-en-22(25)-epoxy-1β,3β,26-triol 26-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside (cambodianoside E) | D. cambodiana | [45] |
| 120 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-3α,5α-cyclofurostane-1β,6β,26-triol 1-O-β-D-fucopyranoside | D. surculosa | [52] |
| 121 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-3α,5α-cyclofurostane-1β,6β,26-triol 1-O-β-D-glucopyranoside | D. surculosa | [52] |
| 122 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-1β,3β,26-triol 1-O-β-D-fucopyranoside | D. surculosa | [31] |
| 123 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-1β,3β,26-triol 1-O-β-D-glucopyranoside | D. surculosa | [31] |
| Number | Compound Name | Source | References |
|---|---|---|---|
| 124 | (22R,25R)-Furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (icogenin) | D. draco | [55] |
| 125 | (14R,22S,25S)-Furost-5-en-22(25)-epoxy-3β,14,26,27-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dracaenoside R) | D. cochinchinensis | [61] |
| 126 | (22R)-26-O-β-D-Glucopyranosyl-furosta-5,25(27)-diene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33] |
| 127 | (22R)-26-O-β-D-Glucopyranosyl-furosta-5,20(22),25(27)-triene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. angustifolia D. cambodiana | [26] [56] |
| 128 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside (alliofuroside A) | D. marginata | [49] |
| 129 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-(4-O-sulfo)-α-L-arabinopyranoside | D. marginata | [49] |
| 130 | (22R,25S)-26-O-β-D-Glucopyranosyl-3-O-sulfo-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-(3,4-O-diacetyl)-α-L-arabinopyranoside sodium salt | D. thalioides | [67] |
| 131 | (14R,22ξ,25R + 14R,22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,14,22,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside (ophipojaponin A + dracaenoside N) | D. cochinchinensis | [61] |
| 132 | (14R, 22ξ,25R and 14R,22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,14,22,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (dracaenoside M) | D. cochinchinensis | [61] |
| 133 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-furosta-5,25(27)-diene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. cambodiana D. draco | [56] [53,] |
| 134 | (22R)-26-O-β-D-Glucopyranosyl-22-methoxy-furosta-5,25(27)-diene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [63] |
| 135 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside | D. surculosa | [31] |
| 136 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-5α-furost-25(27)-ene-1β,3α,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside | D. concinna | [59] |
| 137 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-5α-furost-25(27)-ene-1β,3α,4α,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside | D. concinna | [59] |
| 138 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-5α-furost-25(27)-ene-1β,3β,4α,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-β-D-fucopyranoside | D. concinna | [59] |
| 139 | (22R,25R)-26-O-α-L-Rhamnopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside (afromontoside) | D. afromontana | [68] |
| Number | Compound Name | Source | References |
|---|---|---|---|
| 140 | (25R)-26-O-β-D-Glucopyranosyl-furosta-5,20(22)-diene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-(4-O-acetyl)-α-L-arabinopyranoside (namonin E) | D. angustifolia | [26] |
| 141 | (14R,25R and 14R,25S)-26-O-β-D-Glucopyranosyl-furosta-5,20(22)-diene-3β,14,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside Q) | D. cochinchinensis | [61] |
| 142 | (22R)-26-O-β-D-Glucopyranosyl-furosta-5,25(27)-diene-1β,3β,22,26-tetraol 1-O-(4-O-acetyl)-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33] |
| 143 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-furosta-5,25(27)-diene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. angustifolia | [48] |
| 144 | (22ξ)-26-O-β-D-Glucopyranosyl-22-methoxy-furosta-5,25(27)-diene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-fucopyranoside | D. concinna | [59] |
| 145 | (22S,25S)-26-O-β-D-Glucopyranosyl-furost-5-en-22(25)-epoxy-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside A) | S. trifasciata (D. trifasciata) | [33] |
| 146 | (22R,25R)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. cylindrica | [47] |
| 147 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. thalioides | [67] |
| 147aa | 26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | D. angustifolia | [48] |
| 148 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside E) | S. trifasciata (D. trifasciata) | [33] |
| 149 | (22R,25R)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-xylopyranoside | D. thalioides | [67] |
| 150 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-1β,3β,22,26-tetraol 1-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | D. marginata | [49] |
| 151 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-1β,3β,26-triol 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside (trifasciatoside F) | S. trifasciata (D. trifasciata) | [33] |
| 152 | (12R,15R,22R,25S)-26-O-β-D-Glucopyranosyl-12,15-di-O-α-L-rhamnopyranosyl-furost-5-ene-3β,12,15,22,26-pentaol 3-O-β-D-glucopyranoside | S. cylindrica | [69] |
| 153 | (14R,22ξ,25R and 14R,22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,14,22,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside P) | D. cochinchinensis | [61] |
| 154 | (14R,22ξ,25R and 14R,22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,14,22,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dracaenoside O) | D. cochinchinensis | [61] |
| 155 | (22R,25R)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | D. draco | [28] |
| 156 | (22ξ,25R and 22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | D. cochinchinensis | [61] |
| 157 | (22R,25R)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | D. draco | [28] |
| 158 | (22R,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (protoneodioscin) | D. marginata | [49] |
| 159 | (22ξ,25R and 22ξ,25S)-26-O-β-D-Glucopyranosyl-furost-5-ene-3β,22,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | D. cochinchinensis | [61] |
| 160 | (22ξ,25R)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-3β,26-diol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | D. concinna | [59] |
| 161 | (22R,25R)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-3β,26-diol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (methyl protodioscin) | D. viridiflora | [50] |
| 162 | (22R,25S)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-3β,26-diol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (methyl protoneodioscin) | D. marginata | [49] |
| 163 | (22ξ,25R)-26-O-β-D-Glucopyranosyl-22-methoxy-furost-5-ene-3β,26-diol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | D. concinna | [59] |
| Number | Compound Name | Source | References |
|---|---|---|---|
| 164 | Cambodianoside A | D. cambodiana | [45] |
| 165 | Cambodianoside D | D. cambodiana | [45] |
| 166 | Dracaenoside A | D. cochinchinensis | [70] |
| 167 | Dracaenoside B | D. cochinchinensis | [70] |
| 168 | Pregna-5,16-diene-1β,3β-diol-20-one 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | D. angustifolia | [26] |
| D. cambodiana | [62] | ||
| S. trifasciata (D. trifasciata) | [71] | ||
| 169 | Pregna-5,16-diene-1β,3β-diol-20-one 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-α-L-arabinopyranoside | S. trifasciata (D. trifasciata) | [33,71] |
| 170 | Pregna-5,16-diene-1β,3β-diol-20-one 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranoside | S. trifasciata (D. trifasciata) | [33,71] |
| 171 | Pregna-5,16-diene-1β,3β-diol-20-one 1-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-6-O-acetyl-β–D-glucopyranoside | S. trifasciata (D. trifasciata) | [71] |
| 172 | Pregna-5,16-diene-3β,14α-diol-20-one 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside (dracaenoside D) | D. cochinchinensis | [72] |
| 173 | Pregna-5,16-dien-3β-ol-20-one 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside | D. cambodiana | [45] |
| 174 | Pregna-5,16-diene-3β,14α-diol-20-one 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside (dracaenoside C) | D. cochinchinensis | [72] |
| 175 | (16S)-1-O-α-L-Rhamnopyranosyl-(1→2)-O-α-L-arabinopyranosyl-pregn-5-ene-1β,3β,16-triol 16-O-(4-β-D-glucopyranosyloxymethyl)-pent-4-enoate (namonin F) | D. angustifolia | [26] |
| 176 | (25R)-26-O-β-D-Glucopyranoyl-cholesta-5,17-dien-3β-ol-16,22-dione 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→3)]-β-D-glucopyranoside | D. cambodiana | [45] |
| 177 | 1-O-α-L-Rhamnopyranosyl-5α-cholesta-1β,3β,16β-triol-7,23-dione 16-β-D-glucopyranoside (concinnasteoside A) | D. concinna | [72] |
| 178 | β-Sitosterol 3-O-β-D-glucopyranoside (daucosterol) | D. draco | [29] |
| 179 | β-Sitosterol 3-O-(6-O-palmitoyl)–β-D-glucopyranoside (sitoindoside I) | D. draco | [55] |
| 180 | Stigmasterol 3-O-β-D-glucopyranoside | D. viridiflora | [50] |
| 181 | 1β-Hydroxy-kryptogenin 1-O-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside | S. cylindrica | [60] |
| Species | Plant Parts Extracted | Saponins |
|---|---|---|
| Dracaena afromontana Mildbr. | methanolic extract of the twigs | 139 |
| Dracaena angustifolia Medik, (Roxb.) | methanolic extract of fresh stems; roots and rhizomes | 1,5,11,12,13,16,17,(23),28,29.35,37,39,50,51,53,72,(75),77,83,111,112, 113,127,140,143,147a,168,175 |
| Dracaena arborea (Willd.) Link | methanolic extract of bark | 7,46,97,99,102 |
| Dracaena cambodiana Pierre ex Gagnep | fresh stems; dragon’s blood | 2,11,12,24,50,51,52,54,58,65,76,79,87,97,103,104,106,107,119,127,133, 164,165,168,173,176 |
| Dracaena cochinchinensis (Lour.) S.C. Chen | fresh stems (dragon’s blood) | 43,44,56,86,89,92,93,94,95,96,125,131,132,141,153,154,156,159,166,167,172,174 |
| Dracaena concinna Kunth | fresh stems | 28,36,82,85,88,117,136,137,138,144,160,163,177 |
| Dracaena draco L. | stem bark; aerial parts; leaves; roots | 7,11,14,15,17,18,19,20,21,25,26,45,47,49,53,60,85,88,101,124,133,155, 157,178,179 |
| Dracaena fragrans (L.) Ker Gawl. (syn. D. deisteliana Engl.) | methanolic extract of stems; bark, roots, leaves | 1,2,11,12,25,27,29,58,105 |
| Dracaena mannii Baker | fruit pulp; stem bark | 7,46,97 |
| Dracaena marginata Hort. | bark, roots | 6,28,30,32,40,41,77,81,90,128,129,150,158,162 |
| Dracaena ombet Heuglin ex Kotschy & Peyr. | leaves | 22,73,74 |
| Dracaena surculosa Lindl. | methanolic extract of whole plant | 8,9,10,31,45,48,55,101,120,121,122,123,135 |
| Dracaena thalioides Hort. Makoy ex E. Morren | leaves; fresh underground parts | 1,4,11,32,34,46,58,72,77,80,97,98,100,108,111,112,114,115,118,130,147,149 |
| Dracaena viridiflora Engl. & K. Krause | leaves | 6,25,26,85,88,161,180 |
| Sansevieria cylindrica Bojer ex Hook. | methanolic extract of unflowering aerial parts; leaves | 3,28,33,38,77,146,152,181 |
| Sansevieria ehrenbergii Schweinf. ex Baker | MeOH-CH2Cl2 extract of chipped plant | 25,42,57,84,88,91 |
| Sansevieria trifasciata Prain (syn. D. trifasciata (Prain) Mabb) | aerial parts; methanol extract of the whole plant | 11,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,77,78,79,107,108,109, 110,111,114,116,126,134,142,145,148,151,168,169,170,171 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, Z.M.; Oo, S.M.; Nwe, T.M.; Aung, H.T.; Armijos, C.; Hussain, F.H.S.; Vidari, G. Structures and Bioactivities of Steroidal Saponins Isolated from the Genera Dracaena and Sansevieria. Molecules 2021, 26, 1916. https://doi.org/10.3390/molecules26071916
Thu ZM, Oo SM, Nwe TM, Aung HT, Armijos C, Hussain FHS, Vidari G. Structures and Bioactivities of Steroidal Saponins Isolated from the Genera Dracaena and Sansevieria. Molecules. 2021; 26(7):1916. https://doi.org/10.3390/molecules26071916
Chicago/Turabian StyleThu, Zaw Min, Sann Myint Oo, Thinn Myat Nwe, Hnin Thanda Aung, Chabaco Armijos, Faiq H. S. Hussain, and Giovanni Vidari. 2021. "Structures and Bioactivities of Steroidal Saponins Isolated from the Genera Dracaena and Sansevieria" Molecules 26, no. 7: 1916. https://doi.org/10.3390/molecules26071916
APA StyleThu, Z. M., Oo, S. M., Nwe, T. M., Aung, H. T., Armijos, C., Hussain, F. H. S., & Vidari, G. (2021). Structures and Bioactivities of Steroidal Saponins Isolated from the Genera Dracaena and Sansevieria. Molecules, 26(7), 1916. https://doi.org/10.3390/molecules26071916






