Antioxidant Compounds, Kirenol and Methyl ent-16?, 17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production
Abstract
:1. Introduction
2. Results and Discussion
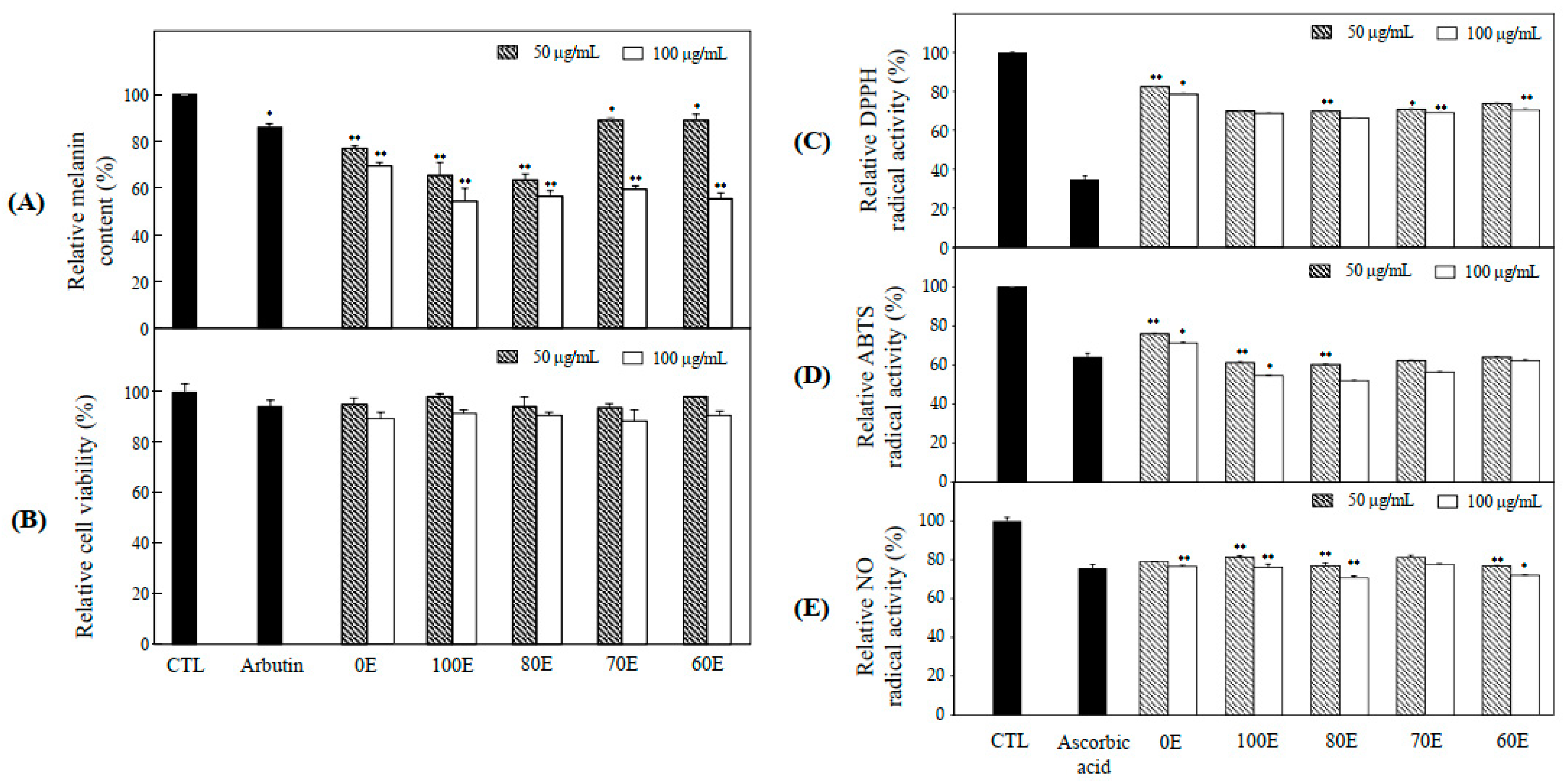
2.1. Anti-Melanogenesis and Anti-Oxidant Effects of Extract
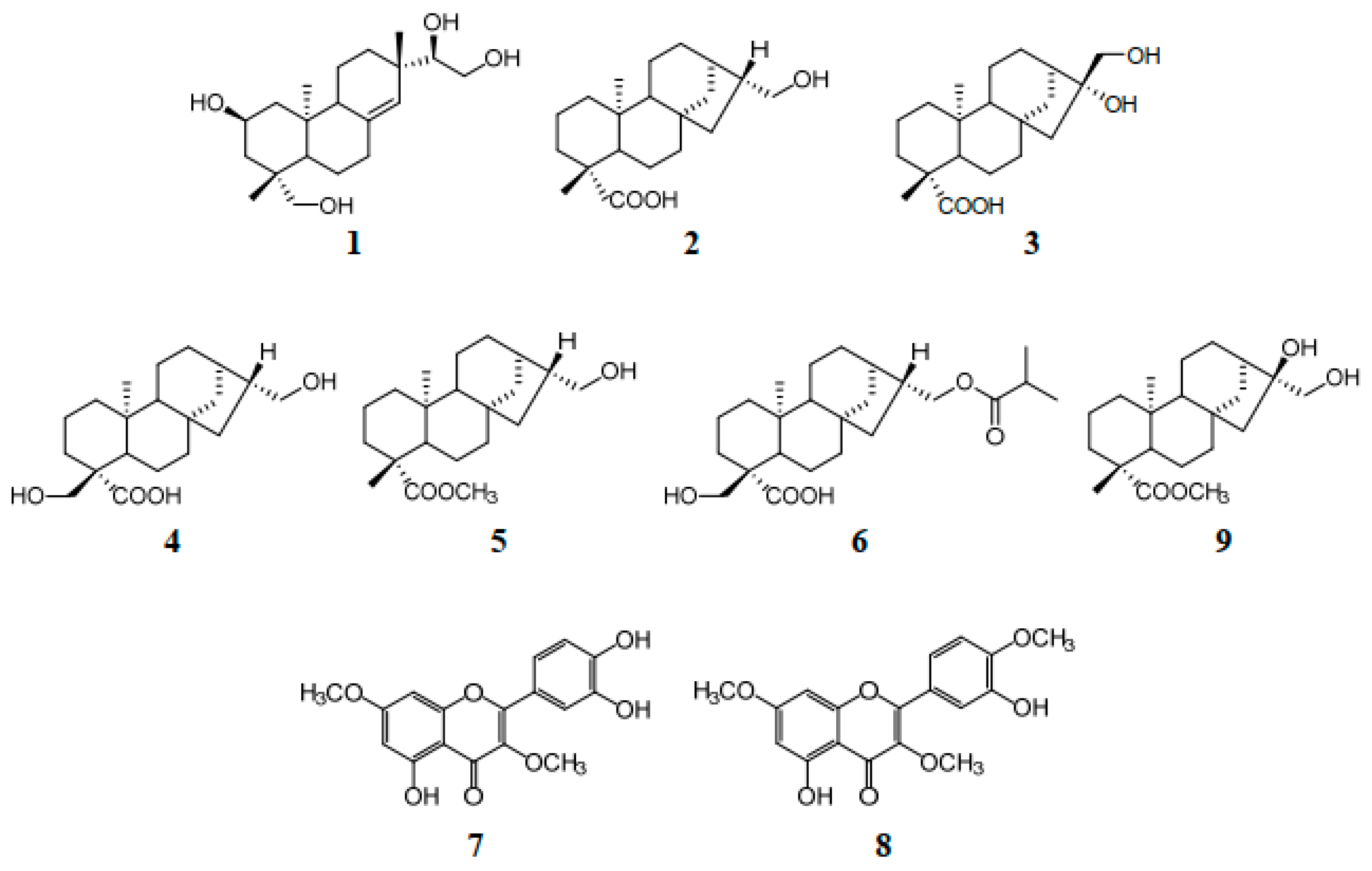
2.2. Bioactivity–Guided Isolation of Active Phytochemicals from S. glabrescens
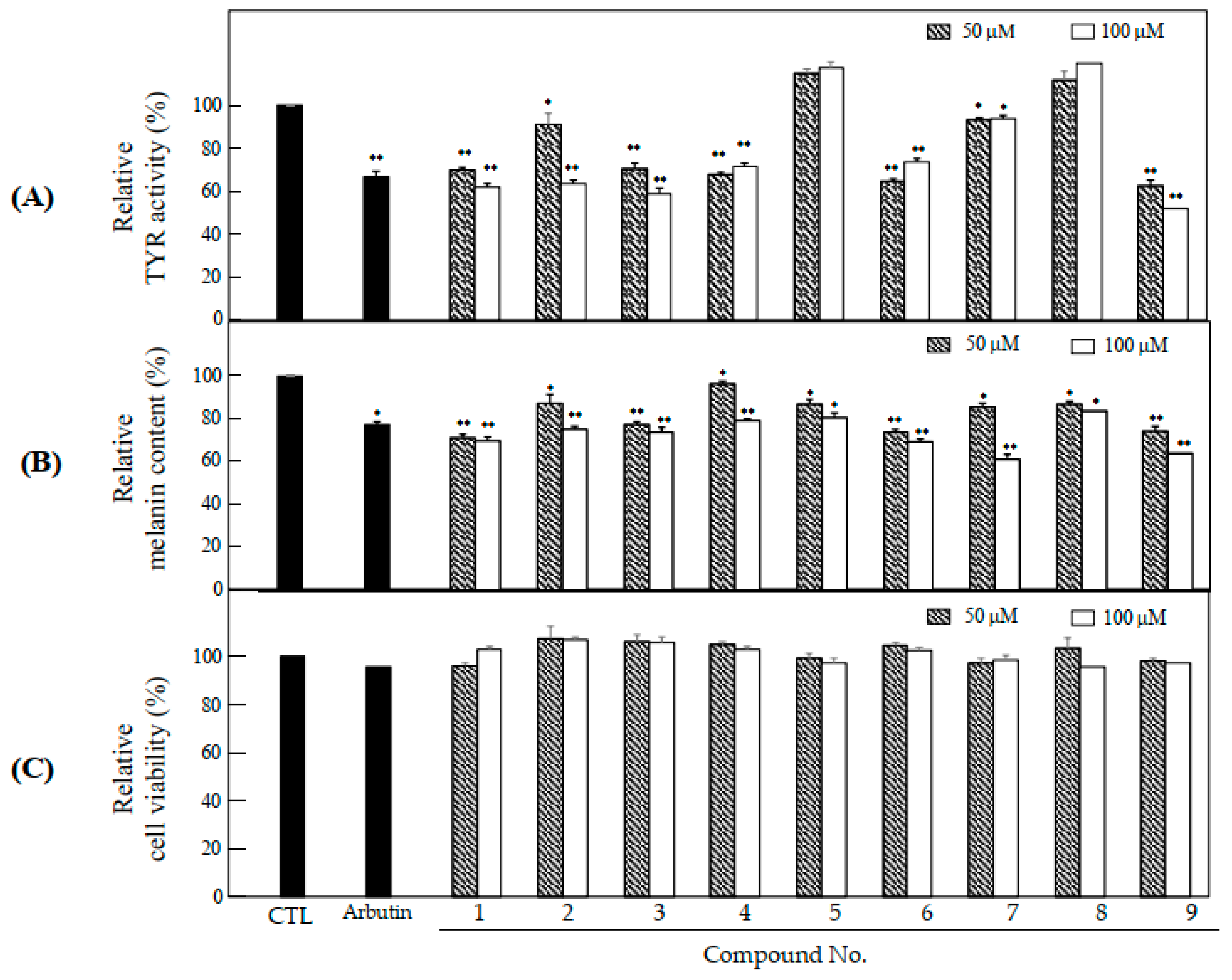
2.3. Anti-Melanogenesis and Anti-Oxidant Effects of Nine Compounds Isolated from S. glabrescens
2.4. Effects of K and MDK on Intracellular ROS Levels
2.5. Effects of K and MDK on TYRP-1, TYRP-2, and MITF Expression
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction and Isolation
3.3. Cell Culture and Viability Assay
3.4. Melanin Content Assay
3.5. Cellular TYR Activity Assay
3.6. Intracellular ROS Assay
3.7. In Vitro Antioxidant Assay
3.7.1. ABTS Assay
3.7.2. DPPH Radical Scavenging Assay
3.7.3. NO Radical Scavenging Assay
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Liu, F.; Fu, Y.; Meyskens, F.L., Jr. MiTF Regulates Cellular Response to Reactive Oxygen Species through Transcriptional Regulation of APE-1/Ref-1. J. Investig. Dermatol. 2009, 129, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S. Recent Development of Signaling Pathways Inhibitors of Melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Fujii, T.; Okuda, T.; Yasui, N.; Wakaizumi, M.; Ikami, T.; Ikeda, K. Effects of Amla Extract and Collagen Peptide on UVB-Induced Photoaging in Hairless Mice. J. Funct. Foods 2013, 5, 451–459. [Google Scholar] [CrossRef]
- Sun, Z.; Hwang, E.; Lee, H.J.; Lee, T.Y.; Song, H.G.; Park, S.; Shin, H.; Lee, D.; Yi, T.H. Effects of Galla Chinensis Extracts on UVB-Irradiated MMP-1 Production in Hairless Mice. J. Nat. Med. 2015, 69, 22–34. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tyminska, A. Skin Melanocytes: Biology and Development. Postepy. Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Korner, A.; Pawelek, J. Mammalian Tyrosinase Catalyzes Three Reactions in the Biosynthesis of Melanin. Science 1982, 217, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V. A Second Tyrosinase-related Protein, TRP-2, is a Melanogenic Enzyme Termed DOPAchrome Tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms Regulating Melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, M.; Berking, C. Principles of Skin Pigmentation. Biochemistry and Regulation of Melanogenesis. Hautarzt 2010, 61, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The Regulation of Skin Pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to Identify Inhibitors of Melanin Biosynthesis Via the Quality Control of Tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, M.V. Signaling Pathways in Melanosome Biogenesis and Pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase Inhibitors: A Patent Review (2011-2015). Expert Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.R.; Zhong, Z.F.; Sang, W.; Xiong, W.; Tao, H.X.; Zhao, G.D.; Li, Z.X.; Ma, Q.S.; Tse, A.K.W.; Hu, Y.J. Comparative Comprehension on the Anti-Rheumatic Chinese Herbal Medicine Siegesbeckiae Herba: Combined Computational Predictions and Experimental Investigations. J. Ethnopharmacol. 2019, 228, 200–209. [Google Scholar] [CrossRef]
- Gao, X.; Wei, J.; Hong, L.; Fan, S.; Hu, G.; Jia, J. Comparative Analysis of Chemical Composition, Anti-Inflammatory Activity and Antitumor Activity in Essential Oils from Siegesbeckia Orientalis, S. Glabrescens and S. Pubescens with an ITS Sequence Analysis. Molecules. 2018, 23, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Ko, H.; Choi, S.; Seo, D. Anti-Angiogenic Effects of Siegesbeckia Glabrescens are Mediated by Suppression of the Akt and p70S6K-Dependent Signaling Pathways. Oncol. Rep. 2015, 33, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, M.; Yun, J.G.; Hwang, J. Protective Effects of Standardized Siegesbeckia Glabrescens Extract and its Active Compound Kirenol Against UVB-Induced Photoaging through Inhtibition of MAPK/NF-κB Pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.; Shin, I.; Shin, N.; Hong, J.; Kwon, O.; Kim, H.; Oh, S.; Myung, P.; Ahn, K. Siegesbeckia Glabrescens Attenuates Allergic Airway Inflammation in LPS-Stimulated RAW 264.7 Cells and OVA Induced Asthma Murine Model. Int. Immunopharmacol. 2014, 22, 414–419. [Google Scholar] [CrossRef]
- Lee, H.N.; Joo, J.; Oh, J.S.; Choi, S.W.; Seo, D. Regulatory Effects of Siegesbeckia Glabrescens on Non-Small Cell Lung Cancer Cell Proliferation and Invasion. Am. J. Chin. Med. 2014, 42, 453–463. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Studies on the Antioxidant Activities of Cinnamon (Cinnamomum Verum) Bark Extracts, through various in Vitro Models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Batista, R.; Humberto, J.L.; Chiari, E.; de Oliveira, A.B. Synthesis and Trypanocidal Activity of Ent-Kaurane Glycosides. Bioorg. Med. Chem. 2007, 15, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Na, M.; Oh, H.; Jang, J.; Sohn, C.B.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. PTP1B Inhibitory Activity of Kaurane Diterpenes Isolated from Siegesbeckia Glabrescens. J. Enzyme. Inhib. Med. Chem. 2006, 21, 379–383. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, F.; Wu, C.; Wang, W.; Wu, Y. New E Nt-Kaurane Diterpenoids with Anti-Platelet Aggregation Activity from Annona s Quamosa. J. Nat. Prod. 2002, 65, 1462–1467. [Google Scholar] [CrossRef]
- Wang, R.; Chen, W.; Shi, Y. Ent-Kaurane and Ent-Pimarane Diterpenoids from Siegesbeckia Pubescens. J. Nat. Prod. 2010, 73, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Murakami, T.; Saiki, Y.; Chen, C. Chemical and Chemotaxonomical Studies of Filices. LIII. Chemical Studies on the Constituents of Dipteris Conjugata Reinw. Chem. Pharm. Bull. 1985, 33, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Etse, J.T.; Gray, A.I.; Waterman, P.G. Chemistry in the Annonaceae, XXIV. Kaurane and Kaur-16-Ene Diterpenes from the Stem Bark of Annona Reticulata. J. Nat. Prod. 1987, 50, 979–983. [Google Scholar] [CrossRef]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W. Regulation of Human Skin Pigmentation and Responses to Ultraviolet Radiation. Pigment. Cell. Res. 2007, 20, 2–13. [Google Scholar] [CrossRef]
- Lu, Y.; Aian, R.; Xiao, J.; Xu, D.; Fu, H.; Chen, Y. Kirenol, a compound from Herba Siegesbeckiae, induces apotosis in human chronic myeloid leukemia K562 cells. Pharmazie 2014, 69, 148–153. [Google Scholar]
- Wang, Y.; Xu, J.; Alarifi, S.; Wang, H. Kirenol inhibited the cell survival and induced apoptosis in human thyroid cancer cells by altering PI3K/AKT and MAP kinase signaling pathways. Environ. Toxicol. 2020. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Boissy, R.E. Tyrp1 and Oculocutaneous Albinism Type 3. Pigment. Cell. Res. 2001, 14, 437–444. [Google Scholar] [CrossRef]
- Fang, D.; Tsuji, Y.; Setaluri, V. Selective Down-regulation of Tyrosinase Family Gene TYRP1 by Inhibition of the Activity of Melanocyte Transcription Factor, MITF. Nucleic Acids Res. 2002, 30, 3096–3106. [Google Scholar] [CrossRef] [Green Version]
- Vachtenheim, J.; Borovanský, J. “Transcription Physiology” of Pigment Formation in Melanocytes: Central Role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Yasumoto, K.; Yokoyama, K.; Shibata, K.; Tomita, Y.; Shibahara, S. Microphthalmia-Associated Transcription Factor as a Regulator for Melanocyte-Specific Transcription of the Human Tyrosinase Gene. Mol. Cell. Biol. 1994, 14, 8058–8070. [Google Scholar] [CrossRef]
- Vance, K.W.; Goding, C.R. The Transcription Network Regulating Melanocyte Development and Melanoma. Pigment. Cell. Res. 2004, 17, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.; Ballotti, R.; Bertolotto, C. Glycogen Synthase Kinase 3β is Activated by cAMP and Plays an Active Role in the Regulation of Melanogenesis. J. Biol. Chem. 2002, 277, 33690–33697. [Google Scholar] [CrossRef] [Green Version]
- Hearing, V.J. Determination of Melanin Synthetic Pathways. J. Investig. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.H.; Tsatsakis, A.M.; Tzanakakis, G.; Kim, H.; Le, B.; Sifaki, M.; Spandidos, D.A.; Tsukamoto, C.; Golokhvast, K.S.; Izotov, B.N. Soyasaponin Ag Inhibits α‑MSH‑induced Melanogenesis in B16F10 Melanoma Cells Via the Downregulation of TRP‑2. Int. J. Mol. Med. 2017, 40, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.; Lim, S.; Lee, B.; Shin, T.; Kim, H. Ethanolic Extract of Sargassum Serratifolium Inhibits Adipogenesis in 3T3-L1 Preadipocytes by Cell Cycle Arrest. J. Appl. Phycol. 2018, 30, 559–568. [Google Scholar] [CrossRef]
- Lin, Y.; Chuang, M.; Chen, C.; Chien, M.; Hou, W. Nicotinic Acid Hydroxamate Downregulated the Melanin Synthesis and Tyrosinase Activity through Activating the MEK/ERK and AKT/GSK3β Signaling Pathways. J. Agric. Food Chem. 2012, 60, 4859–4864. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and their Phytoconstituents. J. Food Process. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, S.-Y.; Lee, Y.E.; Lee, M. Antioxidant Compounds, Kirenol and Methyl ent-16?, 17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production. Molecules 2021, 26, 1940. https://doi.org/10.3390/molecules26071940
Shim S-Y, Lee YE, Lee M. Antioxidant Compounds, Kirenol and Methyl ent-16?, 17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production. Molecules. 2021; 26(7):1940. https://doi.org/10.3390/molecules26071940
Chicago/Turabian StyleShim, Sun-Yup, Ye Eun Lee, and Mina Lee. 2021. "Antioxidant Compounds, Kirenol and Methyl ent-16?, 17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production" Molecules 26, no. 7: 1940. https://doi.org/10.3390/molecules26071940
APA StyleShim, S.-Y., Lee, Y. E., & Lee, M. (2021). Antioxidant Compounds, Kirenol and Methyl ent-16?, 17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production. Molecules, 26(7), 1940. https://doi.org/10.3390/molecules26071940




