Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders
Abstract
:1. Introduction
2. Results and Discussions
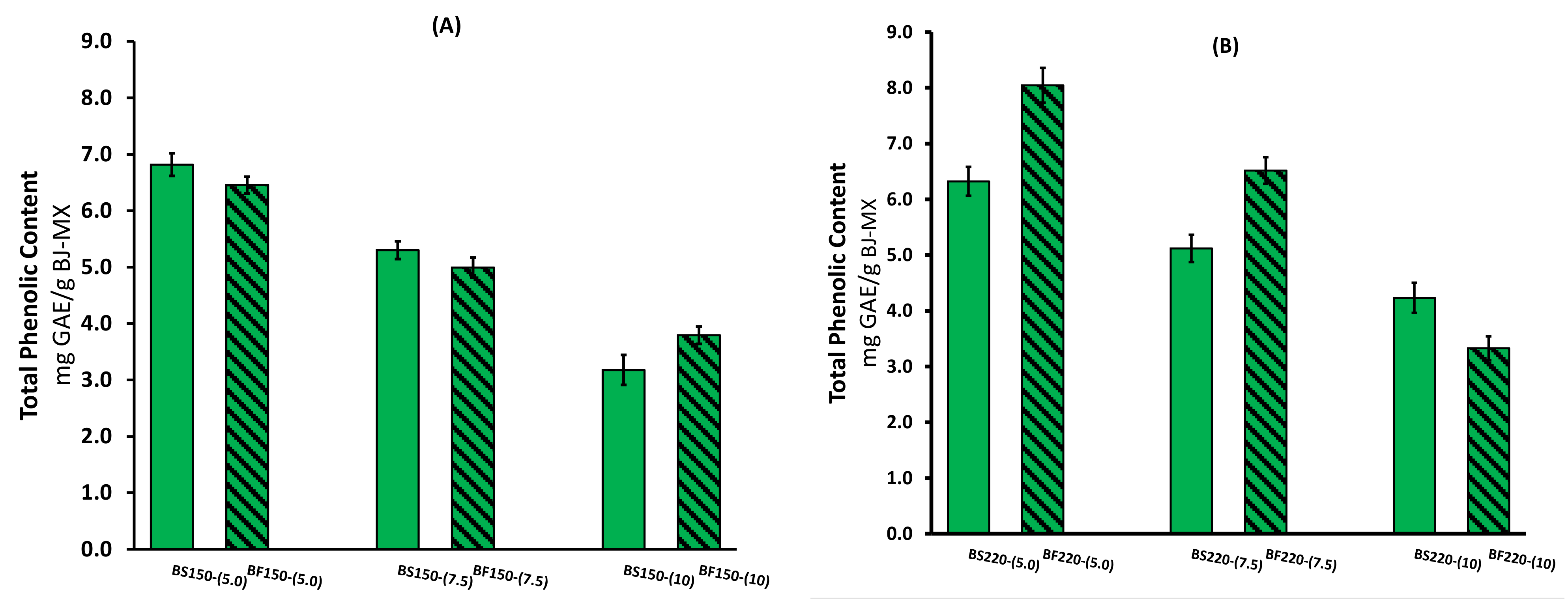
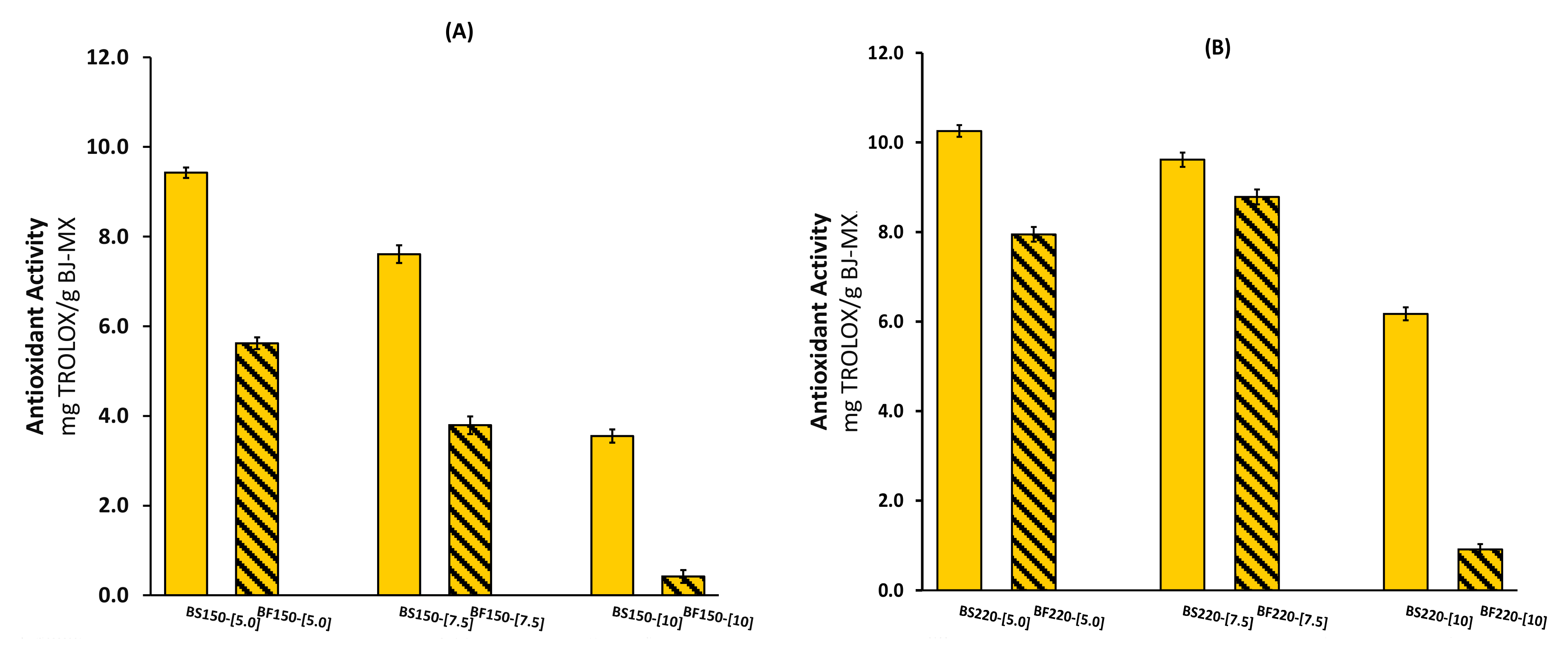
2.1. Total Phenolic Content and Antioxidant Activity
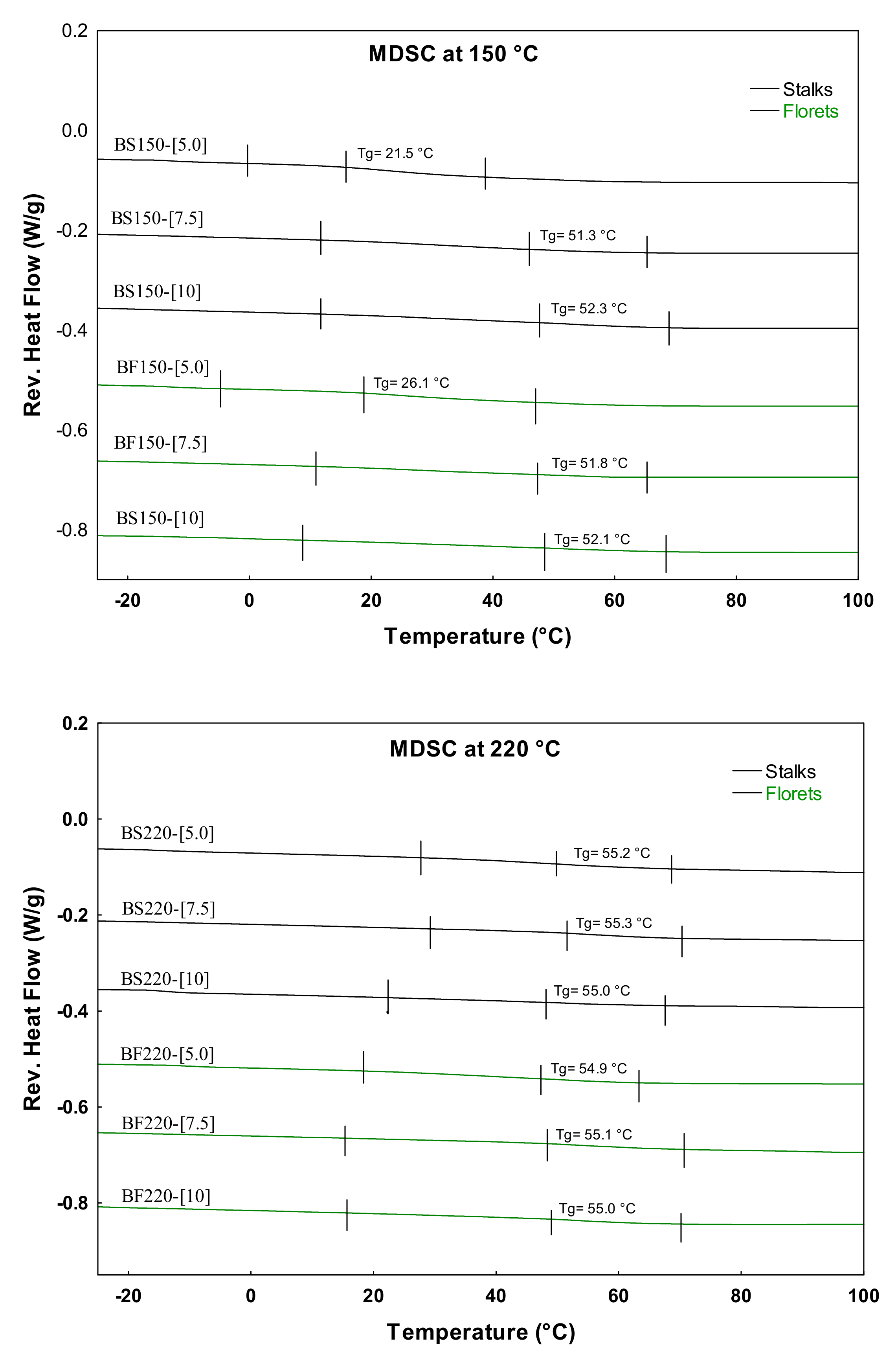
2.2. Thermal Characterization
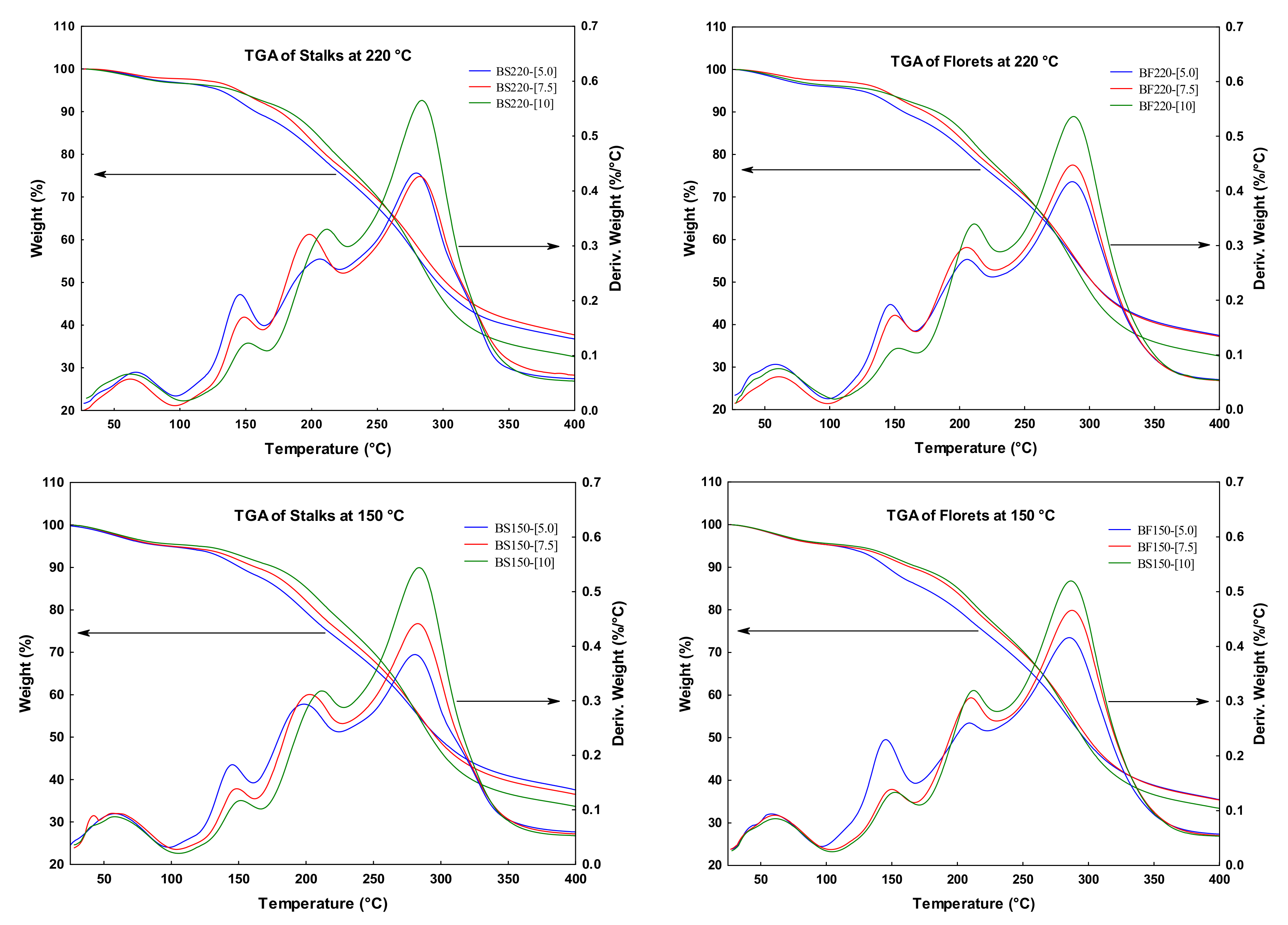
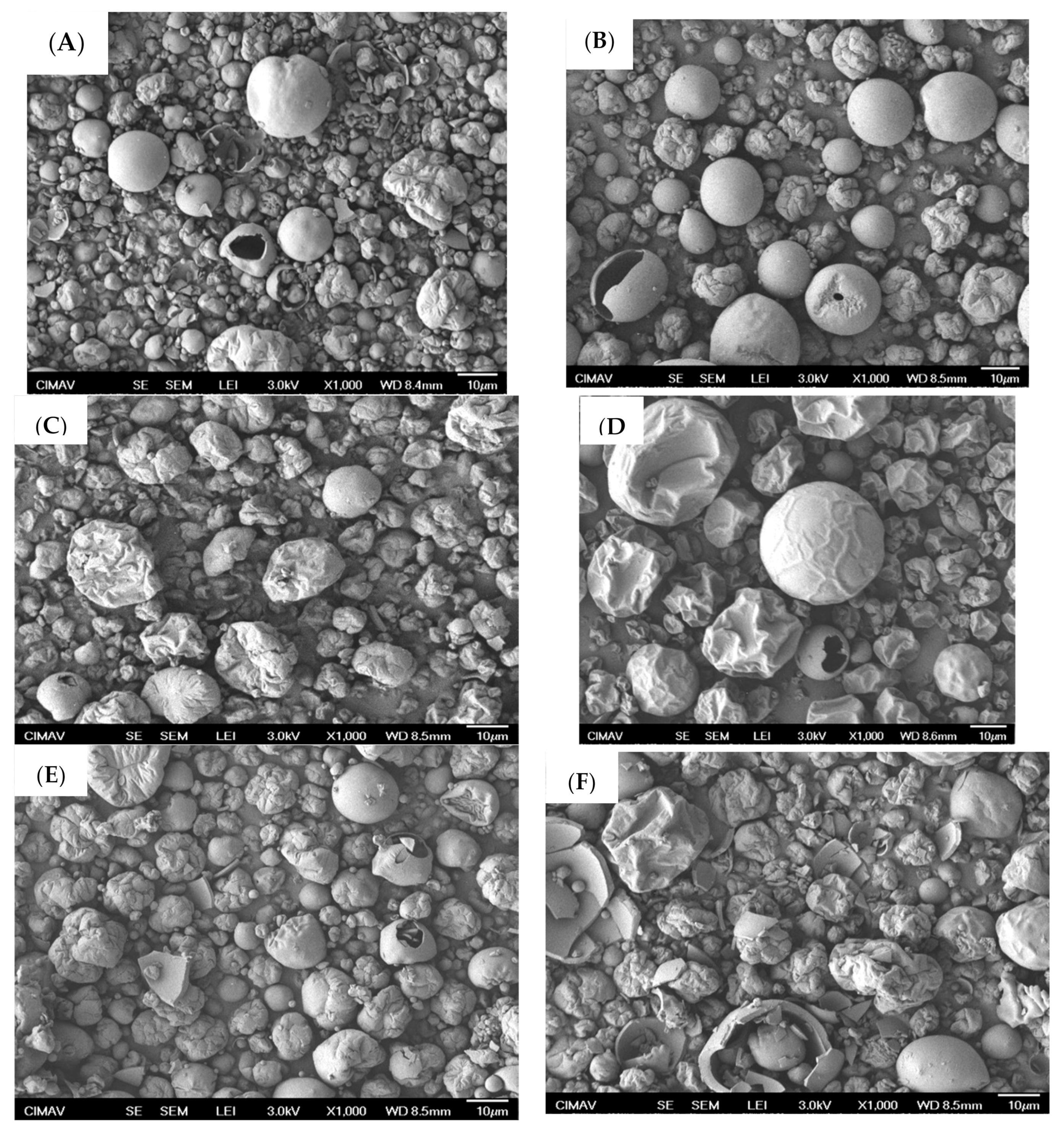
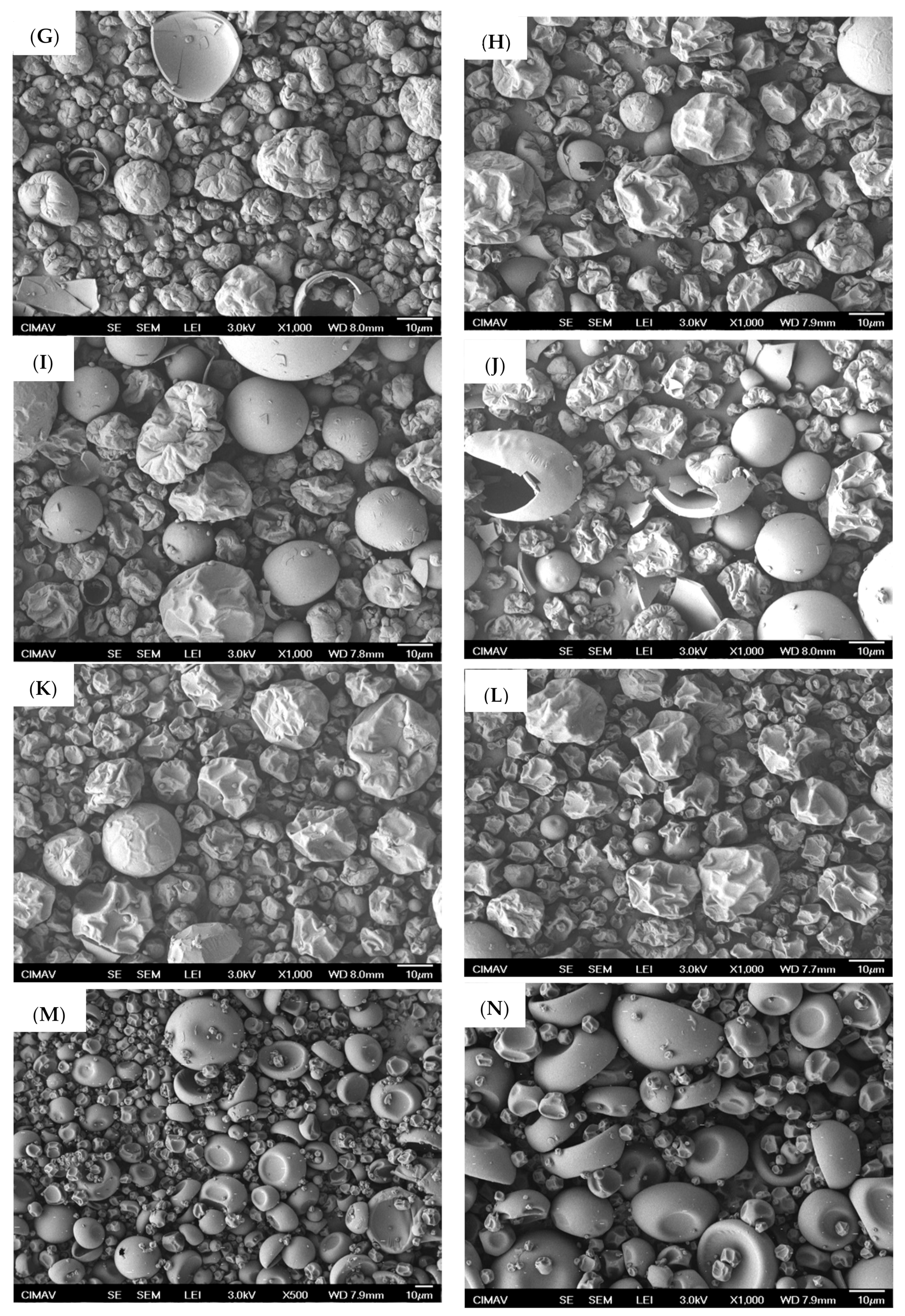
2.3. Microestructural Analysis
3. Materials and Methods
3.1. Materials
3.2. Extraction of Broccoli Floret and Stalk Juice
3.3. Preparation of Spray-Dried Powders
3.4. Antioxidant Activity
3.5. Determination of Total Phenolic Compounds
3.6. Thermal Analysis
3.6.1. MDSC
3.6.2. TGA-DSC-SDT
3.7. Physicochemical Characterization
3.7.1. Scanning Electron Microscopy
3.7.2. X-ray Diffraction
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Branham, S.E.; Stansell, Z.J.; Couillard, D.M.; Farnham, M.W. Quantitative trait loci mapping of heat tolerance in broccoli (Brassica oleracea var. italica) using genotyping-by-sequencing. Theor. Appl. Genet. 2017, 130, 529–538. [Google Scholar] [CrossRef]
- Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT). Broccoli (and Cauliflower) Production in 2019. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 February 2021).
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, H.R.; Mukherjee, S.; Das, D.K. Potential Health Benefits of Broccoli- A Chemico-Biological Overview. Mini Rev. Med. Chem. 2009, 9, 749–759. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Kehoe, K.; Reilly, K.; Rai, D.K.; Barry-Ryan, C. Development of a Novel Functional Soup Rich in Bioactive Sulforaphane Using Broccoli (Brassica oleracea L. ssp. italica) Florets and Byproducts. Food Bioprocess Technol. 2013, 7, 1310–1321. [Google Scholar] [CrossRef] [Green Version]
- Finley, J.W.; Ip, C.; Lisk, D.J.; Davis, C.D.; Hintze, K.J.; Whanger, P.D. Cancer-protective properties of high-selenium broccoli. J. Agric. Food Chem. 2001, 49, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, G.S.; Chouksey, A.; Jaiswal, S.; Jaiswal, A.K. Broccoli. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 5–17. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.W.; Botero-Omary, M.; Chung, F.-L. Disposition of Glucosinolates and Sulforaphane in Humans after Ingestion of Steamed and Fresh Broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T. Targeting cancer stem cells with sulforaphane, a dietary component from broccoli and broccoli sprouts. Future Oncol. 2013, 9, 1097–1103. [Google Scholar] [CrossRef]
- Funamoto, Y.; Yamauchi, N.; Shigenaga, T.; Shigyo, M. Effects of heat treatment on chlorophyll degrading enzymes in stored broccoli (Brassica oleracea L.). Postharvest. Biol. Technol. 2002, 24, 163–170. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry Technol. 2019, 38, 235–258. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; Cervantes-González, E.; Aguirre-Bañuelos, P.; Silva-Cázarez, M.B.; Álvarez-Salas, C. Spray drying of blueberry juice-maltodextrin mixtures: Evaluation of processing conditions on content of resveratrol. Antioxidants 2019, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Syamila, M.; Gedi, M.A.; Briars, R.; Ayed, C.; Gray, D.A. Effect of temperature, oxygen and light on the degradation of beta-carotene, lutein and alpha-tocopherol in spray-dried spinach juice powder during storage. Food Chem. 2019, 284, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Martínez-Guerra, E.; Pérez-García, S.A.; Aguilar-Martínez, J.A.; Álvarez-Salas, C. Physical properties of inulin and inulin-orange juice: Physical characterization and technological application. Carbohydr. Polym. 2014, 105, 10–19. [Google Scholar] [CrossRef]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.R. Taxonomy and Evolution of Broccoli (Brassica Oleracea Var. Italica). Econ. Botany 1982, 36, 397–410. Available online: http://www.jstor.org/stable/4254428 (accessed on 26 February 2021). [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D. Broccoli-derived by-products - a promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, L.; Spinelli, S.; Conte, A.; Del Nobile, M.A. Extract from Broccoli Byproducts to Increase Fresh Filled Pasta Shelf Life. Foods 2019, 8, 621. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Gallagher, E.; Bademunt, A.; Viñas, I.; Bobo, G.; Villaró, S.; Aguiló-Aguayo, I. Bioaccessibility, physicochemical, sensorial, and nutritional characteristics of bread containing broccoli co-products. J. Food Process Preserv. 2019, 43, e13861. [Google Scholar] [CrossRef]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Mrkìc, V.; Cocci, E.; Rosa, M.D.; Sacchetti, G. Effect of drying conditions on bioactive components and antioxidant activity of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2006, 86, 1559–1566. [Google Scholar] [CrossRef]
- Oberoi, D.P.S.; Sogi, D.S. Effect of drying methods and maltodextrin concentration on pigment content of watermelon juice powder. J. Food Eng. 2015, 165, 172–178. [Google Scholar] [CrossRef]
- Islam, Z.; Ayami, O.; Kitamura, Y.; Kokawa, M.; Takeshi, K.; Masayuki, K.; Norihiro, H. Micro wet milling and spray drying of whole mandarin powder and its characterization. Food Meas. 2021, 15, 851–861. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gliszczynska-Swiglo, A.; Ciska, E.; Pawlak-Lemanska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.N.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin−Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Melo-Santiago, F.E.; Rodrigues dos Reis, A.; de Figueiredo, M.A.; Zhou, S.; Thannhauser, T.W.; Li, L. Genotypic variation of flavonols and antioxidant capacity in broccoli. Food Chem. 2021, 338, 127997. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; López-Martínez, L.A.; González-García, R.; Martínez, J.O.; Compeán Martínez, I.; Toxqui-Terán, A. Evaluation of the Spray Drying Conditions of Blueberry Juice-Maltodextrin on the Yield, Content, and Retention of Quercetin 3-d-Galactoside. Polymers 2019, 11, 312. [Google Scholar] [CrossRef] [Green Version]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological Application of Maltodextrins According to the Degree of Polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Maldonado, D.; Espinosa-Solis, V.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Martinez-Gutierrez, F.; Román-Aguirre, M.; Saavedra-Leos, M.Z. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes 2020, 8, 849. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Caldeira-Morzelle, M. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raentos, R.M. Analysis of total phenols and another oxidation substrates anda antioxidants by means Folin-coicalteu. Methods Enzymol. 1999, 152–178. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Peng, H.; Ma, H.; Zeng, X.-A. Effect of inlet air drying temperatures on the physicochemical properties and antioxidant activity of whey protein isolate-kale leaves chlorophyll (WPI-CH) microcapsules. J. Food Eng. 2019, 245, 149–156. [Google Scholar] [CrossRef]
- Namavar, S.S.; Chayjan, R.A.; Parian, J.A.; Zolfigol, M.A. A multi-objective optimization of artichoke (Cynara scolymus L.) leaves aqueous extraction dehydration through a novel spray drying approach using response surface methodology. Iran. J. Chem. Chem. Eng. 2018, 37, 221–236. [Google Scholar]
- Movahhed, M.K.; Mohebbi, M. Spray Drying and Process Optimization of Carrot-Celery Juice. J. Food Process. Preserv. 2015, 40, 212–225. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007, 105, 101–106. [Google Scholar] [CrossRef]

| Run | MX Concentration (%) | Inlet Temperature (°C) | Broccoli Juice Source | Identification |
|---|---|---|---|---|
| 1 | 7.5 | 220 | Stalk | BS220-[7.5] |
| 2 | 7.5 | 220 | Floret | BF220-[7.5] |
| 3 | 7.5 | 150 | Stalk | BS150-[7.5] |
| 4 | 7.5 | 150 | Floret | BF150-[7.5] |
| 5 | 5.0 | 220 | Stalk | BS220-[5.0] |
| 6 | 5.0 | 220 | Floret | BF220-[5.0] |
| 7 | 5.0 | 150 | Stalk | BS150-[5.0] |
| 8 | 5.0 | 150 | Floret | BF150-[5.0] |
| 9 | 10.0 | 220 | Stalk | BS220-[10] |
| 10 | 10.0 | 220 | Floret | BF220-[10] |
| 11 | 10.0 | 150 | Stalk | BS150-[10] |
| 12 | 10.0 | 150 | Floret | BS150-[10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. https://doi.org/10.3390/molecules26071973
Saavedra-Leos MZ, Leyva-Porras C, Toxqui-Terán A, Espinosa-Solis V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders. Molecules. 2021; 26(7):1973. https://doi.org/10.3390/molecules26071973
Chicago/Turabian StyleSaavedra-Leos, María Zenaida, César Leyva-Porras, Alberto Toxqui-Terán, and Vicente Espinosa-Solis. 2021. "Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders" Molecules 26, no. 7: 1973. https://doi.org/10.3390/molecules26071973









