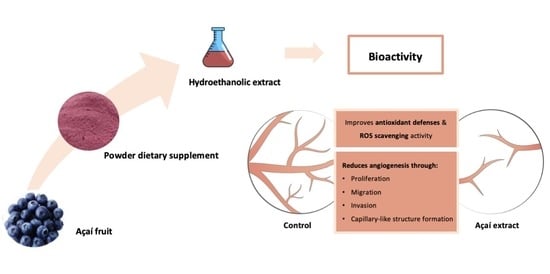
Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Profile of AHE by HPLC-DAD-ESI-MSn
2.2. Effect of AHE on Endothelial Cell Viability and Its Antiproliferative Behavior
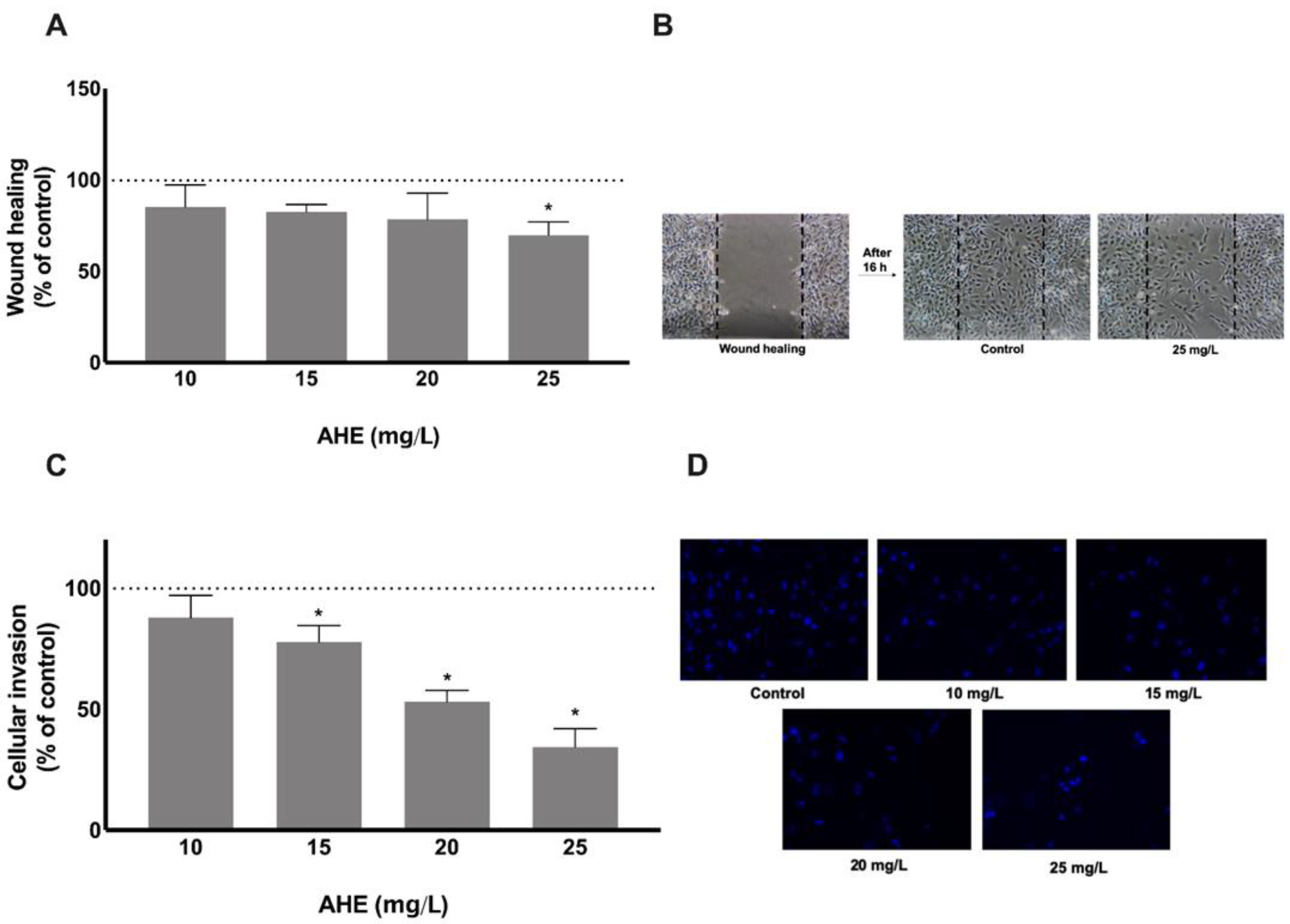
2.3. AHE Decreases Migration and Invasion Potential of Endothelial Cells
2.4. AHE Inhibited the Formation of Capillary-Like Structures Formation
2.5. Antioxidant Potential of AHE on Human Microvascular Endothelial Cells
3. Materials and Methods
3.1. Chemicals
3.2. Sample and Extract Preparation
3.3. HPLC-DAD-ESI-MSn Analysis
3.4. Cell Culture Experiments
3.5. MTS Toxicity Assay
3.6. BrdU Proliferation Assay
3.7. Migration Analysis
3.8. Invasion Assay
3.9. Matrigel Assay for Evaluation of Capillary-Like Structures Formation
3.10. Evaluation of Antioxidant Capacity in HMEC-1
3.10.1. Superoxide Dismutase Activity
3.10.2. Catalase Activity
3.10.3. Reactive Oxygen Species Formation
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- de Almeida Magalhães, T.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Alessandra-Perini, J.; De Moura, R.S.; Dos Santos, T.A.; Pereira, K.G.; Da Silva, Y.M.; Souza, P.J.C.; Nasciutti, L.E.; Perini, J.A. Euterpe oleracea Extract (Açaí) Is a Promising Novel Pharmacological Therapeutic Treatment for Experimental Endometriosis. PLoS ONE 2016, 11, e0166059. [Google Scholar] [CrossRef] [Green Version]
- Alessandra-Perini, J.; Perini, J.A.; Rodrigues-Baptista, K.C.; De Moura, R.S.; Junior, A.P.; Dos Santos, T.A.; Souza, P.J.C.; Nasciutti, L.E.; Machado, D.E. Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer. BMC Complement. Altern. Med. 2018, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nat. Cell Biol. 2011, 473, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.V.; Da Silveira, T.F.F.; Mattietto, R.D.A.; De Oliveira, M.D.S.P.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2016, 97, 1467–1474. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Lichtenthaler, R.; Rodrigues, R.B.; Maia, J.G.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacities of Euterpe oleracea Mart. (Acai) fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and Nutrient Composition of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.-E.; Vincken, J.-P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcott, S.T. Phytochemical Composition and Pigment Stability of Açai (Euterpe oleracea Mart.). J. Agric. Food. Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Gil Cruz, A.P.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; Mattietto, R.D.A.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
- de Jesus, A.L.T.; Cristianini, M.; dos Santos, N.M.; Maróstica Júnior, M.R. Effects of high hydrostatic pressure on the microbial inactivation and extraction of bioactive compounds from açaí (Euterpe oleracea Martius) pulp. Food Res. Int. 2020, 130, 108856. [Google Scholar] [CrossRef] [PubMed]
- Earling, M.; Beadle, T.; Niemeyer, E.D. Açai Berry (Euterpe oleracea) Dietary Supplements: Variations in Anthocyanin and Flavonoid Concentrations, Phenolic Contents, and Antioxidant Properties. Plant Foods Hum. Nutr. 2019, 74, 421–429. [Google Scholar] [CrossRef]
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic content, anti-inflammatory properties, and dermal wound repair properties of industrially processed and non-processed acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef]
- Dias, A.L.S.; Rozet, E.; Larondelle, Y.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. Development and validation of an UHPLC-LTQ-Orbitrap MS method for non-anthocyanin flavonoids quantification in Euterpe oleracea juice. Anal. Bioanal. Chem. 2013, 405, 9235–9249. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Dias, A.; Rozet, E.; Chataigné, G.; Oliveira, A.; Rabelo, C.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. A rapid validated UHPLC–PDA method for anthocyanins quantification from Euterpe oleracea fruits. J. Chromatogr. B 2012, 907, 108–116. [Google Scholar] [CrossRef]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Martins, G.R.; Amaral, F.R.L.D.; Brum, F.L.; Mohana-Borges, R.; de Moura, S.S.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.; Figueiredo, N.G.; da Silva, A.S. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMS(n) and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Schauss, A.G.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- Brunschwig, C.; Leba, L.-J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.-C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2016, 18, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MS(n). Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Mulabagal, V.; Calderón, A.I. Liquid chromatography/mass spectrometry based fingerprinting analysis and mass profiling of Euterpe oleracea (açaí) dietary supplement raw materials. Food Chem. 2012, 134, 1156–1164. [Google Scholar] [CrossRef]
- Costa, R.; Rodrigues, I.; Guardão, L.; Lima, J.Q.; Sousa, E.; Soares, R.; Negrão, R. Modulation of VEGF signaling in a mouse model of diabetes by xanthohumol and 8-prenylnaringenin: Unveiling the angiogenic paradox and metabolism interplay. Mol. Nutr. Food Res. 2017, 61, 4. [Google Scholar] [CrossRef]
- Moeenfard, M.; Cortez, A.; Machado, V.; Costa, R.; Luís, C.; Coelho, P.; Soares, R.; Alves, A.; Borges, N.; Santos, A. Anti-Angiogenic Properties of Cafestol and Kahweol Palmitate Diterpene Esters. J. Cell. Biochem. 2016, 117, 2748–2756. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Machado, V.; Costa, R.; Figueira, M.E.; Sepodes, B.; Barata, P.; Ribeiro, L.; Soares, R. Red Raspberry Phenols Inhibit Angiogenesis: A Morphological and Subcellular Analysis Upon Human Endothelial Cells. J. Cell. Biochem. 2015, 117, 1604–1612. [Google Scholar] [CrossRef]
- Marques, E.d.S.; Froder, J.G.; Oliveira, P.R.d.; Perazzo, F.F.; Rosa, P.C.P.; Gaivão, I.O.N.d.M.; Mathias, M.I.C.; Maistro, E.L. Cytotoxic effects of Euterpe oleraceae fruit oil (açaí) in rat liver and thyroid tissues. Rev. Bras. Farmacogn. 2019, 29, 54–61. [Google Scholar] [CrossRef]
- Marques, E.; Froder, J.; Carvalho, J.; Rosa, P.; Perazzo, F.; Maistro, E. Evaluation of the genotoxicity of Euterpe oleraceae Mart. (Arecaceae) fruit oil (açaí), in mammalian cells in vivo. Food Chem. Toxicol. 2016, 93, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant Capacity and Other Bioactivities of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceaeMart. (Acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Carvalho, M.M.D.F.; Lage, N.N.; Paulino, A.H.D.S.; Pereira, R.R.; De Almeida, L.T.; Da Silva, T.F.; Magalhães, C.L.D.B.; De Lima, W.G.; Silva, M.E.; Pedrosa, M.L.; et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G.; Scherwitz, L.; Beaman, R.; Endres, J.R.; Schauss, A.G. In Vitro and in Vivo Antioxidant and Anti-inflammatory Capacities of an Antioxidant-Rich Fruit and Berry Juice Blend. Results of a Pilot and Randomized, Double-Blinded, Placebo-Controlled, Crossover Study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Liz, S.; Cardoso, A.L.; Copetti, C.L.K.; Hinnig, P.D.F.; Vieira, F.G.K.; da Silva, E.L.; Schulz, M.; Fett, R.; Micke, G.A.; Di Pietro, P.F. Açaí (Euterpe oleracea Mart.) and juçara (Euterpe edulis Mart.) juices improved HDL-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin. Nutr. 2020, 39, 3629–3636. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Kłapcińska, B.; Podgórski, T.; Szade, B.; Tyl, K.; Hadzik, A. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol. Sport 2014, 32, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, P.O.; Pala, D.; Silva, C.T.; de Souza, M.O.; Amaral, J.F.D.; Vieira, R.A.L.; Folly, G.A.D.F.; Volp, A.C.P.; de Freitas, R.N. Açai (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 2016, 32, 674–680. [Google Scholar] [CrossRef]
- Cesar, L.T.; de Freitas Cabral, M.; Maia, G.A.; De Figueiredo, R.W.; De Miranda, M.R.A.; De Sousa, P.H.M.; Brasil, I.M.; Gomes, C.L. Effects of clarification on physicochemical characteristics, antioxidant capacity and quality attributes of açaí (Euterpe oleracea Mart.) juice. J. Food Sci. Technol. 2014, 51, 3293–3300. [Google Scholar] [CrossRef]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in Mesocarp/Epicarp and Endocarp of Fresh Acai (Euterpe oleracea Mart.) and their Antioxidant Activities and Bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Skemiene, K.; Pampuscenko, K.; Rekuviene, E.; Borutaite, V. Protective effects of anthocyanins against brain ischemic damage. J. Bioenerg. Biomembr. 2020, 52, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective effects of Anthocyanins against Amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Torma, P.D.C.M.R.; Brasil, A.V.S.; Carvalho, A.V.; Jablonski, A.; Rabelo, T.K.; Moreira, J.C.F.; Gelain, D.P.; Flôres, S.H.; Augusti, P.R.; Rios, A.D.O. Hydroethanolic extracts from different genotypes of açaí (Euterpe oleracea) presented antioxidant potential and protected human neuron-like cells (SH-SY5Y). Food Chem. 2017, 222, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ereminas, G.; Majiene, D.; Sidlauskas, K.; Jakstas, V.; Ivanauskas, L.; Vaitiekaitis, G.; Liobikas, J. Neuroprotective properties of anthocyanidin glycosides against H2O2-induced glial cell death are modulated by their different stability and antioxidant activity in vitro. Biomed. Pharmacother. 2017, 94, 188–196. [Google Scholar] [CrossRef]




| Peak | Rt (Min) | λmax (nm) | [M]+ (m/z) | MS2 Fragment Ions (m/z) | Proposed Identification | Peak Area (%) |
|---|---|---|---|---|---|---|
| 4 | 30.6 | 280, 517 | 449 | 287 | Cyanidin-3-O-glucoside | 6.2 ± 0.3 |
| 5 | 34.8 | 280, 517 | 595 | 449, 287 | Cyanidin-3-O-rutinoside | 89.0 ± 0.3 |
| 6 | 38.7 | 280, 508 | 579 | 433, 271 | Pelargonidin-3-O-rutinoside | 2.3 ± 0.1 |
| 7 | 41.2 | 280, 517 | 609 | 463, 301 | Peonidin 3-O-rutinoside | 2.6 ± 0.5 |
| Peak | Rt (Min) | λmax (nm) | [M-H]- (m/z) | MS2 Fragments (m/z) | MS3 fragments (m/z) | Proposed Identification | Content (mg/100 g dw) |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||||
| 1 | 18.2 | 260, 290 | 153 | 109 | - | Protocatechuic acid | 9.4 ± 0.9 a |
| Proanthocyanidins | |||||||
| 2 | 22.5 | 278 | 865 | 739, 713, 695, 577, 425, 407 | - | Proanthocyanidin trimer | 6.3 ± 0.1 b |
| 3 | 23.5 | 280 | 577 | 559, 451, 425, 407, 289 | - | Proanthocyanidin dimer | 25.7 ± 0.4 b |
| Total | 32.0 ± 0.5 b | ||||||
| Flavonoids | |||||||
| 8 | 48.9 | 277, 343 | 593 | 575, 503, 473, 383, 353 | 383, 353 | Apigenin-6,8-di-C-hexoside (vicenin-2) | 4.63 ± 0.2 c |
| 9 | 49.6 | 280, 340 | 579 | 561, 519, 489, 459, 429 | 399, 369 | Luteolin-6-C-pentoside-8-C-hexoside isomer 1 | 1.61 ± 0.01 c |
| 10 | 50.6 | 280, 340 | 579 | 561, 519, 489, 459, 429 | 399, 369 | Luteolin-6-C-pentoside-8-C-hexoside isomer 2 | 2.47 ± 0.02 c |
| 11 | 51.4 | 280, 340 | 579 | 561, 519, 489, 459, 429 | 399, 369 | Luteolin-6-C-hexoside-8-C-pentoside | 1.40 ± 0.04 c |
| 12 | 54.4 | 280, 330 | 563 | 545, 503, 473, 443, 383, 353 | 383, 353 | Apigenin-6-C-pentoside-8-C-hexoside | 4.1 ± 0.1 c |
| 13 | 55.8 | 283, 340 | 449 | 287, 269, 151 | 241, 225, 197, 181, 151 | Taxifolin deoxyhexose | 33.1 ± 0.5 c |
| 14 | 56.9 | 270, 348 | 447 | 429, 357, 327 | 327, 299, 285 | Luteolin-6-C-hexoside (orientin) | 12.7 ± 0.5 c |
| 15 | 57.3 | 270, 346 | 447 | 429, 357, 327 | 327, 299, 285 | Luteolin-8-C-hexoside (isoorientin) | 5.4 ± 0.1 c |
| 16 | 59.2 | 270, 340 | 431 | 341, 311 | 283 | Apigenin-8-C-hexoside (vitexin) | 4.79 ± 0.05 c |
| 17 | 62.7 | 290, 340 | 287 | 269, 259, 243 | 241, 215, 173, 151, 125 | Dihydrokaempferol | 9.6 ± 0.1 c |
| 18 | 64.1 | 274, 340 | 461 | 371, 341 | 313, 298 | Scoparin | 5.14 ± 0.02 c |
| 19 | 66.2 | 274, 335 | 689 | 609, 591, 569 | 519, 489, 399, 369 | Luteolin-C-hexoside-C-pentosidederivative | 11.06 ± 0.05 c |
| 20 | 68.3 | 274, 340 | 533 | 473, 443 | 383, 353 | Apigenin-6,8-di-C-pentoside | 2.39 ± 0.02 c |
| 21 | 71.6 | 275, 330 | 673 | 593, 575, 503 | 473, 383, 353 | Apigenin-di-C-hexoside sulfate | 11.6 ± 0.2 c |
| 22 | 75.4 | 280, 370 | 301 | 179, 151 | 151 | Quercetin | 2.54 ± 0.01 c |
| 23 | 77.2 | 260, 285, 350 | 285 | 241, 217, 199, 175, 151, 133 | - | Luteolin | 2.30 ± 0.06 c |
| 24 | 79.8 | 268, 350 | 315 | 300 | 283, 271, 227, 151 | Isorhamnetin | 5.10 ± 0.04 c |
| 25 | 80.0 | 268, 343 | 329 | 314 | 299 | Tricin | 3.46 ± 0.08 c |
| Total | 123.3 ± 0.7 c | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.; Azevedo, D.; Barata, P.; Soares, R.; Guido, L.F.; Carvalho, D.O. Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement. Molecules 2021, 26, 2011. https://doi.org/10.3390/molecules26072011
Costa R, Azevedo D, Barata P, Soares R, Guido LF, Carvalho DO. Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement. Molecules. 2021; 26(7):2011. https://doi.org/10.3390/molecules26072011
Chicago/Turabian StyleCosta, Raquel, Daniela Azevedo, Pedro Barata, Raquel Soares, Luís F. Guido, and Daniel O. Carvalho. 2021. "Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement" Molecules 26, no. 7: 2011. https://doi.org/10.3390/molecules26072011
APA StyleCosta, R., Azevedo, D., Barata, P., Soares, R., Guido, L. F., & Carvalho, D. O. (2021). Antiangiogenic and Antioxidant In Vitro Properties of Hydroethanolic Extract from açaí (Euterpe oleracea) Dietary Powder Supplement. Molecules, 26(7), 2011. https://doi.org/10.3390/molecules26072011